Abstract
Colorectal cancer is one of the most frequent solid tumors in the Western world. Treatment options are dependent on the stage of the disease, the performance status of the patient, and increasingly the molecular makeup of the tumor. In countries with surveillance programs, the incidence rate as well as the mortality rate has gone down because of the earlier stages at which the tumors are detected. For rectal cancer, standard of care differs from that of colon cancer with regard to perioperative treatment. In the metastatic setting, treatment options are uniform for colorectal cancer. Over the years, treatment options have emerged from single-agent 5-fluorouracil (5-FU) treatment to combination regimens using 5-FU and oxaliplatin or irinotecan or both. Treatment efficacy in the metastatic setting has been increased with the introduction of targeted substances. These include (a) the anti-vascular endothelial growth factor-A (anti-VEGF-A) antibody bevacizumab, (b) the anti-epidermal growth factor receptor (anti-EGFR) antibodies cetuximab and panitumumab, (c) the anti-angiogenic multi-kinase inhibitor regorafenib, and (d) the anti-angiogenic compound aflibercept. Anti-EGFR antibodies have shown efficacy only in the subpopulations of tumors that do not have any mutation in KRAS and NRAS exon 2, 3, 4. Physicians have the choice in the first line to use anti-EGFR or anti-VEGF inhibitors in combination with chemotherapy based on treatment goals and patient performance. In recent years, tumor location has been shown to be prognostic and predictive for clinical outcome. Right-sided sporadic colon cancers differ significantly in molecular characteristics and, with the exception of microsatellite instability (MSI-H) tumors, are associated with poor prognosis. Tumors based on hereditary non-polyposis colorectal cancer, on the other hand, have excellent prognosis in stage II and III disease. Recent efforts have focused on the molecular classification of colorectal cancer with the purpose of establishing molecularly defined subgroups.
Epidemiology
Colorectal cancer (CRC) is the third most common cancer in males and females, accounts for 8% of new cancer cases in the United States (US), and is responsible for 8% to 9% of the estimated cancer deaths in the US in 2014 [1]. The lifetime probabilities of developing invasive CRC in the US are 5% in males (1 in 20) and 4.6% in females (1 in 22), and the median age at time of diagnosis is about 70 years. Worldwide incidence rates of CRC vary widely; the incidence rate is 10-fold higher in the US and Europe than in African and Asian countries. First-generation immigrants have the incidence rates of their home country, whereas in second-generation immigrants, incidence rates adapt to the rates of the country of immigration. Western lifestyle, with its known risk factors of red meat (beef and pork), alcohol consumption, and obesity, is associated with a higher risk of CRC. Patients with inflammatory bowel disease (ulcerative colitis and Crohn's disease) also have a higher risk of CRC and warrant close surveillance programs. Hereditary syndromes that are known to be associated with the development of CRC, such as FAP (familial adenomatous polyposis) and HPNNC (hereditary non-polyposis CRC), account for 5% of CRC cases. Familial clustering is assumed to account for another 20% of cases. Sporadic CRC cases account for the vast majority (about 75%) [2].
Cure and survival rates depend on the stage of CRC. Staging is done by the size of the primary tumor (T stage), the involvement of lymph nodes (N stage), and the occurrence of distant metastases (M stage). Table 1 gives the 5-year survival rates depending on the stage in CRC [3].
Table 1. Stage-specific survival in colorectal cancer according to Union for International Cancer Control.
| UICC stage | TNM T stage | N stage | M stage | 5-year survival rates [3] |
|---|---|---|---|---|
| 0 | TIS | N0 | M0 | |
| I | T1, T2 | N0 | M0 | 93.2% |
| IIA | T3 | N0 | M0 | 84.7% |
| IIB | T4 | N0 | M0 | 72.2% |
| IIIA | T1,T2 | N1 | M0 | 83.4% |
| IIIB | T3, T4 | N1 | M0 | 64.1% |
| IIIC | T1-4 | N2 | M0 | 52.3% |
| IV | T1-4 | N1-2 | M1 | 8.1% |
Abbreviations: M, distant metastases; N, lymph node involvement; T, tumor size; UICC, Union for International Cancer Control.
Primary prevention strategies of sporadic CRC include increased consumption of whole grains and fruits and vegetables [4,5], increased physical activity, and, for adipose patients, weight reduction [6]. Owing to inconsistent study results, no recommendations with regard to nutrition can be made. Although chemoprevention of colorectal polyps with low-dose aspirin or other COX2 inhibitors has been shown to be effective [7,8], no reduction in the incidence rate of CRC could be established. With the known gastrointestinal side effects of aspirin, such as gastric ulcers and gastrointestinal bleeding, and the increased risk of cardiovascular events associated with the long-term intake of COX2 inhibitors, there is no consensus on chemoprevention.
