Abstract
Correct patterning and polarization of epithelial and mesenchymal cells are essential for morphogenesis and function of all organs and organisms. Epithelial cells are generally polarized in two axes: (a) the ubiquitous apical-basal axis and (b) polarity within the plane of the epithelium. The latter is generally referred to as planar cell polarity (PCP) and also is found in several contexts of mesenchymal cell patterning. In Drosophila, all adult structures display PCP features, and two conserved molecular systems (the Fat [Ft]/Dachsous [Ds] system and the Frizzled [Fz]/PCP pathway) that regulate this process have been identified. Although significant progress has been made in dissecting aspects of PCP signaling within cells, much remains to be discovered about the mechanisms of long-range and local PCP cell-cell interactions. Here, we discuss the current models based on Drosophila studies and incorporate recent insights into this long-standing cell and developmental biology problem.
Introduction
Establishment and maintenance of cellular polarization are critical features for development and organ function. Apical-basolateral (A/B) polarity of epithelial cells serves to perform vectorial functions, like transport of fluid or directed secretion of specific proteins [1]. Besides the ubiquitous epithelial A/B polarization, most epithelial tissues present a second polarity axis within the epithelial plane, referred to as PCP.
PCP was initially discovered in insects [2–4] and studied in Drosophila, where all adult cuticular structures display PCP [5–9]. Many processes and organs requiring PCP have been subsequently discovered in vertebrates ranging from skin and body hair orientation, positioning of primary cilia, and sensory epithelium in the inner ear to directed movement of mesenchymal cell populations during gastrulation among many other processes (for example, [10–14]). Other vertebrate PCP processes can easily be envisioned [15].
Unraveling the mechanisms of PCP establishment is a key developmental and cell biological question. How individual cells that are hundreds of cell diameters apart acquire the same polarity within the plane of an epithelial organ is a fascinating cell biological problem. Although much progress has been made, the mechanistic aspects of PCP establishment are far from being understood.
PCP regulatory systems: the Ft/Ds pathway and the Fz core group
Initial molecular insights identifying PCP signaling components came from genetic screens in Drosophila (Table 1) [5,16–19]. Functional studies in several tissues placed a set of the PCP regulators into different categories. These consist largely of two groups: (a) the Fz-PCP core group and (b) the Ft/Ds group. Whereas the latter revolves around the heterophilic adhesion of the two protocadherins Ft and Ds and the associated secreted Golgi kinase Four-jointed (Fj), the so-called Fz-PCP core genes historically comprise six proteins that interact with each other inter- and intracellularly to separate into two complexes on opposing sides of each cell, which provides a cell with a planar orientation (see Table 1 and Figure 1 for molecular details and published reviews for further insight on pathways and regulators associated with these core PCP systems). The transmembrane interacting components of the Fz core system are, besides Fz itself, the four transmembrane protein Van Gogh (Vang, also known as Stbm/Strabismus, Vangl in vertebrates) and the atypical cadherin Flamingo (Fmi, also known as Stan/Starry Night, Celsr in vertebrates) [7–9,13].
Table 1. General planar cell polarity regulatory systems in Drosophila.
| Drosophila component | Molecular features (intracellular) | Intercellular non-autonomous function/requirement | |
|---|---|---|---|
| Frizzled (Fz) core group | |||
