Abstract
The cryopreservation and autotransplantation of ovarian tissue is emerging as a powerful approach for preserving fertility. However, for cancer patients, it may not be possible to transplant ovarian tissue due to the risk of re-seeding disease. We investigated strategies for transplantation of individually isolated follicles to minimize the risk of re-introducing cancer cells present within the vasculature of ovarian stroma. Procedures for large-scale isolation of early-stage follicles and their encapsulation into fibrin hydrogels were developed. For in vivo validation studies, mice were ovariectomized and transplanted with encapsulated follicles into the ovarian bursa. A substantial increase in the number of secondary follicles was observed in the graft at 9 days after transplantation, and antral follicles by day 21, demonstrating primordial follicle recruitment into the growing pool. Initially, elevated follicle-stimulating hormone levels declined substantially by day 21, indicating feedback from the graft; presence of corpora lutea showed the graft's capability of restoring hormone cyclicity. Taken together, the transplanted follicles were able to engraft, mature, and restore ovarian function in an infertile mouse. This biomaterial may, thus, provide a platform for follicle transplantation with a low risk of cancer contamination and for developing strategies that preserve fertility for women facing a cancer diagnosis.
Introduction
Cancer patients' concern for their future fertility ranks second only to concerns about mortality, which has motivated the pursuit of strategies that preserve fertility.1 Unfortunately, many chemotherapies are fertility threatening; in particular, alkylating agents and platinum-based drugs are highly associated with post-treatment infertility, as they cause DNA damage to the oocytes that comprises the ovarian reserve.2–4 Most young women with cancer are interested in trying to preserve their fertility so they might have children in the future.5 While there have been promising results with fertility preservation, some of these techniques can require more time than is available to the patient or are not feasible depending on the patient's age or relationship status, among other variables.6 The traditional approach for fertility preservation involves hormonal stimulation, and subsequent egg or embryo banking; however, this option has constraints on its applicability, such as the nature of the disease, and availability of a male partner or willingness to use a sperm donor. Furthermore, egg retrieval requires hormonal stimulation that is time consuming and may have unwanted effects on women with hormone-responsive cancers. Moreover, pediatric patients cannot use in vitro fertilization technology for oocyte or embryo banking.
The cryopreservation and autotransplantation of ovarian tissue is emerging as a powerful approach for preserving fertility for patients who are losing ovarian function.7 Ovarian tissue transplantation has resulted in at least 24 published live births,8–20 20 of which were preceded by a freeze-thaw cycle,21 and at least 1 legal pregnancy termination after cancer recurrence.22 The number of live births demonstrates the feasibility of the approach, yet also indicates that advances are needed to make the procedure more widespread and safe. A primary focus of transplantation is the primordial follicle, which is the most immature follicle stage and is the most abundant follicle stage in the ovary (>70%) for women before perimenopause.23 In addition to primordial follicles, the ovary contains follicles at multiple stages of development (primary, preantral, and antral), along with stromal tissue. Harnessing the potential of these earliest-stage follicles to develop and produce mature eggs may provide greater opportunities for fertility preservation. In addition, transplantation of tissue with a large number of follicles could both restore endocrine function and produce multiple cycles for conception.
In this article, we investigated the engraftment and function of transplanted ovarian follicles as an alternative to transplantation of ovarian tissue. We focused on the transplantation of primordial follicles, which are the earliest-stage follicles and are present in the ovary in the greatest numbers, and aimed at supporting their engraftment and resumption of endocrine function. Follicles along with a small number of passenger stromal cells were delivered into the ovarian bursa in mice, and were encapsulated within fibrin hydrogels that support the isolated follicles in the absence of intact stromal tissue. Fibrin has been employed as a surgical sealant; however, it is also employed as a versatile scaffold for tissue engineering24 and as a vehicle for cell and tissue transplantation,24–27 which is due, in part, to its gelation under physiological conditions (isotonic, 37°C, and a pH 7.4). Human ovarian follicles have been transplanted into immunocompromised mice,11,28 with the follicles able to enter the growing pool. However, the functionality of the transplanted follicles was not addressed, in part, due to the long times for development and differences in hormones and cycles between mice and humans. Here, the functionality of the transplanted grafts was assessed through histology, hormone production, and resumption of the estrous cycle of the recipient. Systems for the efficient transplantation of ovarian follicles may ultimately provide novel options to preserve fertility for women facing a cancer diagnosis.
Materials and Methods
Fibrinogen preparation
Fibrinogen (Calbiochem) was reconstituted in water at 62.5 mg/mL. The fibrinogen solution was aliquoted in dialysis tubing (MWC 6–8 kDa; SpectraPor) and placed in excess Tris buffered saline overnight. Purified fibrinogen solution was adjusted to 40 mg/mL and stored until use at −20°C.
Ovarian tissue digestion
The procedure for large-scale isolation of early-stage follicles was based on three rounds of alternating chemical and mechanical digestion. Ovaries were dissected from 6-day-old F1 hybrid (C57Bl/6J×CBA/Ca) female pups, which have large numbers of primordial follicles, and transferred to Leibovitz L-15 (Gibco) media containing 0.15 Wünsch units/mL Liberase TM (Roche). Liberase, a purified enzyme blend, has previously been shown to yield highly viable and morphologically unaltered primordial and primary follicles in contrast to conventional collagenase blends containing endotoxins and other impurities.29 Ovaries were digested for 1 h, and gently sheared with a P10 pipette tip at 15-min intervals during the digest. Before each shearing, tissue was washed in 400 μL L-15 media before shearing in 400 μL L-15 containing 1% fetal bovine serum (FBS). Digestion, washing, and shearing were done in a 9-cavity Pyrex plate (Electron Microscopy Sciences) on a 30°C heating stage, as physiological temperature (37°C) leads to excessive evaporation and media hypertonicity during the procedures. The digestion was quenched by adding 50 μL FBS to the shearing well.