The implementation of screening programs in some countries has led to the detection of CRC in earlier stages. In combination with the development of more effective treatment options for the metastatic stage, 5-year survival rates have improved throughout all stages, from about 51% in the '70s to about 65% in the 2000s when all races are taken together [1]. However, survival rates differ by ethnicity, and rates are lower in African-Americans. As displayed in Table 1, patients with a small tumor (Union for International Cancer Control [UICC] stage I and II) have an excellent 5-year survival rate after surgery alone and are not treated with adjuvant chemotherapy. Locally advanced stages in which the primary tumor has managed to metastasize to the local lymph nodes (UICC stage III) are treated with adjuvant treatment to reduce the risk of recurrence after surgery. In the metastatic setting (UICC stage IV), even with modern and targeted chemotherapeutic regimens and the advances in secondary metastasectomy, 5-year survival rates remain low at about 15%, even though rates up to 32% have been reported in specialized centers in the US [9].
Molecular carcinogenesis
The principle of the adenoma-carcinoma sequence was introduced in 1975 by Day and Morson [10]. They described CRC carcinogenesis from benign precursors to invasive carcinomas in hereditary and sporadic cases. Vogelstein and colleagues [11] proposed the accumulation of genetic alterations, in particular APC, TP53, and KRAS mutations, to be responsible for CRC development. Next to the hereditary syndromes such as FAP, which is caused by APC gene defects, and HNPCC (hereditary non-polyposis colorectal cancer), which is caused by a defective DNA mismatch repair that leads to microsatellite instability (MSI-H), most CRCs are sporadic. They have been divided into three molecularly different types: chromosomal instable (CIN), MSI-H [12], and CpG-island methylated phenotype (CIMP) [13] tumors. Next to the classic adenoma-carcinoma pathway, which is classified by Wnt pathway dysregulation [14], the development of CRC via serrated polyps with early BRAF mutations has been described [15]. The understanding of CRC carcinogenesis has been extended as a result of technical developments. In 2006, first-generation sequencing in 11 colorectal tumors revealed 11 recurrent mutations per tumor [16]. In the landmark publication of The Cancer Genome Atlas Network in 2012 [17] applying next-generation sequencing technique on 97 colorectal tumors, it has become clear that CRC is made up of a complex network of genetic alterations leading to the dysregulation of multiple pathways. At the 2014 American Society of Clinical Oncology Annual Meeting, four major molecularly distinguishable subtypes of CRC were proposed by the CRC subtyping consortium [18]. For this classification, microarray data on more than 4500 CRC tumors of all UICC stages were used to propose four major molecular subtypes of CRC, leaving 21% of the tumors unclassified [18] (Table 2).
Table 2. Molecular subclassification of colorectal cancer tumors [18].
| Type | Clinical characterization | Molecular features |
|---|---|---|
| CMS1 | Females, older age, right colon | MSI, hypermutation, BRAF mutant, immune activation |
| CMS2 | Left colon | Epithelial, MSS, high CIN, TP53 mutant, WNT/MYC pathway activation |
| CMS3 | Epithelial, heterogeneous CIN/MSI, KRAS mutant, IGFBP2 overexpression | |
| CMS4 | Younger age, stage III/IV | Mesenchymal, CIN/MSI, TGFβ/VEGF activation, NOTCH3 overexpression |
| Unclassified | Immune and stromal infiltration, variable epithelial-mesenchymal activation |
BRAF, proto-oncogene BRAF; CIMP, CpG-island methylated phenotype; CIN, chromosomal instable; CMS, consensus molecular subtype; IGFBP2, insulin-like growth factor binding protein 2; KRAS, Kirsten Ras; MSI, microsatellite instable; MSS, microsatellite stable; TGFβ, transforming growth factor beta; TP53, tumor protein 53; VEGF, vascular endothelial growth factor.
It remains to be seen whether this classification will stand the test of time. Until now, it has been unclear whether those molecular subtypes, with the exception of MSI-H tumors, are associated with treatment outcomes in CRC.