| Frizzled (Fz) | Fz is a seven-pass transmembrane protein/receptor that binds and recruits Disheveled (Dsh) to the membrane. | Fz binds Wnt ligands via cysteine rich domain (CRD) region. CRD also acts as a “ligand” (signal sending in non-autonomous signaling) by binding to Van Gogh (Vang) across cell membranes. FzCRD-Vang interaction is regulated by Wg/Wnt4. |
|
| Disheveled (Dsh) | Dsh is a cytoplasmic protein containing DIX, PDZ,and DEP domains; is recruited to the membrane by binding to Fz and lipids; binds Fz, Prickle (Pk), Vang, and Diego (Dgo); and recruits Dgo to the membrane. |
None | |
| Diego (Dgo) | Dgo is a cytoplasmic ankyrin repeat protein; is recruited to the membrane by Dsh and Fz; binds Dsh, Vang, and Pk; and protects Dsh from Pk binding. | None | |
| Vang (also known as Strabismus (Stbm) |
Vang is a four-pass transmembrane protein, binds and recruits Pk to the membrane, and can also interact with Dsh and Dgo. |
Vang acts as a receptor for FzCRD in “signal receiving” cells in non- autonomous signaling across cell membranes. This interaction is regulated by Wg/Wnt4. |
|
| Prickle (Pk) | Pk is a cytoplasmic protein with three LIM domains and a PET domain, exists in two isoforms —called Pk and Sple (Spiny legs)—that act in distinct tissues, binds and is recruited by Vang to the membrane, can also physically interact with Dsh and Dgo, and competes with Dgo for Dsh binding. |
None | |
| Flamingo (Fmi) (also known as Starry night, or Stan) |
Fmi is an atypical cadherin with seven-pass transmembrane receptor features, co- immunoprecipates with Fz and Vang, and stabilizes these near adherens junctions. |
Fmi is a homophillic cell adhesion molecule that participates in intercellular interactions of Fz and Vang. |
|
| Furrowed (Fw) | Fw is a single-pass transmembrane protein, Drosophila member of the Selectin family, co- immunoprecipitates with Fz, and stabilizes Fz at the plasma-membrane. |
Fw is a homophilic cell adhesion molecule that participates in interactions of Fz and Vang. | |
| VhaPRR | VhaPRR is a Vha proton-pump subunit and homologue of PRR (pro-renin receptor), physically associates with the HRM (hormone-receptor domain) extracellular region of Fmi, and can be co- immunoprecipated with Fmi (and also Fz). |
None | |
| Fat/Dachsous systema | |||
| Fat (Ft) | Ft is a large proto-cadherin with many intracellular binding partners established (including Atrophin). Its signaling relay for PCP is unknown. Ft inhibits Dachs (D) membrane association. | Ft has heterophillic interaction with Ds and serves as a “receptor” in Ft-Ds interaction. |
|
| Dachsous (Ds) | Ds is a large proto-cadherin that stabilizes D at the plasma membrane. | Ds has heterophilic interaction with Ft and acts as a “ligand” in Ft-Ds interaction. | |
| Four-jointed (Fj) | Fj is a Golgi-resident kinase that phosphorylates extracellular cadherin domains of Ft and Ds. |
Fj modulates Ft-Ds intercellular binding. | |
| Dachs (D) | D is an atypical myosin that associates with Ds at the membrane and is an excellent marker for polarity orientation in the Ft/Ds system. | None |
All Drosophila genes have equivalent functional orthologues in vertebrates (often more than one). The homologous vertebrate genes for Fw and VhaPRR have not yet been functionally characterized in the planar cell polarity (PCP) context.
aSeveral other intracellular binding partners exist with yet-to-be-defined functions in PCP.
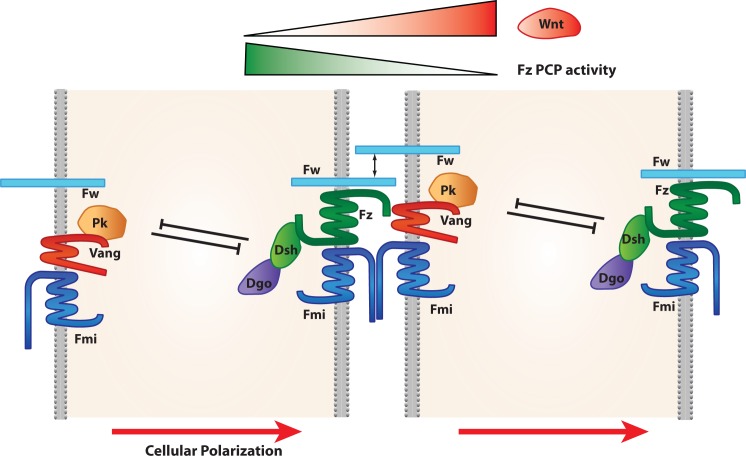
Figure 1. Schematic cartoons of the Fz/PCP core components across a two-cell border.
Note that Fz and Vang act in both the interpretation of the long-range signal(s) – Wg and dWnt4 - as well as the short-range/local cell-cell transmembrane interactions. The latter is also mediated by homophilic interactions of Fmi and Fw. The cytoplasmic components Dsh, Dgo, and Pk are essential for the negative feedback loops within individual cells, as they antagonize each other. Dgo, Diego; Dsh, Dishevelled; Fmi, Flamingo; Fw, Furrowed; Fz, Frizzled; PCP, planar cell polarity; PK, Prickle; Vang, Van Gogh.