Ovarian tissue digestion and follicle isolation
Digested tissue was recovered under an inverted dissecting microscope by collecting 200 μL from the base of the cavity and transferring to a centrifuge tube for sedimentation to separate follicles from digested tissue. The sedimentation velocity of a primordial follicle (r=20 μm), from a suspension of digested, single cells, was estimated to be 7.8×10−4 m/s from the following equation:
 |
where v is the sedimentation velocity, r is the radius of the follicle or cell, ρ is the known density of Chinese Hamster Ovary cells (1.06 g/cm2), and ρ0 and μ are the density and viscosity of water.30 The sedimentation time is dependent on the height of 200 μL volume in a 0.6 mL centrifugation tube, which was determined to be ∼1.3 cm. Based on the earlier approximation, a primordial follicle would fall this distance in 17 min in pure water. In contrast, an arbitrary cell type with a radius of 5 μm would be expected to sink only 0.33 cm in 17 min. Empirically, a 30 min sedimentation time was used to account for potential error in approximations and assumptions. One hundred ninety microliters of supernatant is discarded after sedimentation. The follicle pellet is re-suspended in the remaining 10 μL volume.
Encapsulation in fibrin hydrogels
The follicle suspension was mixed at a 1:1 volume with 40 mg/mL fibrinogen solution. Ten microliters of this mixture was injected into an isotonic Tris encapsulation buffer (17.5 mM Tris, 105 mM NaCl, and 40 mM CaCl2) at 37°C containing 50 IU/mL thrombin (Sigma-Aldrich). The gelation period was 5 min before the formed hydrogels were transferred to L-15 media at 37°C, where they were maintained until transplantation.
Animal husbandry and care
Female C57Bl/6J mice (Jackson) were paired with male CBA/Ca (Harlan) mice to obtain C57Bl/6J×CBA/Ca F1 hybrids that were both used as pups for tissue donation and weaned as transplant recipients. The animals were maintained in a temperature-controlled room, on a 14:10 light:dark schedule with access to food and water ad libitum. Animals were treated in agreement with the NIH Guide for the Care and Use of Laboratory Animals and the IACUC protocol at Northwestern University.
Ovariectomy and transplantation
Before any surgical procedure, anesthesia was provided by an intraperitoneal injection of 100 mg/kg ketamine and 5 mg/kg xylazine. Ovaries were removed from the ovarian bursa as previously described31 in adult females (12–16 weeks of age) with some modification. Briefy, a midline incision was made in the abdominal wall. The intraperitoneal space was exposed with an abdomen retractor. Below the fat pad, a small incision was made in the bursal membrane, and the host ovary was removed at the hilum. To prevent collapse of the bursal cavity before re-transplantation, a 10 μL alginate (1.0% w/v) bead was placed into the membrane. The bursal membrane was then closed with 10-0 sutures. Animals were kept on a heated stage during surgery and recovery to prevent hypothermia. Females who did not have an estrous cycle, as determined by daily vaginal cytology, received a graft in both orthotopic sites at 14–21 days after ovariectomy as described earlier after removal of the alginate bead.
Vaginal cytology was performed daily starting at 5 days after ovariectomy for at least 8 days, a time over which normally cycling mice would be expected to have two cycles. After transplantation, vaginal cytology was again begun after 5 days, and was monitored for 2 weeks. The appearance of at least one cycle was counted as resumed cycling.
Serum follicle-stimulating hormone levels
Tail blood was collected weekly in a 5¾′ glass pasteur pipette. At the time of sacrifice, blood was collected via heart puncture. Blood was drained into a gel StatSampler centrifuge tube (Iris) and immediately sealed to prevent evaporation. The sample was allowed to clot for 1 h at room temperature. Serum (20 μL) was aspirated from the sample after centrifugation at 5400 rpm for 15 min and stored at −20°C. The sample was diluted 10-fold for determination of follicle-stimulating hormone (FSH) levels via radioimmunoassay (Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction).
Histological tissue analysis
Tissue and fibrin hydrogels were fixed in 4% buffered formaldehyde at 4°C overnight and transferred to 70% EtOH at 4°C until they were processed. Samples were embedded in paraffin, serially sectioned at a 5 μm thickness, and stained with hematoxylin and eosin (H&E). All developmental stages of follicles were quantified by counting the nucleolus every-other section to prevent counting a follicle more than once. Individual tracking of follicles through sequential slides confirmed that a nucleolus appears in an average of 1.9 and 2.0 sections in the fixed fibrin hydrogels (n=31) and ovarian tissue (n=37), respectively. A follicle was classified as primordial only if the surrounding granulosa cells were squamous. Follicles with a mix of squamous and cuboidal granulosa cells, or a single layer of cuboidal cells, were considered primary follicles. Two or more layers of cuboidal granulosa cells defined a secondary follicle, and the presence of a fluid-filled antrum defined an antral follicle. Corpora lutea were evident based on extensive permeation of red blood cells into the ovulated follicle.