Management of rectal cancer
Management of rectal cancer, which accounts for about 35% of all CRC, differs in early stages, as anatomic conditions are distinctive from the rest of the colon, and local recurrence is a major problem for morbidity and quality of life. Prognosis after surgery is dependent on the circumferential resection margin, emphasizing the importance of the surgical quality on outcome [19]. Preoperative magnetic resonance imaging scans to estimate the involvement of the rectum wall and local lymph node metastases are standard in most centers. Well-differentiated (G1/G2) small tumors (<3 cm) (ultrasound staging classification uT1, N0, and M0) can be cured with local excision. After surgical removal of pT1 low-risk tumors, no adjuvant chemotherapy is recommended [20]. In case of a higher postoperative T stage, an additional total mesorectal excision (TME) should be performed. To reduce the possibility of local recurrence in locally advanced rectum cancers (T2-4, N0-2, and M0), neoadjuvant radio-chemotherapy using a fluoropyrimidine followed by TME and adjuvant chemotherapy is considered standard [21]. In this setting, capecitabine has shown superior survival rates when compared with infusional 5-fluorouracil (5-FU) in one trial [22], but no difference could be detected in the NSAPB-R04 trial [23], so infusional and oral 5-FU administrations are being used interchangeably. For neoadjuvant radiation, a dose of 50.4 Gy in fractions of 1.8 Gy a day for 5 days a week for 5 weeks is prescribed. In cases in which time to surgery is an issue (for example, tumor bleeding), a shorter radiation protocol consisting of the application of 5 × 5 Gy has shown efficacy in several studies [24,25]. As higher single-dose radiation is known to affect long-term functionality, the longer protocol should be preferred. The German CAO/ARO/AIO-94 randomized phase III study compared preoperative radio-chemotherapy with postoperative chemo-radiotherapy in locally advanced (cT3-4, N+) rectal cancer. Preoperative therapy with 50.4 Gy delivered in 1.8 Gy fractions over 5 weeks, combined with 5-FU in weeks 1 and 5, showed a significantly better local recurrence-free survival (5-year relapse rate of 6% versus 13%; P = 0.006) and had less toxicity when compared with postoperative radio-chemotherapy [21]. With a longer follow-up, the difference in 10-year relapse rate was significant (P = 0.048) with cumulative incidences of local relapse of 7.1% in the preoperative and 10.1% in the postoperative chemoradiation arm [26]. The primary endpoint of the study, overall survival (OS), was not met. The frequency of distant metastases was comparable in the two treatment arms.
In one study, the addition of oxaliplatin to this infusional 5-FU treatment resulted in a significantly higher rate of pathological response [27] and a significantly longer 3-year disease-free survival (DFS) [28]. However, the recently presented PETACC-06 study tested oxaliplatin in combination with capecitabine and radiotherapy in the same setting and failed to demonstrate a better outcome for the oxaliplatin-treated cohort [29]. This is in line with previous published data from phase III trials (STAR01 [30] and ACCORD/PRODIGE2 [31]). Therefore, the addition of oxaliplatin to the neoadjuvant radio-chemotherapy cannot be recommended. The ADORE study tested 5-FU bolus versus 5-flourouracil, leucovorin, oxaliplatin (FOLFOX) as adjuvant treatment after surgery for rectal cancer. 5-FU bolus resulted in a significantly shorter DFS [32] and should not be administered any longer.
To summarize, in locally advanced rectal cancer, capecitabine during radio-chemotherapy is superior to bolus 5-FU [22] as a combination partner for radiotherapy. Within this concept, the addition of oxaliplatin to capecitabine was not shown to be of superior outcome [29]. In high-risk patients with positive lymph nodes and no major patho-histological response after neoadjuvant treatment, the addition of oxaliplatin to 5-FU in the adjuvant treatment might be considered [32].
In larger T4 tumors, a gynecological and urological examination should be performed to exclude other organ involvement before treatment is started. A simplified workflow of the treatment of local or locally advanced rectal cancer is presented in Figure 1.
Figure 1. Simplified workflow of the management of localized rectal cancer.
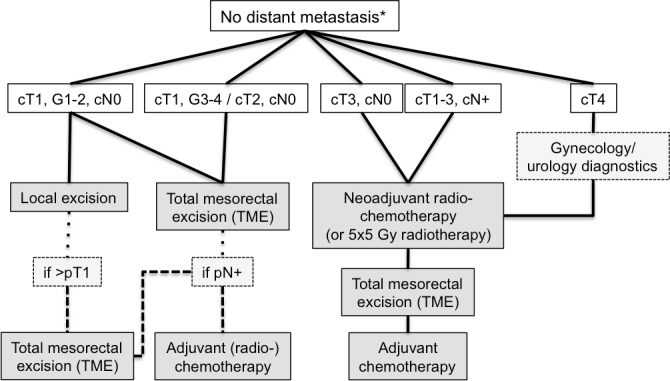
Asterisk indicates results of computed tomography scan/magnetic resonance imaging. Abbreviations: c, clinical; G, grading; Gy, gray; N, lymph node stage; N+, lymph nodes involved; p, pathological; T, tumor stage.
