Abstract
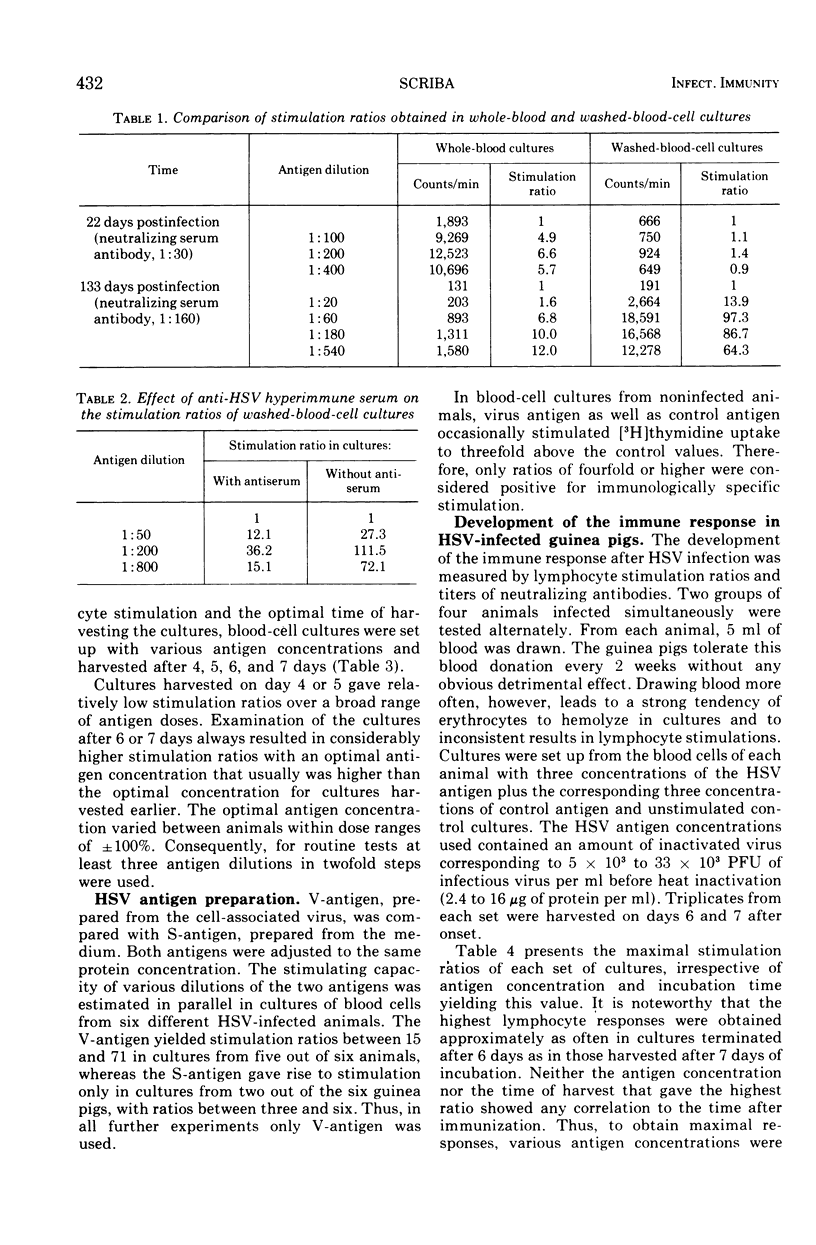
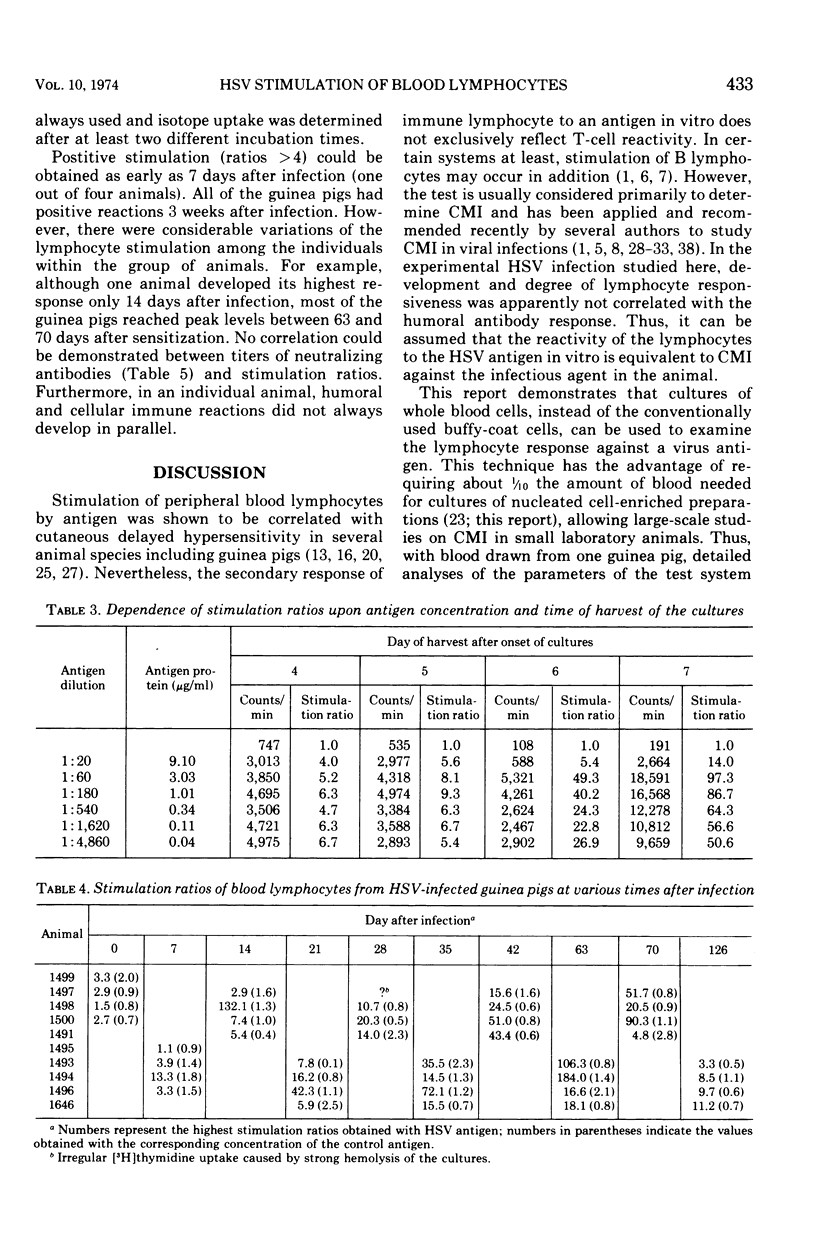
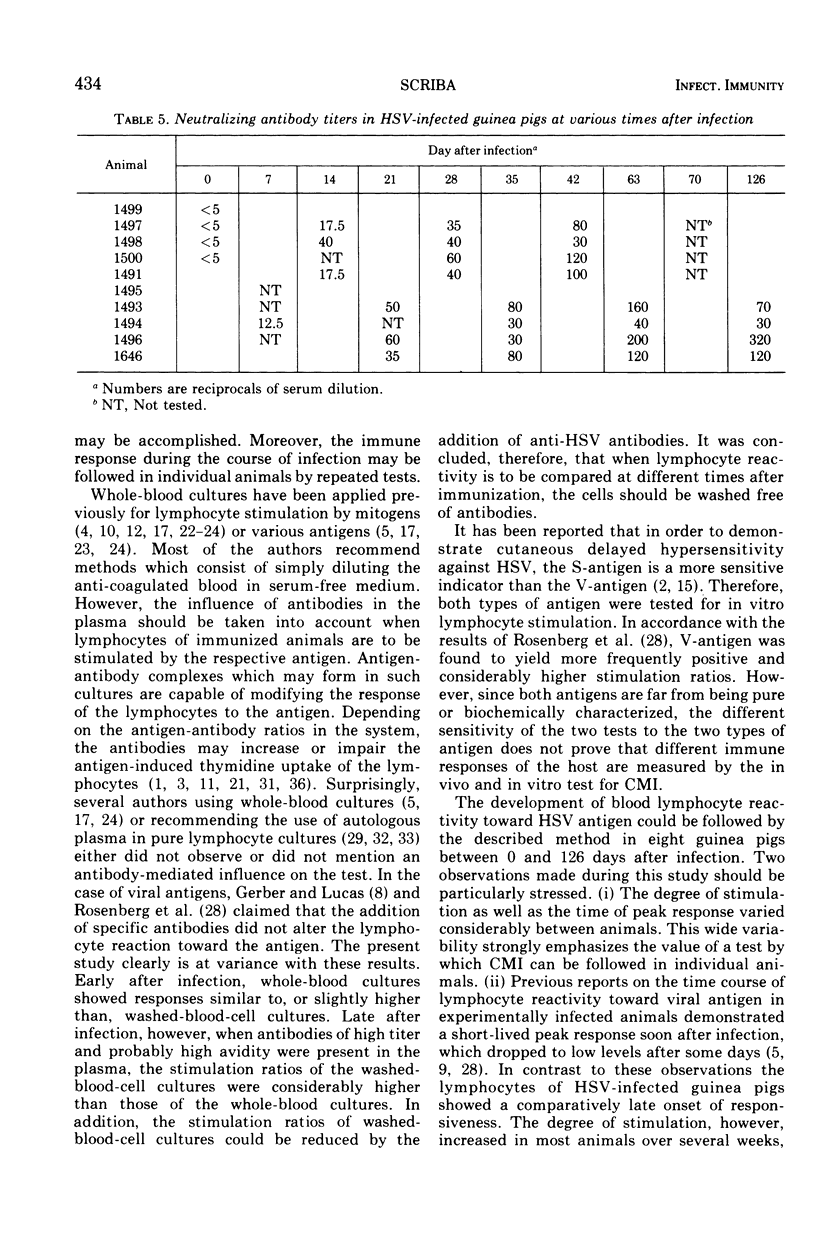
A method for stimulating sensitized lymphocytes by inactivated herpes simplex virus was established by using cultures of washed whole blood cells. The development of the immune response of herpes simplex virus-infected guinea pigs was examined at different times in the 4-month period after infection. Humoral and cellular immune responses were compared in individual animals by measuring lymphocyte stimulation ratios and neutralizing serum antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON W. A., KILBOURNE E. D. A herpes simplex skin test diagnostic antigen of low protein content from cell culture fluid. J Invest Dermatol. 1961 Jul;37:25–28. [PubMed] [Google Scholar]
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Banks K. L. The effect of antibody on antigen-induced lymphocyte transformation. J Immunol. 1973 Mar;110(3):709–716. [PubMed] [Google Scholar]
- Bryan J. H., Hybertson R. L. The in vitro stimulation of lymphocytes from peripheral blood and lymph nodes of the laboratory mouse. Cytogenetics. 1972;11(1):25–34. doi: 10.1159/000130173. [DOI] [PubMed] [Google Scholar]
- Davies D. H., Carmichael L. E. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infect Immun. 1973 Oct;8(4):510–518. doi: 10.1128/iai.8.4.510-518.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein G. J., Rosenberg G. L. In vitro proliferation of rabbit bone marrow-derived and thymus-derived lymphocytes in response to vaccinia virus. Cell Immunol. 1973 Jun;7(3):516–521. doi: 10.1016/0008-8749(73)90216-5. [DOI] [PubMed] [Google Scholar]
- Elfenbein G. J., Shevach E. M., Green I. Proliferation by bone marrow-derived lymphocytes in response to antigenic stimulation in vitro. J Immunol. 1972 Oct;109(4):870–874. [PubMed] [Google Scholar]