CM-DiI staining and visualization
After 15 min of sedimentation, 100 μL was aspirated from the digestion product; 0.5 μL of a 2 mg/mL stock of CellTracker CM-DiI (Invitrogen) was added, incubated for 3 min at 37°C, and then placed on ice for 15 min for further sedimentation.32,33 DiI-labeled follicles were encapsulated in fibrin hydrogels as described earlier and immediately transplanted. After sacrifice, the ovary and surrounding tissue was fixed and processed as described earlier. Samples were de-paraffinized and labeled with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories). Samples were counterstained with hematoxylin for follicle pool quantification and images using a Nikon Eclipse E600 microscope (Nikon).
Statistical analysis
Follicles in stained histology samples were counted and classified by developmental stage, and average numbers and standard error of the mean were reported (Tables 1 and 2). Statistical analyses (one-way ANOVA) were performed using the software Prism 4.0 (GraphPad Software). A difference was considered significant if the p-value was <0.05.
Table 1.
Follicle Population Delivered in Fibrin Hydrogels
| Follicle developmental stage | |||||
|---|---|---|---|---|---|
| Primordial | Primary | Secondary | Antral | Sum | |
| Number (Avg±SEM) | 277±96 | 13.5±4.4 | 4.3±2.8 | 0±0 | 295±102 |
| Distribution (% of total) | 94±1 | 4.7±0.6 | 1.2±0.6 | 0±0 | 100 |
Fibrin hydrogels with encapsulated follicles were immediately fixed, rather than transplanted (n=6), and the population was characterized by manual counting after histological preparation.
SEM, standard error of the mean.
Table 2.
Follicle Development Distribution Within the Graft
| Time post-Tx (days) | Primordial (%) [follicle no.] | Primary (%) [follicle no.] | Secondary (%) [follicle no.] | Antral (%) [follicle no.] |
|---|---|---|---|---|
| 0 (n=6) | 94±1a [1661] | 5±1d [81] | 1.2±0.6f [29] | 0h [0] |
| 3 (n=4) | 89±3a [657] | 11±3d [101] | 0f [4] | 0h [0] |
| 9 (n=6) | 49±7b [318] | 46±6e [326] | 5±2f [31] | 0h [0] |
| 21 (n=5) | 17±4c [88] | 34±4e [179] | 41±6g [265] | 8h±1i [48] |
Percentages of primordial, primary, secondary, and antral follicles as a function of time after transplantation. Statistical analysis within each column was performed as a one-way ANOVA. Different letters indicate a significant different within that column (p<0.05). No statistical comparison was made between columns.
Results
Maintaining the orthotopic site after ovariectomy
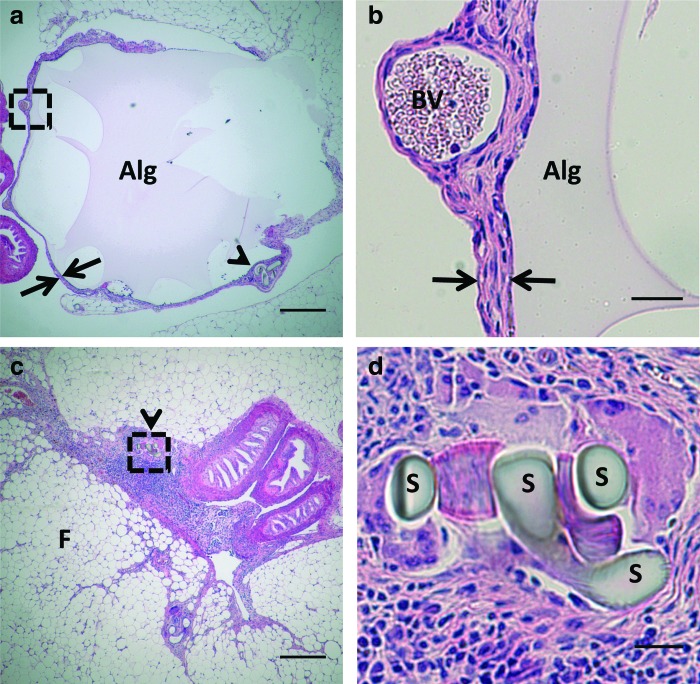
Mice were bilaterally ovariectomized, which was performed 2 weeks before transplantation. The bursa was opened, the ovary was removed, and this orthotopic site was preserved until follicle transplantation by implanting an alginate bead (Fig. 1a). Histology images confirm that no cellular infiltration into the material occurred, nor was inflammation present at the host-material interface (Fig. 1b). Maintenance of the space within the bursa is crucial to support blood vessel ingrowth to the graft. If either no biomaterial or a degradable biomaterial was inserted, the bursa collapsed and adhesions developed that made surgical placement of the graft impossible (Fig. 1c, d). The 2-week period between ovariectomy and transplantation was used to disrupt the negative feedback loop of the hypothalamus-pituitary-gonad axis, which stops cycling in the mice and leads to elevated levels of FSH. FSH levels were below the detection limit (<20 ng/mL) before ovariectomy and reached an average of 61.2±12.9 ng/mL (n=15) by the day of transplantation.
FIG. 1.