Metastatic rectal cancer is treated according to metastatic CRC (mCRC).
Management of colon cancer
Depending on the stage of CRC, recurrence rates, survival times, and management are different. In early-stage tumors (UICC stage I), radical hemicolectomy with lymph node resection without any additional treatment is appropriate. In low-risk carcinomas (pT1, G1-2, L0, and R0), local procedures, such as endoscopic mucosal resection or a laparoscopic segment resection, may be discussed. Tumors invading the serosa (T3) or spreading to local lymph nodes (N+) have a higher risk of recurrence, so adjuvant treatment is recommended.
Adjuvant setting
UICC stage II
In UICC stage II patients without risk factors, the gain in 5-year survival rate (2% to 3%) by adjuvant chemotherapy 5-FU or capecitabine is small and was established in only a single study in which the nodal staging was considered inadequate by current standards [33]. The addition of oxaliplatin to 5-FU or capecitabine in the adjuvant treatment of UICC stage II patients without risk factors cannot be recommended, as no survival gain could be demonstrated. In patients with the risk factor of T4 tumor, tumor perforation, emergency surgical procedure, or fewer than 12 removed lymph nodes, the addition of oxaliplatin to the adjuvant treatment can be discussed. Tumors with a defective mismatch repair do not appear to benefit from a 5-FU adjuvant monotherapy and have excellent prognosis and are not recommended for adjuvant therapy [34].
To guide the decision in stage II disease, considerable efforts have been made to develop molecular-based scores for the prediction of recurrence [35–38]. Gene expression of tumor samples has been measured by microarray or quantitative reverse transcription-polymerase chain reaction in fresh-frozen or formalin-fixed paraffin-embedded material, and predictive scores have been developed accordingly. But tests add only little to the known risk factors. Reasons for this observation are manifold but taking tumor heterogeneity into account, the cell clone ultimately responsible for tumor recurrence may only account for a fraction of the investigated tissue sample and its signature cannot be reliably detected. Until now, the use of those tests could not be recommended.
UICC stage III
Patients with UICC stage III disease (lymph node involvement) should be treated with adjuvant chemotherapy to improve survival. 5-FU-based regimens led to an increase in 5-year survival rates of about 10% to 15%. The addition of oxaliplatin to a capecitabine regimen added another 4% of 3-year DFS to a bolus 5-FU-based regimen [39] and increased benefit in stage III disease in combination with infusional 5-FU [40,41]. Standard regimens are FOLFOX (12 cycles for 24 weeks) [40] or capecitabine plus oxaliplatin (XELOX) (8 cycles for 24 weeks) [39]. For patients older than 70 years of age, single-agent 5-FU-based therapy has shown a benefit [42]; however, a meta-analysis of the ACCENT database was not able to show a benefit for the addition of oxaliplatin to 5-FU-based chemotherapy [43]. In this age group, only highly motivated patients with an excellent performance status (Eastern Cooperative Oncology Group score of 0) should be considered for the addition of oxaliplatin in adjuvant treatment.
Metastatic setting
Patients with UICC stage IV (mCRC) disease have poor outcomes; 5-year survival rates are only about 6%. In metastatic, unresectable, untreated disease, a median OS of 6 months has been reported, which was increased to 12 months by treatment with 5-FU and leucovorin [44]. Development of continuous 5-FU infusional regimens, in combination with leucovorin, decreased toxicity and increased efficacy of 5-FU treatment. The addition of irinotecan (5-fluorouracil, leucovorin, irinotecan [FOLFIRI]) and oxaliplatin (FOLFOX) raised OS to a median of about 20 months [45]. The addition of biologicals, such as the vascular endothelial growth factor-A (VEGF-A) antibody bevacizumab or the epidermal growth factor receptor (EGFR) antibodies cetuximab and panitumumab, to those standard regimens led to OS times of about 24 months [46–48]. The exposure to all active agents over time in sequence appears to give the best overall outcomes as exemplified by the recent data from CALGB 80405 and FIRE-3, in which 88% and 78% of patients received subsequent therapy; a median OS of 30 months and beyond can be reached in molecularly defined subpopulations [49,50].
First-line treatment
Choice of first-line treatment is important, as it is the most active treatment line when it comes to tumor response, progression-free survival (PFS), and OS. Additionally, substances administered in the first line trigger the possibilities of further-line treatment. For example, aflibercept is approved only after the failure of an oxaliplatin-based therapy [51]. An experienced surgeon should be consulted to discuss the probability of resectability as this is the only way to achieve cure [9].