- Gerber P., Lucas S. J. In vitro stimulation of human lymphocytes by Epstein-Barr virus. Cell Immunol. 1972 Oct;5(2):318–324. doi: 10.1016/0008-8749(72)90057-3. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Cellular immune response to viral infection: in vitro studies of lymphocytes from mice infected with Sindbis virus. Cell Immunol. 1973 Dec;9(3):426–434. doi: 10.1016/0008-8749(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Han T., Pauly J. Simplified whole blood method for evaluating in vitro lymphocyte reactivity of laboratory animals. Clin Exp Immunol. 1972 May;11(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Harris G. Further studies of antigen stimulation of deoxyribonucleic acid synthesis in rabbit spleen cell cultures. II. The effects of specific antibody. Immunology. 1968 Mar;14(3):415–423. [PMC free article] [PubMed] [Google Scholar]
- Heiniger H. J., Wolf J. M., Chen H. W., Meier H. A micromethod for lymphoblastic transformation of mouse lymphocytes from peripheral blood. Proc Soc Exp Biol Med. 1973 May;143(1):6–11. doi: 10.3181/00379727-143-37242. [DOI] [PubMed] [Google Scholar]
- Hinz C. F., Jr, Daniel T. M., Baum G. L. Quantitative aspects of the stimulation of lymphocytes by tuberculin purified protein derivative. Int Arch Allergy Appl Immunol. 1970;38(2):119–129. doi: 10.1159/000230265. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Murphy F. A. Effects of anti-lymphoid sera on viral infections. Lancet. 1968 Jul 6;2(7558):37–40. doi: 10.1016/s0140-6736(68)92904-8. [DOI] [PubMed] [Google Scholar]
- JAWETZ E., COLEMAN V., ALLENDE M. F. Studies on herpes simplex virus. II. A soluble antigen of herpes virus possessing skin-reactive properties. J Immunol. 1951 Sep;67(3):197–205. [PubMed] [Google Scholar]
- Jevitz M. A., Ekstedt R. D. Correlation of lymphocyte transformation with the in vivo immune responsiveness of rabbits. J Immunol. 1971 Feb;106(2):494–505. [PubMed] [Google Scholar]