Maintenance of the ovarian orthotopic site with an alginate bead. (a) An alginate bead (Alg) was placed in the ovarian bursa (arrows) so that ovarian tissue could be transplanted at 14 days after ovariectomy. The incision is denoted by the presence of sutures (arrow head). (b) Neither cellular infiltration nor inflammation is observed at the host material interface of the alginate (Alg) and ovarian bursa (arrows). Blood vessels (BV) are observed within the bursa, which may initially support the transplant. (c) The absence of an alginate bead leads to collapse of the bursa, resulting in dense connective tissue. Sutures that identify the bursal tissue are denoted by arrow heads. (d) Magnification of the sutures (S) used to identify connective tissue as the collapsed bursa. Scale bars are 250 μm (a, c) or 25 μm (b, d), F denotes fat tissue, Color images available online at www.liebertpub.com/tea
Encapsulation of an immature follicle pool in a fibrin hydrogel
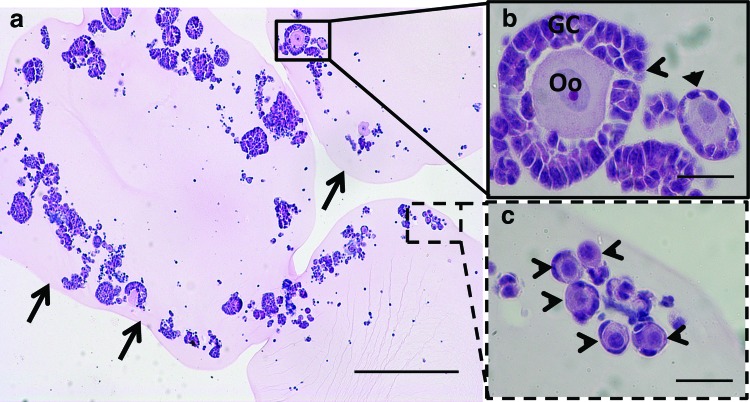
We subsequently developed procedures for large-scale isolation of early-stage follicles and their encapsulation into fibrin hydrogels. Follicles were isolated by a combination of mechanical and enzymatic digestion, and after digestion, the follicle population was enriched relative to individual cells by sedimentation without centrifugation (Table 1). The number of stromal cells associated with the follicles averaged 40±20 stromal cells per follicle. The isolated follicles were then encapsulated within fibrin hydrogels, which were examined by H&E staining to verify maintenance of follicle morphology (Fig. 2a). Factors considered in analyzing the morphology were a centrally located oocyte (the germ cell) enclosed by somatic granulosa cells and lack of fragmentation of the oocyte (Fig. 2b, c). Analysis of the fibrin-encapsulated follicles indicated morphologically intact follicles, with a population distribution of 94% primordial follicles, 4.7% primary follicles, and 1.2% secondary follicles. These results demonstrate the potential for large-scale isolation and encapsulation of morphologically intact follicles.
FIG. 2.
Encapsulation of an ovarian follicle pool in a fibrin hydrogel. (a) Representative image of a cross-section of follicles encapsulated within the fibrin hydrogel. The edge of the fibrin hydrogel is indicated with black arrows. (b) A secondary follicle (open arrow head) and primary follicle (closed arrow head) with a centrally located oocyte (Oo) and surrounding cuboidal granulosa cells (GC). (c) Primordial follicles with an oocyte and squamous granulosa cells. Scale bars are 250 μm (a) or 25 μm (b, c). Color images available online at www.liebertpub.com/tea
DiI confirms the donor origin of grafted tissue
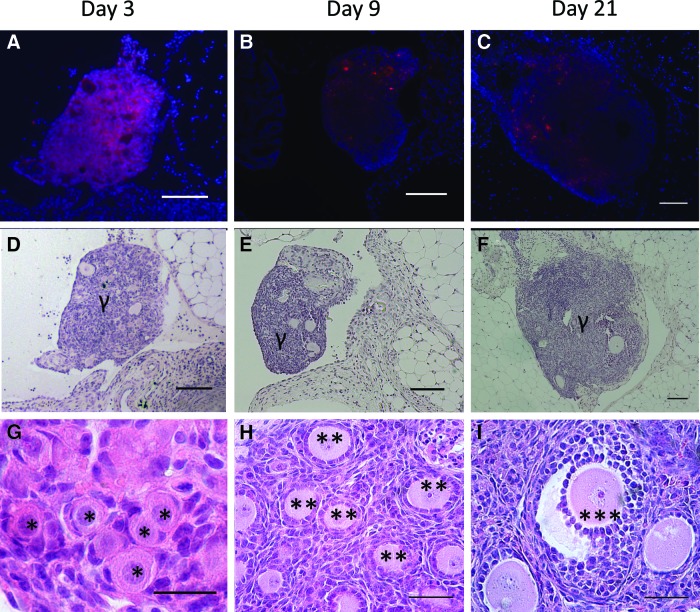
Follicle survival, morphology, and distribution of classes were subsequently investigated after transplantation of DiI-labeled follicles. DiI-labeled follicles (Fig. 3A–C) were readily observed in histological sections and distinguishable from native ovarian tissue at days 3, 9, and 21 post-transplantation, with numerous early-stage follicles readily seen within the transplant (Fig. 3D–I). Host ovarian tissue was negative for DiI fluorescence (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). The population distribution at day 3 was similar to the distribution reported after fibrin encapsulation (Fig. 4A), which was expected as the transition from an activated primordial to a primary follicle takes ∼1 week. Although the follicle distribution was similar pre- and post-transplantation, the graft morphology at day 3 differed significantly from that observed in the fibrin hydrogel pre-transplantation. Before transplantation, the follicles were dispersed throughout the hydrogel. At day 3, however, the ovarian follicles had coalesced into one or more small pieces of tissue, and an intact fibrin hydrogel was not observed (Fig. 3D, G) likely due to the degradation of fibrin. No significant effect of ischemic injury was apparent at day 3, as the population had not significantly changed between isolation and day 3.