In a carefully selected, younger, and medically fitter patient population, the combination of 5-FU, irinotecan, and oxaliplatin (FOLFOXIRI) has shown better efficacy than FOLFIRI [52]. As both study arms combined chemotherapy with bevacizumab, the effect of bevacizumab cannot be evaluated. This concept might be of special interest for the molecularly defined subgroup of BRAF mutant tumors. An exploratory analysis pooling BRAF mutant patients treated with FOLFOXIRI plus bevacizumab showed an OS of 24 months [53], which is promising when compared with subgroup analyses of FOLFIRI-treated patients in the CRYSTAL and FIRE-3 trials, in which an OS of 16 to 17 months was reported [46,49,54]. As toxicity is higher than in doublet chemotherapy, FOLFOXIRI should be considered only in young patients with an excellent performance status.
Multiple phase III trials showed that doublet chemotherapy regimens consisting of a fluoropyrimidine (5-FU, tegafur-uracil [UFT], or capecitabine) and irinotecan or oxaliplatin (FOLFOX/capecitabine and oxaliplatin [CAPOX] or FOLFIRI/capecitabine and irinotecan [CAPIRI]) are more active than a monotherapy with fluoropyrimidine [55–57]. Therefore, doublet chemotherapy containing a fluoropyrimidine and oxaliplatin or irinotecan is considered standard for most patients with mCRC. With the addition of irinotecan or oxaliplatin to 5-FU, toxicity is rising, so not all patients qualify for those combinations. For patients with concomitant diseases or a poor performance status, in the absence of contraindications, bevacizumab might be added to infusional 5-FU [58] or capecitabine [59] to prolong OS. The recently published Avastin in Elderly With Xeloda (AVEX) study demonstrated this OS benefit in an older study population [60].
For patients qualifying for doublet chemotherapy, combination with an antibody against VEGF-A or EGFR has been shown to increase first-line treatment efficacy [46–48,61,62]. Since the pivotal investigation by Sartore-Bianchi and colleagues [63], who showed that the benefit of adding cetuximab to a chemotherapy is restricted to patients with no KRAS exon 2 mutation, approval for the use of cetuximab and panitumumab has been restricted to patients bearing KRAS exon 2 (codon 12 and 13) wild-type tumors. Data from clinical trials more recently showed that no benefit was derived when cetuximab or panitumumab was administered if the tumor was mutant in one of the more rare locations in KRAS exons 3 and 4 (codon 59, 61, 117, and 146) and NRAS exons 2, 3, and 4 (codon 12, 13, 59, 61, 117, and 146) [54,64–67]. Those additional mutations account for approximately 10% of all mCRC cases, so only 50% of the mCRC population qualifies for anti-EGFR treatment. Furthermore, in patients with a RAS mutant tumor, the combination of oxaliplatin and an anti-EGFR antibody had the detrimental effect of shorter OS [65,67] than chemotherapy alone. In Europe and elsewhere, the label for the use of panitumumab and cetuximab has changed accordingly. For patients bearing a RAS mutant tumor, the only available biological is bevacizumab, which can be combined with either FOLFOX [47], FOLFIRI [68], or FOLFOXIRI [52]. If an anti-EGFR antibody treatment is considered, extended RAS analysis should be performed early on.
For patients with a RAS wild-type tumor, bevacizumab and cetuximab or panitumumab can be used. Three studies are testing the efficacy of bevacizumab versus cetuximab or panitumumab in combination with FOLFIRI or FOLFOX or both. The CALGB 80405 study tested the addition of cetuximab or bevacizumab to chemotherapy in 1137 patients. Whereas the chemotherapeutic backbone was chosen by the respective physician, patients were randomly assigned to either bevacizumab or cetuximab. The primary endpoint was OS in the KRAS exon 2 wild-type population. The first results have been presented, and the primary endpoint was not met. Cetuximab-treated patients had a median OS of 29.9 months, and bevacizumab-treated patients reached 29.0 months (P = 0.34) [50]. A subgroup analysis of FOLFOX-treated patients showed a trend (P = 0.09) toward longer OS in cetuximab-treated patients versus bevacizumab-treated patients, with OS 30.1 versus 26.9 months, respectively. In FOLFIRI-treated patients, comparable OS times were achieved: 33.4 months in the bevacizumab arm and 28.9 months in the cetuximab arm (P = 0.28). The extended RAS test is ongoing, and data are urgently awaited. The FIRE-3 trial tested bevacizumab versus cetuximab by using FOLFIRI as a chemotherapeutic backbone in 592 patients. This phase III trial did not meet its primary endpoint: objective tumor response (P = 0.183). Although there was no difference in PFS, a difference in OS with a benefit of 3.7 months in cetuximab-treated patients was seen in the KRAS exon 2 wild-type population (P = 0.017) [69]. After extended RAS testing, this difference grew to 7.5 months with a hazard ratio of 0.7 (P = 0.011) [54]. The PEAK trial was a phase II trial that tested panitumumab against bevacizumab in combination with FOLFOX. The exploratory endpoint, PFS, was met after an extended RAS test was applied and showed a significant benefit from panitumumab (13.0 versus 9.5 months; P = 0.029) [70]. Data on OS are still immature but showed a trend (P = 0.058) toward benefit for the panitumumab arm. When the results of CALGB 80405, FIRE-3, and PEAK are taken together, there is circumferential evidence of a longer OS by using cetuximab or panitumumab rather than bevacizumab in the extended RAS wild-type population. For a final decision, results of the extended RAS analysis of the CALGB 80405 have to be awaited. A proposed decision tree for first-line patients is presented in Figure 2. The individual decision may be different because of concomitant diseases and toxicity reasons.