- Junge U., Hoekstra J., Wolfe L., Deinhardt F. Microtechnique for quantitative evaluation of in vitro lymphocyte transformation. Clin Exp Immunol. 1970 Sep;7(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed Proc. 1971 Nov-Dec;30(6):1822–1830. [PubMed] [Google Scholar]
- Oppenheim J. J. Modulation of in vitro lymphocyte transformation by antibodies: enhancement by antigen-antibody complexes and inhibition by antibody excess. Cell Immunol. 1972 Mar;3(3):341–360. doi: 10.1016/0008-8749(72)90243-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- Park B. H., Good R. A. A new micromethod for evaluating lymphocyte responses to phytohemagglutinin: quantitative analysis of the function of thymus-dependent cells. Proc Natl Acad Sci U S A. 1972 Feb;69(2):371–373. doi: 10.1073/pnas.69.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paty D. W., Hughes D. Lymphocyte transformation using whole blood cultures: an analysis of responses. J Immunol Methods. 1972 Nov;2(1):99–114. doi: 10.1016/0022-1759(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Sokal J. E. A simplified technique for in vitro studies of lymphocyte reactivity. Proc Soc Exp Biol Med. 1972 May;140(1):40–44. doi: 10.3181/00379727-140-36391. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Zweiman B. Characteristics of the in vitro response of guinea pig blood lymphocytes to PHA and antigen. J Immunol. 1970 Jul;105(1):204–214. [PubMed] [Google Scholar]
- Plummer G. Isolation of herpesviruses from trigeminal ganglia of man, monkeys, and cats. J Infect Dis. 1973 Sep;128(3):345–347. doi: 10.1093/infdis/128.3.345. [DOI] [PubMed] [Google Scholar]