FIG. 3.
DiI staining confirms origin of grafted tissue. (A) CM-DiI stained follicles in retrieved tissue at day 3 (A), day 9 (B), and day 21 (C). The corresponding hematoxylin counterstaining is shown in (D–F). DAPI and CM-DiI are blue and red, respectively. Graft is denoted by γ. (G) Hematoxylin and eosin staining of histological sections identified primordial follicles (denoted by an *) within the graft at day 3 h. At day 9, primary follicles (**) are observed within the graft. (F) After 21 days, some antral follicles are present (***). Scale bars are 100, 25, and 50 μm in (A–F, G), and (H–I), respectively. Color images available online at www.liebertpub.com/tea
FIG. 4.
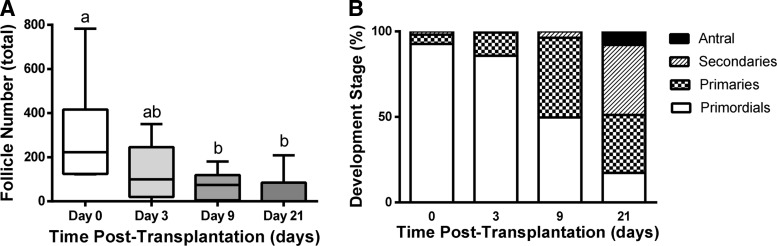
Follicle development and attrition. (A) Number of follicles as a function of time in the graft. Different letters above bars indicate significant difference (p<0.05). (B) Percentage of follicles in each developmental stage within the graft. The n was 6, 6, 10, and 12 for day 0, 3, 9, and 21, respectively.
At day 9, the graft was identified with DiI labeling, though the morphology of the graft also distinguished it from the host (Fig. 3B, E). The graft contained primordial follicles; however, a substantial increase in the number of secondary follicles was observed (Fig. 4B). The primordial follicles were recruited into the growing pool. A decrease in follicle survival was observed at 9 days post-transplantation (Fig. 4A). The follicle numbers did not decrease further between 9 and 21 days, suggesting that the first week after transplantation is the critical time for influencing follicle survival.
At day 21 of transplantation, grafts could be identified morphologically and with DiI staining (Fig. 3C, F), though the fluorescence intensity from DiI had significantly decreased relative to day 3. Day 21 was selected as that is the time reported for a primordial follicle to develop into a fully mature pre-ovulatory follicle, or a corpus luteum (CL) if an luteinizing hormone surge occurs.34 The grafts contained primordial, primary, secondary, and antral follicles. Antral follicles, the final stage of follicular development before ovulation, were identified by the presence of a fluid-filled cavity called the antrum (Fig. 3I). Less than 20% of the total follicle pool remained in primordial stage of development, and the remainder of the pool consisted of primary, secondary, and antral follicles (Fig. 4B). Between the time of transplantation and day 21, an 83% reduction of the follicle pool was observed (Fig. 4A). Interestingly, the grafts attached exclusively to the bursal tissue, as opposed to the ovarian hilum, which supplies the majority of the blood to the ovary.
Assessment of graft function
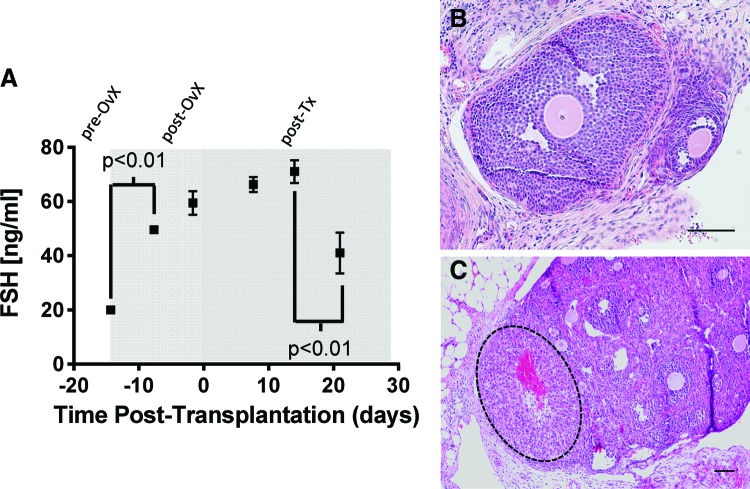
The function of the graft was assessed with serum hormone levels of FSH in transplant recipients. The number of grafts retrieved from mice in which the biomaterial and surviving follicles were observed was high at the initial time points, but declined to ∼40% of the mice by day 21 (Fig. 5). This percentage reflects the challenge in identifying fibrin in the implantation site due to its degradation. The FSH levels were relatively low (≈20 ng/mL) at the time of ovariectomy and increased to ∼60 ng/mL before transplantation (Fig. 6A). The FSH levels remained relatively constant during the first 2 weeks after follicle transplantation. However, by day 21 post-transplantation, the FSH levels had declined substantially. The levels remained greater than the initial levels before transplantation. Graft function, as observed with the reduction in FSH levels, was further confirmed by daily vaginal cytology, where 8 of 10 mice that were previously luteal (noncycling) for more than 1 month resumed cyclicity. In addition, large antral follicles (Fig. 6B) and post-ovulatory follicles or CL (Fig. 6C) were observed in histological sections for four out of five mice at 21 days post-transplantation. The CL was positive for CM-DiI staining, confirming the transplant origin, and had the typical hallmarks: centrally located blood clot, alignment of the stromal cells relative to the blood clot, and the lack of an oocyte and a basement membrane (Fig. 6C). Thus, the follicle pool survival and activation observed in histological analysis was in agreement with physiological changes of the mouse, indicating a functional graft.