The two systems are thought to mediate both long-range and cell-to-cell (short range) interactions in all tissues where PCP has been studied in Drosophila. The Fz/PCP core group appears fully conserved in vertebrate PCP contexts, although additional components, like receptor tyrosine kinases of the Ror family, are involved in vertebrate models. The function of the Ft/Ds group in vertebrates is less well defined, although there is good evidence in some tissues/organs for their requirement in PCP establishment [20–22]. Nevertheless, the study of both PCP systems in vertebrates is complicated by redundancy issues of multiple related proteins for each component. Also, whereas the Fz/PCP core group appears fully dedicated to PCP regulation, Ft/Ds signaling has also been linked to growth control and to Hippo signaling as an upstream activator module [23,24]. Despite these complications, an absolutely key question in PCP regulation is the molecular relationship of the two PCP systems or how their function is integrated within individual cells or groups of cells. Several interaction models have been proposed, but these remain controversial and are an active area of research (see below).
Despite the prevalence of studies on the Fz/PCP core group and Ft/Ds signaling in PCP establishment, it is noteworthy that additional cellular mechanisms function in cellular planar polarization contexts that appear to be largely (or fully) independent of these two molecular cassettes. Alone in Drosophila, the planar cellular polarization in the embryo that drives germband extension (a process comparable to vertebrate “convergence and extension”) and that is based on polarized myosin II localization seems to occur independently of either system [25,26].
New components of the Fz core group?
The six components of the Fz core group have been known for quite some time, the last of these—Diego (Dgo, related to Inversin and Diversin in vertebrates)—being added in 2001 [27]. However, recent work in Drosophila identified two additional factors that might be linked to PCP establishment in a similar manner and thus could also be considered members of the Fz core group.
These include the VhaPRR accessory subunit of the proton pump V-ATPase [28–30] and Furrowed (Fw), a Drosophila selectin family member [31]. Both affect PCP in a manner similar to the core components in all tissues analyzed (wing, eye, and thorax) and can be co-immunoprecipitated with Fz and Fmi (VhaPRR) or specifically with Fz (Fw). At least for Fw, its function appears to be similar to that of Fmi, as it is required to stabilize Fz in plasma membrane complexes, and it appears also to promote homophilic cell adhesion [31,32]. Moreover, Fw can mediate an intercellular binding/interaction between Fz and Vang, again a function related to what Fmi is doing [33], but unlike Fmi, Fw appears to affect only Fz stability and associates only with Fz (Figure 1). Strikingly, Fw and Fmi could be partially redundant in mediating an Fz-Vang interaction or Fz membrane association, as the tissues where Fmi mutants have a weaker phenotype (thorax) are where fw loss of function (LOF) is most severe; for the eye, the opposite is true. Importantly, the PCP defects in fw, fmi double mutants are not more severe than those in fz null flies, suggesting that both require the presence of Fz for their function. The role of the VhaPRR protein remains to be clarified, but functional studies suggest that it can affect the trafficking of Fmi and possibly Fz to or from the membrane (or both). Whereas VhaPRR can be co-immunoprecipitated with both Fz and Fmi, it appears to directly physically associate with Fmi only [30]. While the function of Fw is restricted largely to PCP (with only a mild overgrowth of the eye disc and a potential role in cytoskeletal regulation), VhaPRR appears generally required for trafficking, affecting also Fz2 (canonical Wingless [Wg] signaling), Notch, and E-cad [29,30].
Short- and long-range signaling in PCP establishment
The biggest conceptual questions in PCP establishment are how long-range signals might regulate the process and how this is relayed to and reinforced through local cell-to-cell interactions. From early studies with LOF mutants, it was clear that cellular orientation was non-random (best visualized in the wing) and that cellular orientation would indeed follow/adjust to the neighbor's patterns, easily observed in fz [3,34], ds [35], Ft [36], fmi [32,37], or Vang mutants [38,39]. This behavior was further clarified with clonal analyses and the discovery of non-cell autonomous effects. An example is the non-autonomous behavior of the fz mutant or overexpression clones affecting the polarity of wildtype cells surrounding the clones in the wing, eye, and abdomen [34,40,41]. These observations set the stage for analyses of local and global signals to work on PCP orientation: local signals with short-range abilities to communicate on a cell-to-cell basis and global signals that coordinate the process at the organ level over a long range. What are the characteristics for such signals? First, there needs to be spatial restriction or directional signaling for a given “signal”. Second, a connection between the long-range signal and the cell-cell communication mechanism(s) should exist. Two global clues had been associated with long-range PCP signaling: (a) the Ds and Fj expression gradients in the Ft/Ds system [36,42] and more recently (b) the long-range effects of localized Wnt expression on the Fz/PCP core factors [43,44]. How are the Ft/Ds or Fz-PCP core pathways interpreting these signals, and how are these two systems integrating the information?