Figure 2. Proposed decision tree for first-line treatment in patients with metastatic colorectal cancer.
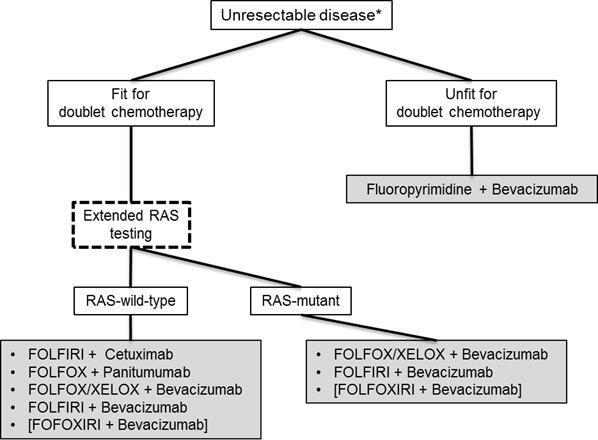
Individual decision may be different because of concomitant diseases and toxicity. Asterisk indicates results of an experienced surgeon. FOLFIRI, 5-fluorouracil, leucovorin irinotecan; FOLFOX, 5-flourouracil, leucovorin, oxaliplatin; FOLFOXIRI, 5-fluorouracil, leucovorin irinotecan, oxaliplatin; RAS, KRAS and NRAS exon 2, 3, 4; XELOX, capecitabine, oxaliplatin.
Owing to the cumulative toxicity of oxaliplatin, causing limiting polyneuropathy, most of the patients cannot be treated continuously for more than 4 months. In clinical practice, a reduction of the oxaliplatin dose or the de-escalation to a less toxic regimen, such as 5-FU plus bevacizumab, is commonly done. Several studies tested the impact of planned drug holidays [71] or different maintenance strategies after an induction therapy [72,73] on PFS and OS. As anticipated, both trials, CAIRO-3 and AIO KRK-0207, demonstrated that any treatment after an induction therapy with FOLFOX plus bevacizumab led to a longer PFS or maintenance time than no treatment. This supports the clinical practice of a de-escalation strategy. The impact of a drug holiday on OS, which was a secondary endpoint in both trials, cannot be definitely determined. In both trials, the difference in OS was not significant, but owing to the small numbers of events, data are still immature.
Second-line treatment
For second-line decisions, evidence is less clear than for first-line treatment. After irinotecan-based first-line treatment, the addition of bevacizumab to FOLFOX showed a significant survival benefit [74]. Similar survival benefits have been shown for the use of bevacizumab beyond progression irrespective of the chemotherapeutic combination [75]. Aflibercept, an anti-angiogenic component targeting VEGF-A, VEGF-B, and placental growth factor (PlGF), in combination with FOLFIRI has been shown to prolong OS significantly after the failure of an oxaliplatin-containing first-line therapy [51]. Trials testing the effect of anti-EGFR treatment in the second line were not able to prove an OS benefit irrespective of the chemotherapeutic backbone [76–78].
Later-line treatment
In later-line treatment, prolongation of survival with regimens with low toxicity is the main focus. Anti-EGFR antibodies with or without the combination of irinotecan have been shown to prolong survival significantly [79,80]. Furthermore, the kinase inhibitor regorafenib was able to prolong OS significantly when tested against best supportive care [81]. Promising new compounds interfering with thymidylate metabolism, such as TS-102 and TS-114, have shown promising results in phase II trials in later-line treatment. TS-102 (trifluridine and tipiracil hydrochloride), which is acting as a thymidylate-synthetase inhibitor, was tested in a global phase III trial (RECOURSE) in refractory mCRC and demonstrated a significant OS benefit of 1.8 months (hazard ratio 0.68; stratified log-rank test P <0.001) against placebo [82].