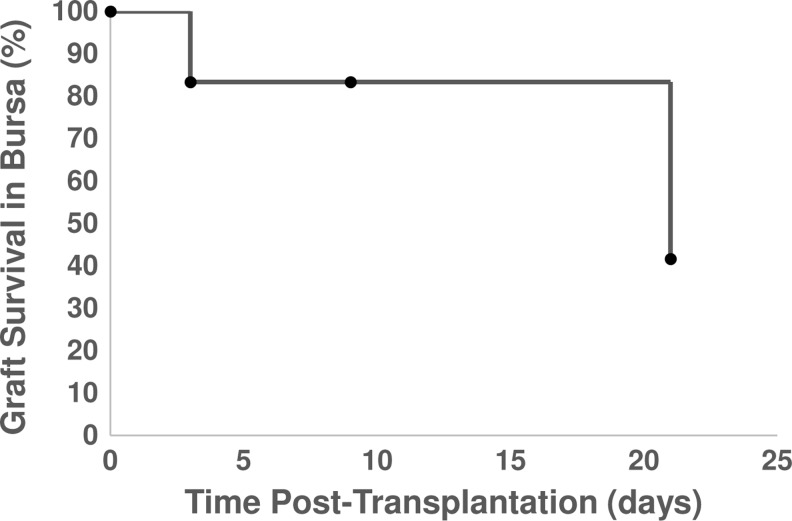
FIG. 5.
Graft survival. Graft survival was determined by the presence of transplanted follicles within the harvested bursa. Note that samples were harvested at each time point for analysis by histology, and the n for each time point was 6, 6, 10, and 12 for day 0, 3, 9, and 21, respectively.
FIG. 6.
Graft function. (A) Follicle-stimulating hormone (FSH) levels within the serum were measured pre-ovariectomy, post-ovariectomy but pre-transplantation, and post-transplantation. Statistical analysis was performed using a one-way ANOVA. (B) Large antral follicles and (C) corpora lutea were observed at 21 days post-transplantation in four of five mice, in agreement with the FSH levels. The corpus (dashed outline) luteum supports the occurrence of ovulation. Scale bars=100 μm. Color images available online at www.liebertpub.com/tea
Discussion
This article demonstrated the use of fibrin hydrogels for the transplantation of isolated ovarian follicles, which survived, were recruited into the growing pool, and were able to restore endocrine function that was usually performed by the ovary. The early-stage follicles that were transplanted developed into antral follicles, and corpora lutea were observed, suggesting an ovulatory event. The presence of antral follicles at day 21 is consistent with the time for primordial follicle activation, in which primordial follicles require ∼21 days to reach maturity as a pre-ovulatory antral follicle. Interestingly, the presence of antral follicles also correlated with a decline in FSH levels. In healthy mice, antral follicles produce high levels of estrogens in their granulosa cell compartment, which feedback to reduce FSH secretion by the pituitary gland in a cycling, fertile mouse. This time at which FSH levels began to decline is consistent with a previous report using the same mouse model that investigated the transplantation of ovarian tissue.27 Other groups have similarly had success with transplants and here, we extend this work to show the functionality of the graft through endocrine hormone assessment. The long-term goal of this work is to develop a bioengineered transplant approach for restoring human ovarian function in the location of the original ovary. The methods described here represent the first step toward this ultimate goal.
The limited stromal support was anticipated to be a significant factor limiting follicle survival and function, and a biomaterial was employed as a temporary support to maintain follicular structure until ingrowth of the host stromal cells. Fibrin enabled follicle encapsulation under conditions that maintained high cell viability. The isolation process can disrupt some cell–cell interactions within the follicle, though oocytes and somatic cells can reform connections, which are likely facilitated by encapsulation within the biomaterial.35,36 The isolation process resulted in modest levels of passenger stromal cells; nevertheless, the gels also provided a physical connectivity between the graft and host, and, thus, stromal cells infiltrate the graft and surround the follicles. The stromal cells, both endogenous and passenger, may contribute to engraftment and function of the transplanted follicles. Fibrin hydrogels, which are degradable and support cell ingrowth and integration with the host rather than isolation, have been widely used as surgical sealants, tissue engineering scaffolds, drug delivery, and cell and tissue transplantation.24–27,37 At the time of encapsulation, follicles are distributed throughout the hydrogel; however, they were observed in close proximity at days 9 and 21 post-transplantation, which may have resulted from fibrin degradation. The distribution of follicles within the implant is a critical design consideration that will require further study. The initially sparse density may have enabled follicle activation into the growing pool, as primordial follicles are usually at high density in the ovary, which may serve to modulate their growth.38 However, the subsequent aggregation between days 9 and 21 may have contributed to follicle survival and growth,38,39 as paracrine signaling influences follicle growth. While follicle transplantation has many challenges that are noted earlier, a potential advantage of this approach is the delivery of a known number and density of follicles. In tissue transplantation, the number of follicles may be unknown or heterogeneous, and isolation of follicles for transplantation in a biomaterial offers the opportunity to standardize the transplant procedures.