Ft/Ds pathway short- and long-range
The Ft/Ds pathway covers both the (long-range) spatial restriction and the (short-range) cell-cell communication features of PCP establishment. Related to long-range effects, two of its key components have a graded/localized expression pattern: in the wing, fj is expressed in an ascending proximo-distal gradient [45–47] and Ds is expressed at higher levels in the proximal part, opposite to the Fj gradient [42], although its slope is rather steep as compared with other gradients. The third main component, Ft, has a rather uniform expression in the wing [42,48]. Within the current model, Ds acts as a ligand for Ft, inhibiting its function and creating a distal-to-proximal Ft activity gradient [42] (reflected in anisotropic subcellular distribution of Ft, along with the fj expression gradient). This model applies also for the eye, where fj is expressed in a graded manner with its peak at the equator, ds forms gradients with peaks at the poles and Ft is uniformly expressed [36,49]. Importantly, it seems that only one of the graded expression features is necessary. For example, uniform Ds expression rescued PCP in the wing [50]. Similarly, in the eye, flat Ds expression rescued the ds mutant as long as fj was expressed in a gradient [49].
Next, polarity needs to be “translated” from the long-range (body) axes to the local (short-range) cell-to-cell communication. How is the long-range Ft/Ds pathway information transmitted to the short-range cell-to-cell interactions? At the single-cell level, the atypical cadherins Ft and Ds are localized subapically in epithelial cells near but not directly at the adherens junctions, where they form heterodimers [42,51]. This heterophilic cell-cell adhesion promotes their stabilization mutually across cell membranes [50]. Fj functions as a kinase at the Golgi, affecting the localization of Ft and Ds and the strength of their interactions [52,53]. Clones lacking Ft or Ds have opposing effects on neighboring wildtype cells. For example, Ft mutant tissues induce surrounding cells to “point” toward the clone, whereas ds clones reorient cells away from the mutant patch [40,54], and fj mutant patches also reorient cells toward the clone [40,54,55]
Several unresolved mysteries exist in Ft/Ds “signaling”. Heterodimer formation, though seemingly essential for cell-to-cell communication, is itself not required for Ft/Ds PCP “signaling” to work, since Ds binding to Ft is not necessary for Ft activity, as shown by the electron capture dissociation (ECD)-Ft (Ft lacking the extracellular domain) [56]. The mirror experiment adds even more mystery, as the Ds extracellular region is sufficient to generate PCP defects, whereas its intracellular region is thought to stabilize Dachs, an atypical myosin that serves as a good marker for asymmetric Ft/Ds interactions [56]. The open questions remain (at least in part) because downstream effectors of the Ft/Ds pathway in PCP are functionally largely not defined, although several proteins are known to bind to Ft, for example. Dachs is currently the only known asymmetric PCP “marker” downstream of these complexes, exhibiting a polarized localization (already at the third instar larval stage), with Ds recruiting Dachs to the membrane and Ft antagonizing its membrane localization [51,57,58]. Although Dachs localization is a very useful marker for this pathway, its function in PCP establishment is still elusive, largely because its LOF phenotype is very mild (and this is further complicated by the observation that the intracellular domain of Ds, where Dachs interacts, seems dispensable for Ds PCP signaling [56]). Another downstream player that could provide answers is Par-1, which might be the connection between Ft/Ds and microtubule plus-ends orientation [59]. However, the molecular bridges to downstream cellular effects/effectors remain unknown.
Fz-PCP core factor short- and long-range regulation
Again, both PCP systems need to be “translated” from global/body axis-related polarity to short-range cell-to-cell communication/relay and ultimately to single-cell responses. In the developing wing, each epithelial cell shows polarized core PCP protein complexes of Fz and Vang early in the prepupal stage or even late third instar [60,61], Fz/Dishevelled (Dsh)/Dgo on one side and Vang/Pk on the opposite side, with both complexes also containing Fmi. At early stages, the PCP axis is perpendicular to the wing margin, displaying a radial pattern [60]. As mentioned above, the respective complexes are stabilized via feedback loops among themselves (Figure 1) in a manner that, disturbing the localization of one component (via either LOF or gain of function), affects the localization of all the others, even from the “opposite complex” [18,62] (Figure 1). There are documented intercellular interactions between Fmi-Fmi and Fz-Vang [32,33,63]. Since Fmi also interacts with both Fz and Vang, it had been suggested that homophilic Fmi bridges across cell membranes facilitate intercellular Fz-Vang interactions that are essential to propagate PCP [33,63–66].