Summary
The development of new drugs and drug combinations and the implementation of an interdisciplinary management of mCRC resulted in a meaningful increase of median OS from 6 months to more than 30 months during the last two decades. With a better understanding of CRC carcinogenesis and molecular biomarkers leading to a more individualized treatment, personalized therapy will enhance survival and decrease toxicity for patients with mCRC. The investigation of mechanisms of secondary resistance and the evaluation of the best therapy sequence are the next tasks that may improve treatment possibilities.
Abbreviations
- 5-FU
5-fluorouracil
- CRC
colorectal cancer
- DFS
disease-free survival
- EGFR
epidermal growth factor receptor
- FAP
familial adenomatous polyposis
- FOLFIRI
5-fluorouracil, leucovorin, irinotecan
- FOLFOX
5-flourouracil, leucovorin, oxaliplatin
- mCRC
metastatic colorectal cancer
- MSI-H
microsatellite instability high
- OS
overall survival
- PEAK
Panitumumab Efficacy in Combination with mFOLFOX6 Against bevacizumab plus mFOLFOX6 in mCRC subjects with wild-type KRAS tumors
- PFS
progression-free survival
- TME
total mesorectal excision
- UICC
Union for International Cancer Control
- US
United States
- VEGF-A
vascular endothelial growth factor-A
Disclosures
Sebastian Stintzing has received honoraria or travel support from Roche/Genentech, Merck KgAa, Amgen, Sanofi, and Siflex Europe.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/108
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute: Genetics of Colorectal Cancer (PDQ®) http://www.cancer.gov/cancertopics/pdq/genetics/colorectal/HealthProfessional
- 3.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 4.Campos FG, Logullo Waitzberg AG, Kiss DR, Waitzberg DL, Habr-Gama A, Gama-Rodrigues J. Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp. 2005;20:18–25. [PubMed] [Google Scholar]
- 5.Doyle VC. Nutrition and colorectal cancer risk: a literature review. Gastroenterol Nurs. 2007;30:178–82. doi: 10.1097/01.SGA.0000278165.05435.c0. quiz 182-3. [DOI] [PubMed] [Google Scholar]
- 6.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, Haboubi N, Renehan AG. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11:689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 7.Dubé C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 8.Rostom A, Dubé C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–89. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1070888
- 9.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey J, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day DW, Morson BC. The adenoma-carcinoma sequence. Major Probl Pathol. 1978;10:58–71. [PubMed] [Google Scholar]
- 11.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 12.Ilyas M, Straub J, Tomlinson IP, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002. doi: 10.1016/S0959-8049(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 13.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–6. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 15.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009;4:343–64. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 16.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson James KV, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV Krishna, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1096958
- 17.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717950661
- 18.Dienstmann R, Guinney J, Delorenzi M, De Reynies A, Roepman P, Sadanandam A, Vermeulen L, Schlicker A, Missiaglia E, Soneson C, Marisa L, Homicsko K, Wang X, Simon I, Laurent-Puig P, Wessels LFA, Medema JP, Kopetz S, Friend SH, Tejpar S. Colorectal Cancer Subtyping Consortium (CRCSC) identification of a consensus of molecular subtypes [abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 19.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–12. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 20.Schmiegel W, Pox C, Adler G, Fleig W, Fölsch UR, Frühmorgen P, Graeven U, Hohenberger W, Holstege A, Junginger T, Kühlbacher T, Porschen R, Propping P, Riemann JF, Sauer R, Sauerbruch T, Schmoll HJ, Zeitz M, Selbmann HK. S3-Leitlinienkonferenz “Kolorektales Karzinom” 2004. Z Gastroenterol. 2004;42:1129–77. doi: 10.1055/s-2004-813699. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721622510
- 21.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens J, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1119252
- 22.Hofheinz R, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, Fritz E, Hieber U, Lindemann HW, Grunewald M, Kremers S, Constantin C, Hipp M, Hartung G, Gencer D, Kienle P, Burkholder I, Hochhaus A. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579–88. doi: 10.1016/S1470-2045(12)70116-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720488777
- 23.Allegra CJ, Yothers Y, O'Connell MJ, Roh MS, Lopa SH, Petrelli NJ, Beart RW, Sharif S, Wolmark N. Final results from NSABP protocol R-04: Neoadjuvant chemoradiation (RT) comparing continuous infusion (CIV) 5-FU with capecitabine (Cape) with or without oxaliplatin (Ox) in patients with stage II and III rectal cancer [abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 24.Peeters Koen CMJ, Marijnen Corrie AM, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde Cornelis JH. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1108873