While transplanted follicles form mature follicles over time, the number of follicles and percentage of primordial follicles in the graft declines throughout 21 days. Recent reports have focused on relative short-term studies, with a reduction of 50% of follicles through 7 days.40,41 An 83% reduction of the follicle pool was observed through 21 days, with most of the decrease occurring during the first 9 days post-transplantation. The decline in follicle numbers was expected to result from limited follicle survival and follicle atresia after activation. Interestingly, this decline in follicle numbers is greater than what is observed with ovarian tissue transplantation (unpublished observations). A major factor in cell survival is revascularization, which has been reported to occur at about 2–5 days post-transplantation.42,43 Ischemic injury has been estimated to significantly decrease the size of the follicle pool.44 Interestingly, the ovary receives the majority of its blood supply from the ovarian hilum; however, the surviving follicles were observed adjacent to the bursal tissue. Follicle atresia after follicle activation likely also contributes to the decline in follicle number, as not all follicles that enter the growing follicle population develop to antral follicles.45 The number of primordial follicles that enter the growing pool is not likely to be influenced by the high levels of FSH for the 14 days after transplantation as receptors for this ligand are not present.27 Local peptide hormones such as Kit Ligand or anti-Mullerian hormone have also been implicated in follicle activation after transplantation46 and could be supplemented through peptide delivery from the biomaterial. Moreover, systems biology analysis of follicles across various stages of development are identifying key factors. Using time series microarrays, we recently identified cartilage oligomeric matrix protein (COMP) as a key follicle survival factor,47 and incorporation of these factors into the biomaterial provides an avenue to further support and promote follicle development. Finally, these studies investigated transplantation of follicles without significant stroma. Stromal cells secrete a range of factors that influence follicle activation and also follicle survival,48–52 and the hydrogel could be loaded with these cells as a means to augment the transplant environment.53
Transplantation of individually isolated follicles was investigated as a means to reduce the risk of re-seeding cancer cells that may be present within the vasculature or stroma of the ovary. Ovarian follicle transplantation is viewed as an intermediate approach within the spectrum of transplant procedures that are aimed at restoring fertility. At one end of the spectrum, ovarian tissue is cut into modest size pieces (i.e., no enzymatic digestion of the tissue) for subsequent transplant.27 At the other end of the spectrum, the ovary is completely dissociated into individual cells, and the oocytes are re-aggregated with somatic cells for transplantation.36 Our approach involves a partial digestion of the tissue, which also results in modest numbers of passenger stromal cells with the follicles. Ovarian tissue transplantation has been successful at fertility preservation. However, transplanted ovarian tissue may also contain cancer cells that pose a significant risk for translation to cancer patients.6,54–62 Cancer cells have the potential to metastasize to the ovary,63–66 or circulating tumor cells may be present within the vasculature of the ovary, both of which pose the risk of reimplanting malignant cells.67 Non-Hodgkins lymphomas, colon, melanoma, pancreas, gastric, and breast cancer, often called Krukenberg tumor, can metastasize to the ovary. Our transplanted follicles contained a modest number of stromal cells (40±20 stromal cells per follicle). While this number of stromal cells may reduce the risk of re-seeding disease relative to ovarian tissue transplantation or the transplantation of dissociated cells that were re-aggregated,28,36 further modifications to the isolation procedure are likely necessary to further minimize the risk.
Conclusions
Fibrin gels for the transplantation of early-stage ovarian follicles supported their engraftment and, ultimately, development to antral follicles. The fibrin hydrogels provide a platform for follicle transplantation that provides a support for the follicle; however, it enables cellular infiltration of cells from the host that leads to integration of the graft. Furthermore, the graft was able to reduce systemic FSH levels and restore cyclicity in a previously noncycling, infertile mouse, which has not been reported in recent reports with follicle transplantation.40,41 The significant decrease in follicle numbers and robust activation of primordial follicles into the growing pool may be addressed through the design of the local environment, as ovarian tissue transplants do not demonstrate the same decline in follicle numbers. Thus, designer environments for follicle transplantation provide the opportunity for female cancer patients to preserve their fertility while minimizing their risk of re-seeding disease.
Supplementary Material
Acknowledgment
This work was funded by the National Institutes of Health grant U54 HD076188.
Disclosure Statement
No competing financial interests exist.