At the short-range level, cell-cell communication is based on sending and receiving signals between neighboring cells. Clonal cells lacking Fz reorient adjacent cells toward the clone, whereas cells lacking Vang reorient neighboring cells to point away; double-mutant clones for fz and Vang reorient hairs to point toward the clone [38,63,67]. These data suggest that Fz is required for sending a polarity signal and Vang for its “reception” (Table 1). Nonetheless, cells can and probably do communicate in both directions, possibly also via Fmi-Fmi bridges. However, although Fmi is needed on both sides, the ability of cells to communicate via Fmi-Fmi bridges depends largely on the presence of Fz and Vang [63,66]. Nevertheless, it will be interesting to determine whether other PCP regulators are directly involved in modifying Fmi-Fmi interactions. It is important to mention that dsh, pk, or dgo mutant clones affect PCP within mutant clones only [27,41,68], suggesting that Dsh, Pk, and Dgo are involved mainly in the intracellular interactions and interpretation of PCP signals [69].
What about long-range regulation? In the wing and eye, PCP axes are orientated toward the prospective margins, the source of Wg/dWnt4 expression [43,60,70]. Recently, gain-of-function experiments demonstrated that dWnt4 and Wg reorient PCP, altering the Fz-PCP core complex localization and polarity axis orientation, and thus indicated that these Wnts serve an instructive function in defining PCP axes. Moreover, through complicated genetic assays, it was possible to show that Wg and dWnt4 are indeed necessary for PCP establishment but that they act redundantly [43]. Like Lawrence and colleagues (who mentioned that “to understand the machinery, one needs to define its components and then work out what they do” [71]), we were able to show that Wg/Wnt4 modulates the intercellular Fz-Vang interaction [43]. In agreement with the proposed graded Fz activity (instructive capacity to interact with other core PCP components) that directs PCP orientation toward lower Fz activity [40,63,67] (Figure 1), co-overexpression of Fz and Wnt4 in vivo buffers each other's effects, confirming the cell culture-based modulation of Fz-Vang interactions by dWnt4 and Wg [43]. Thus, the current model for long-range core Fz-PCP axis establishment includes Wg/dWnt4 as instructive regulators of Fz-Vang polarization in a graded manner in Drosophila imaginal discs (Figure 1) (this view applies at least for the wing and eye, but other tissues are more complex). Along these lines, in vertebrates, particularly in zebrafish, Wnt5a and Wnt11 are involved in PCP establishment, but their mechanism for PCP establishment remains unclear [44,72–75]; one mechanism involving Wnt5a-mediated Vangl2 phosphorylation during digit formation in mouse limbs [72] also involves Ror2, a receptor tyrosine kinase that does not appear to have a PCP function in Drosophila, suggesting potential additional players within the Fz-PCP network.
Nonetheless, the Drosophila experiments provide strong evidence that Wnts serve as an instructive input in regulating Fz-PCP axis definition [43]. Although in vertebrates the “mechanistic mix” is likely to be more complex as Wnt5a and Wnt11 might employ distinct functions, an instructive role for Wnt11 has also been suggested [44].
Coordination of Ft/Ds and Fz-PCP pathways
It is intriguing to think about Wg/Wnts as the master regulators for PCP: (a) Wg itself, through canonical Wg signaling, regulates graded fj and ds expression in eyes and wings (in the eye, Wg downregulates Fj and upregulates Ds; in the wing, Wg upregulates Fj and downregulates Ds) [36,57,76], and (b) Wg/Wnt4 regulates Fz-PCP activity/polarity axis establishment through regulating the interaction between Fz and Vang [43]. In addition, Wg might contribute through an additional function to PCP establishment. Strikingly, Sagner and colleagues [70] show in Drosophila wings that PCP can be affected by “morphogenetic organizers” and oriented cell divisions. In the larval wing disc, Notch and Wg signaling sets up the dorso-ventral organizer and Hedgehog (Hh) and decapentaplegic signaling sets up the antero-posterior organizer [77,78]. As the morphogens control wing proliferation, size, and axes within the wing, their positional information could provide the earliest cues for PCP orientation [70]. This is a point of view supported by the fact that uniform expression of morphogens during wing development can generate PCP defects [70]. An Hh-and-Wg/Wnt4 interplay has been also demonstrated for PCP establishment in the abdomen and thus might be a more general mechanism (reviewed in [71]).