- Ricci M., Romagnani S., Passaleva A., Biliotti G. Lymphocyte transformation and macrophage migration in guinea-pigs immunized with Freund's complete adjuvant. Clin Exp Immunol. 1969 Dec;5(6):659–667. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G., Gotoff S. P. Humoral and cellular response in humans after immunization with influenza vaccine. Infect Immun. 1973 Apr;7(4):594–596. doi: 10.1128/iai.7.4.594-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S., Maini R. A., Bailey M., Dumonde D. C. Cell-mediated immunity to Varicella-Zoster antigen in acute Herpes zoster (shingles). Clin Exp Immunol. 1973 Jun;14(2):181–185. [PMC free article] [PubMed] [Google Scholar]
- Simons M. J., Fitzgerald M. G. Rubella virus and human lymphocytes in culture. Lancet. 1968 Nov 2;2(7575):937–940. doi: 10.1016/s0140-6736(68)91167-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Chess L., Mardiney M. R., Jr The characteristics of lymphocyte tritiated thymidine incorporation in response to mumps virus. Cell Immunol. 1972 Dec;5(4):597–603. doi: 10.1016/0008-8749(72)90111-6. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Chess L., Mardiney M. R., Jr The relationship between rubella hemagglutination inhibition antibody (HIA) and rubella induced in vitro lymphocyte tritiated thymidine incorporation. Cell Immunol. 1973 Aug;8(2):321–327. doi: 10.1016/0008-8749(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Steele R. W., Hensen S. A., Vincent M. M., Fuccillo D. A., Bellanti J. A. A 51 Cr microassay technique for cell-mediated immunity to viruses. J Immunol. 1973 Jun;110(6):1502–1510. [PubMed] [Google Scholar]
- Thorbecke G. J., Siskind G. W. Effect of specific antibody on the antigen-induced proliferative response of rabbit lymph node cells. J Immunol. 1973 Mar;110(3):648–651. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Herpesvirus neutralization: induction of the persistent fraction by insufficient antibody. Virology. 1970 Sep;42(1):128–137. doi: 10.1016/0042-6822(70)90245-x. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]