References
- 1.Partridge A.H., et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 22,4174, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Oktem O., and Oktay K.Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110,2222, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Anderson R.A., and Cameron D.A.Assessment of the effect of chemotherapy on ovarian function in women with breast cancer. J Clin Oncol 25,1630, 2007; author reply 1632 [DOI] [PubMed] [Google Scholar]
- 4.Anderson R.A., et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 21,2583, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Schover L.R., et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 20,1880, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Jeruss J.S., and Woodruff T.K.Preservation of fertility in patients with cancer. N Engl J Med 360,902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolmans M.M.Safety of ovarian autotransplantation. Blood 120,4275, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Meirow D., et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 353,318, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Andersen C., et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod 23,2266, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Demeestere I., et al. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist 12,1437, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dolmans M.M., et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online 16,705, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Donnez J., et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364,1405, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Donnez J., et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril 95,1787.e1–;4, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ernst E., et al. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Human Reprod 25,1280, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Piver P., et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. Hum Reprod i15,2009 [Google Scholar]
- 16.Roux C., et al. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril 93,2413.e15, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Serrano M., et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril 93,268.e11, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Silber S.J., et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Human Reprod 23,1531, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Silber S.J.Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod 18,59, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Donnez J., et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 99,1503, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Donnez J., et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril 98,720, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Ernst E.H., et al. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet 30,975, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebar R.W.Endocrinology of the female. In: Becker K., ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott Williams & Wilkins, 2001, pp. 744–753 [Google Scholar]
- 24.Breen A., O'Brien T., and Pandit A.Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B Rev 15,201, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Deponti D., et al. Fibrin-based model for cartilage regeneration: tissue maturation from in vitro to in vivo. Tissue Eng Part A 18,1109, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chen X., et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A 15,1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shikanov A., et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A 17,3095, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolmans M.M., et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 134,253, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Dolmans M.M., et al. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod 21,413, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Harrison R.G., et al. Bioseparations Science and Engineering. New York: Oxford University Press, 2002 [Google Scholar]
- 31.Liu L., et al. Restoration of fertility by orthotopic transplantation of frozen adult mouse ovaries. Hum Reprod 23,122, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Kruyt M.C., et al. Application and limitations of chloromethyl-benzamidodialkylcarbocyanine for tracing cells used in bone tissue engineering. Tissue Eng 9,105, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Mimura T., et al. Transplantation of corneas reconstructed with cultured adult human corneal endothelial cells in nude rats. Exp Eye Res 79,231, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Pedersen T.Determination of follicle growth rate in the ovary of the immature mouse. J Reprod Fertil 21,81, 1970 [DOI] [PubMed] [Google Scholar]
- 35.Eppig J.J., Wigglesworth K., and Pendola F.L.The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A 99,2890, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll J., and Gosden R.G.Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod 8,1163, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Jackson M.R.Fibrin sealants in surgical practice: an overview. Am J Surg 182(2 Suppl),1S, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Da Silva-Buttkus P., et al. Inferring biological mechanisms from spatial analysis: prediction of a local inhibitor in the ovary. Proc Natl Acad Sci U S A 106,456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornick J.E., et al. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145,19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luyckx V., et al. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril 101,1149, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Gavish Z., et al. Follicle activation and ‘burn-out’ contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod 29,989, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Dissen G.A., et al. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology 134,1146, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Van Eyck A.S., et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril 93,1676, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Donnez J., Squifflet J., and Dolmans M.M.Frozen-thawed ovarian tissue retransplants. Semin Reprod Med 27,472, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Bristol-Gould S.K., et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol 298,149, 2006 [DOI] [PubMed] [Google Scholar]
- 46.David A., et al. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod 27,1088, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Skory R.M., et al. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev 80,132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magoffin D.A., and Magarelli P.C.Preantral follicles stimulate luteinizing hormone independent differentiation of ovarian theca-interstitial cells by an intrafollicular paracrine mechanism. Endocrine 3,107, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Parrott J.A., and Skinner M.K.Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev 55,55, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Huang C.T., et al. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod 64,451, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Nilsson E.E., and Skinner M.K.Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol 214,19, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Orisaka M., et al. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod 75,734, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Vanacker J., et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33,6079, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Silber S.J., Woodruff T.K., and Shea L.D.To transplant or not to transplant—that is the question. Cancer Treat Res 156,41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meirow D., et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod 23,1007, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Falcone T., et al. Ovarian function preservation in the cancer patient. Fertil Steril 81,243, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Lobo R.A.Potential options for preservation of fertility in women. N Engl J Med 353,64, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Sklar E.M.Post-transplant neurotoxicity: what role do calcineurin inhibitors actually play? AJNR Am J Neuroradiol 27,1602, 2006 [PMC free article] [PubMed] [Google Scholar]
- 59.Schroder C.P., et al. An in vitro model for purging of tumour cells from ovarian tissue. Hum Reprod 19,1069, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Nakayama K., et al. Gonadal failure after treatment of hematologic malignancies: from recognition to management for health-care providers. Nat Clin Pract Oncol 5,78, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Jemal A., et al. Cancer statistics, 2008. CA Cancer J Clin 58,71, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Kyono K., et al. Potential indications for ovarian autotransplantation based on the analysis of 5,571 autopsy findings of females under the age of 40 in Japan. Fertil Steril 93,2429, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Perlman S., et al. Non-Hodgkin's lymphoma presenting as advanced ovarian cancer—a case report and review of literature. Int J Gynecol Cancer 15,554, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Elharroudi T., et al. Primary lymphoma of the ovary. J Cancer Res Ther 4,195, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Kim J.W., et al. Ovarian and multiple lymph nodes recurrence of acute lymphoblastic leukemia: a case report and review of literature. Pediatr Surg Int 24,1269, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Monterroso V., et al. Malignant lymphomas involving the ovary. A clinicopathologic analysis of 39 cases. Am J Surg Pathol 17,154, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Dolmans M.M., et al. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril 99,1514, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.