Although it is clear that both Ft/Ds and Fz-PCP pathways are linked to Wg/Wnt gradients, it remains open as to how these two PCP pathways interact or get integrated at the cellular level. Initial proposed models suggested that Ft/Ds acts upstream of the Fz-PCP core factors, where the Ft/Ds system would provide “global cues” that orient the initial polarity of Fz-PCP complexes [36,42]; this was based largely on the observation that, at the cellular level, in Ft or ds mutants, Fz-PCP core protein complexes remained with polarized distribution in individual cells but with abnormal orientation [42]. Different views emerged when overexpression or LOF clones of Ft or Ds displayed non-autonomy in null mutant backgrounds of fmi or fz [54,71]. This opened two possibilities, either that Ft/Ds signals to other “tissue-specific modules” but not to the core module (called the bypass pathway [5]) or that the Ft/Ds and Fz-PCP systems act in parallel. The latter is supported by many genetic experiments in the fly abdomen [54,71] and also in the late embryonic/larval abdominal denticle belt orientations [79]. In denticle belts, orientation of denticles is PCP-dependent, but the Ft/Ds and Fz-PCP systems act in a redundant manner. Whereas fz or Ft/ds mutants display normal denticle orientations [54,80], the double-mutant combinations affecting both systems resulted in marked denticle PCP defects [79], indicating a clear redundancy in this context.
Consistent with two parallel pathways (possibly affecting different cellular features/functions) are the following observations. It was suggested that Ds expression was necessary for correct PCP throughout the wing, but rescue experiments of ds mutants with exogenous transgenic constructs suggested that it was not the case [50], concluding that the Ft/Ds system was redundant with Fz-PCP signaling in much of the wing and pointed out a major phenotypic difference between the two systems: whereas flies carrying mutations in Fz/PCP core components exhibit PCP defects throughout the wing, the Ft/Ds system affects mainly polarity in the proximal wing half (Figure 2), the wing area where PCP strongly depends on cellular realignments and rotations during the transition from the radial to the proximo-distal PCP axis (Figure 2). In fact, the realignment from the radial to the proximo-distal PCP axis is dependent on Ft/Ds-induced mechanical forces, and Ds in the wing hinge is essential for the wing to respond to the associated anisotropic mechanical stress [60]. Thus, the different PCP systems appear to regulate distinct cellular features, both affecting the final PCP establishment and orientation.
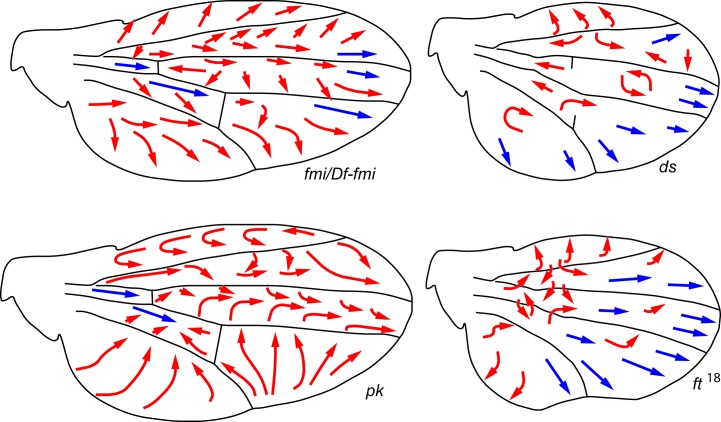
Figure 2. Schematic presentations of the wing PCP phenotypes of components of the Fz/PCP core pathway (left) and the Ft/Ds system (right).
The mutations in Fz/PCP core components affect the whole wing blade. This is seen in both intercellularly acting factors—for example, fmi, the fmi-stan3 allele (top left)—or the cytoplasmic intracellular components—for example, pk- (bottom left). However, mutations in ds and Ft affect largely the proximal wing half. Consistently, ds and Ft also influence mitotic figure orientations near the hinge at the larval stage [70]. Ds, Dachsous; Fmi, Flamingo; Ft, Fat; Fz, Frizzled; PCP, planar cell polarity; PK, Prickle; Stan, Starry night.
A potential link between the two pathways might be provided via the polarization/orientation of microtubules. The Uemura lab has shown that the Ft/Ds system can regulate microtubule orientation [59] and that Fz and Fmi can be seen moving along planar microtubule arrays in pupal wings [81]. Moreover, most recently, it has been suggested that the different protein isoforms of the Fz/core group gene pk, Pk and Sple, provide a different bias toward microtubule “−/+”-end polarization [82]. In this context, it is noteworthy that recently the two distinct Pk-Sple isoforms were suggested to provide a link between the Fz/PCP core group and the Ft/Ds system [83]: as the Sple isoform specifically binds to Ds (and Dachs) through its N-terminal extension, it can coordinate (co)localization between the Ds and Vang complexes (by binding to both), whereas the Pk isoform (lacking the N-terminal domain) only physically interacts with Vang. As such, the tissues where Sple is expressed and genetically more important (eye and leg) would align the Vang and Ds complexes on the same cellular side, whereas those tissues using mainly Pk (wing and thorax) would not align these complexes (allowing Fz and Ds to cohabit on the same side of cells). Although this requires a more physiological and functional dissection, it provides an intriguing potential mechanism for cross-talk and coordination of the two systems. In conclusion, we support the view that the two main molecular systems regulating PCP establishment act in parallel by using distinct effectors and affecting different cellular responses; as such, they can act together in a non-redundant (imaginal discs) or redundant (embryo) manner. Importantly, such a setup provides a high level of fidelity and potential for corrective measures if needed. Along these lines, the non-autonomous defects associated with mutant clones of Fz core components are expanded in Ft/ds mutant backgrounds as compared with a wildtype background in wings [42], arguing that the two pathways can indeed help each other in “correcting” aberrant cell-to-cell interactions in the respective other system. Such observations and hypotheses are all in favor of two parallel pathways, to provide corrective functions when needed and fidelity to the process. As it appears that both systems act through different effectors, they should converge somewhere downstream [63,71]. Could it be at the level of microtubules? Cortical microtubules align perpendicular to the margin in proximal wing regions, supporting a general role for the orientation of cortical microtubules in PCP [59,81] and in the realignment of PCP later in development. It will be for future functional dissections to establish the specific intersection and integration points between the two systems.
Acknowledgments
The authors would like to thank all Mlodzik lab members for critical and helpful discussions. The related work in the Mlodzik lab is funded by the National Institutes of Health/National Institute of General Medical Sciences.
Abbreviations
- A/B
apical-basolateral
- Dgo
Diego
- Ds
Dachsous
- Dsh
Dishevelled
- Fj
Four-jointed
- Ft
Fat
- Fw
Furrowed
- Fz
Frizzled
- Hh
Hedgehog
- LOF
loss of function
- PCP
planar cell polarity
- Wg
Wingless
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/98
References
- 1.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–42. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence PA. Cellular differentiation and pattern formation during metamorphosis of the milkweed bug Oncopeltus. Dev Biol. 1969;19:12–40. doi: 10.1016/0012-1606(69)90068-2. [DOI] [PubMed] [Google Scholar]
- 3.Gubb D, García-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 4.Lawrence PA, Crick FH, Munro M. A gradient of positional information in an insect, Rhodnius. J Cell Sci. 1972;11:815–53. doi: 10.1242/jcs.11.3.815. [DOI] [PubMed] [Google Scholar]
- 5.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27:2207–20. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maung, SM, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7:165–79. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–99. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15(Spec No 2):R227–34. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–58. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 12.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 13.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton S. Planar polarization of Drosophila and vertebrate epithelia. Curr Opin Cell Biol. 1997;9:860–6. doi: 10.1016/S0955-0674(97)80089-0. [DOI] [PubMed] [Google Scholar]
- 16.Adler PN. Planar Signaling and Morphogenesis in Drosophila. Developmental Cell. 2002;2:525–35. doi: 10.1016/S1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 17.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/S0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 18.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–13. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, McNeill H. Fat and Dachsous cadherins. Prog Mol Biol Transl Sci. 2013;116:215–35. doi: 10.1016/B978-0-12-394311-8.00010-8. [DOI] [PubMed] [Google Scholar]
- 20.Zakaria S, Mao Y, Kuta A, Ferreira de Sousa Catia, Gaufo GO, McNeill H, Hindges R, Guthrie S, Irvine KD, Francis-West PH. Regulation of neuronal migration by Dchs1-Fat4 planar cell polarity. Curr Biol. 2014;24:1620–7. doi: 10.1016/j.cub.2014.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718482818
- 21.Saburi S, Hester I, Goodrich L, McNeill H. Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development. 2012;139:1806–20. doi: 10.1242/dev.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/715747804
- 22.Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, Basson MA, Francis-West P, Irvine KD. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–57. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/11820957
- 23.Irvine KD. Integration of intercellular signaling through the Hippo pathway. Semin Cell Dev Biol. 2012;23:812–7. doi: 10.1016/j.semcdb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vichas A, Zallen JA. Translating cell polarity into tissue elongation. Semin Cell Dev Biol. 2011;22:858–64. doi: 10.1016/j.semcdb.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–93. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- 27.Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/S1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1001472
- 28.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–8. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720461573
- 29.Hermle T, Saltukoglu D, Grünewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–76. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720461575
- 30.Hermle T, Guida MC, Beck S, Helmstädter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 2013;32:245–59. doi: 10.1038/emboj.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717970292
- 31.Chin M, Mlodzik M. The Drosophila selectin furrowed mediates intercellular planar cell polarity interactions via frizzled stabilization. Dev Cell. 2013;26:455–68. doi: 10.1016/j.devcel.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–95. doi: 10.1016/S0092-8674(00)80046-X. [DOI] [PubMed] [Google Scholar]
- 33.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–64. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720462492
- 34.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–51. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 35.Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–68. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- 36.Yang C, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–88. doi: 10.1016/S0092-8674(02)00658-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1007399
- 37.Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–9. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- 38.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–59. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–64. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1021602
- 41.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–63. doi: 10.1016/S1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1011043
- 42.Ma D, Yang C, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–7. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1012282
- 43.Wu J, Roman A, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–55. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718058256
- 44.Gros J, Serralbo O, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–93. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1147179
- 45.Brodsky MH, Steller H. Positional information along the dorsal-ventral axis of the Drosophila eye: graded expression of the four-jointed gene. Dev Biol. 1996;173:428–46. doi: 10.1006/dbio.1996.0038. [DOI] [PubMed] [Google Scholar]
- 46.Villano JL, Katz FN. four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development. 1995;121:2767–77. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
- 47.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–96. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 48.Garoia F, Guerra D, Pezzoli MC, López-Varea A, Cavicchi S, García-Bellido A. Cell behaviour of Drosophila fat cadherin mutations in wing development. Mech Dev. 2000;94:95–109. doi: 10.1016/S0925-4773(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 49.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–84. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721975618
- 50.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–94. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721976106
- 51.Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol. 2012;22:907–14. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–7. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720461642
- 53.Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–10. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720461640
- 54.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1053700
- 55.Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–72. doi: 10.1016/S0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 56.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–24. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721977403
- 57.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1020310
- 58.Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–5. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1122968
- 59.Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720988434
- 60.Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J, Jülicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/5562957
- 61.Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell. 2011;20:511–25. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720988347
- 62.Seifert Jessica RK, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–9. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1115465
- 65.Lawrence PA, Struhl G, Casal J. Planar cell polarity: A bridge too far? Curr Biol. 2008;18:R959–61. doi: 10.1016/j.cub.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–74. doi: 10.1242/dev.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–9. doi: 10.1016/S0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- 68.Tree David RP, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–81. doi: 10.1016/S0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1006710
- 69.Krasnow RE, Wong LL, Adler PN. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development. 1995;121:4095–102. doi: 10.1242/dev.121.12.4095. [DOI] [PubMed] [Google Scholar]
- 70.Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Jülicher F, Eaton S. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol. 2012;22:1296–301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717954073
- 71.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–63. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/9872956
- 73.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–76. doi: 10.1016/S0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721983679
- 74.Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718393699
- 75.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721984330
- 76.Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dahmann C, Basler K. Compartment boundaries: at the edge of development. Trends Genet. 1999;15:320–6. doi: 10.1016/S0168-9525(99)01774-6. [DOI] [PubMed] [Google Scholar]
- 78.Cohen SM. Controlling growth of the wing: vestigial integrates signals from the compartment boundaries. Bioessays. 1996;18:855–8. doi: 10.1002/bies.950181102. [DOI] [PubMed] [Google Scholar]
- 79.Donoughe S, DiNardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–9. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13248956
- 80.Repiso A, Saavedra P, Casal J, Lawrence PA. Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat. Development. 2010;137:3411–5. doi: 10.1242/dev.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–22. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1030826
- 82.Olofsson J, Sharp KA, Matis M, Cho B, Axelrod JD. Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development. 2014;141:2866–74. doi: 10.1242/dev.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718485958
- 83.Ayukawa T, Akiyama M, Mummery-Widmer JL, Stoeger T, Sasaki J, Knoblich JA, Senoo H, Sasaki T, Yamazaki M. Dachsous-dependent asymmetric localization of spiny-legs determines planar cell polarity orientation in Drosophila. Cell Rep. 2014;8:610–21. doi: 10.1016/j.celrep.2014.06.009. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718482812