- 25.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, Metz C de, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1159563
- 26.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab H, Villanueva M, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 27.Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab H, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–87. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717697919
- 28.Rodel C, Liersch T, Fietkau R, Hohenberger W, Graeven U, Hothorn T, Arnold D, Raab H, Wittekind C, Hess CF, Staib L, Becker H, Sauer R. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: Results of the German CAO/ARO/AIO-04 randomized phase III trial [abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 29.Schmoll H, Haustermans K, Price TJ, Nordlinger B, Hofheinz R, Daisne J, Janssens J, Brenner B, Schmidt P, Reinel H, Hollerbach S, Caca K, Fauth FWB, Hannig C, Zalcberg JR, Tebbutt NC, Mauer ME, Messina CGM, Lutz MP, Van Cutsem E. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis [abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 30.Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/11732965
- 31.Gérard J, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne P, Vendrely V, François E, de La Roche Guy, Bouché O, Mirabel X, Denis B, Mineur L, Berdah J, Mahé M, Bécouarn Y, Dupuis O, Lledo G, Seitz J, Bedenne L, Juzyna B, Conroy T. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717962497
- 32.Hong YS, Nam B, Kim K, Lee J, Park JO, Park YS, Kim SY, Kim T, Kim JH, Ahn JB, Jung KH, Kim TW. Adjuvant chemotherapy with oxaliplatin/5-fluorouracil/leucovorin (FOLFOX) versus 5-fluorouracil/leucovorin (FL) for rectal cancer patients whose postoperative yp stage 2 or 3 after preoperative chemoradiotherapy: Updated results of 3-year disease-free survival from a randomized phase II study (The ADORE) [Abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 33.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 34.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, Zaniboni A, Seitz J, Sinicrope F, Gallinger S. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/3920956
- 35.O'Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J, Cowens JW, Wolmark N. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol. 2010;28:3937–44. doi: 10.1200/JCO.2010.28.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/5524959
- 36.Jiang Y, Casey G, Lavery IC, Zhang Y, Talantov D, Martin-McGreevy M, Skacel M, Manilich E, Mazumder A, Atkins D, Delaney CP, Wang Y. Development of a clinically feasible molecular assay to predict recurrence of stage II colon cancer. J Mol Diagn. 2008;10:346–54. doi: 10.2353/jmoldx.2008.080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh SC, Park Y, Park ES, Lim JY, Kim SM, Kim S, Kim J, Kim SC, Chu I, Smith JJ, Beauchamp RD, Yeatman TJ, Kopetz S, Lee J. Prognostic gene expression signature associated with two molecularly distinct subtypes of colorectal cancer. Gut. 2012;61:1291–8. doi: 10.1136/gutjnl-2011-300812. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/721948208
- 38.Kennedy RD, Bylesjo M, Kerr P, Davison T, Black JM, Kay EW, Holt RJ, Proutski V, Ahdesmaki M, Farztdinov V, Goffard N, Hey P, McDyer F, Mulligan K, Mussen J, O'Brien E, Oliver G, Walker SM, Mulligan JM, Wilson C, Winter A, O'Donoghue D, Mulcahy H, O'Sullivan J, Sheahan K, Hyland J, Dhir R, Bathe OF, Winqvist O, Manne U, et al. Development and independent validation of a prognostic assay for stage II colon cancer using formalin-fixed paraffin-embedded tissue. J Clin Oncol. 2011;29:4620–6. doi: 10.1200/JCO.2011.35.4498. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721945319
- 39.Haller DG, Tabernero J, Maroun J, Braud F de, Price T, van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll H. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/9113956
- 40.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, Gramont A de. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718720156
- 41.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721704314
- 42.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718723143
- 43.McCleary NJ, Meyerhardt JA, Green E, Yothers G, Gramont A de, van Cutsem E, O'Connell M, Twelves CJ, Saltz LB, Haller DG, Sargent DJ. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600–6. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/721633819
- 44.Petrelli N, Douglass HO, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419–26. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 45.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, Gramont A de. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721624660
- 46.van Cutsem E, Köhne C, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721627570
- 47.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720158512
- 48.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–55. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718345543
- 49.Heinemann V, von Weikersthal Ludwig Fischer, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes H, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 50.Venook AP, Niedzwiecki D, Lenz H, Innocenti F, Mahoney MR, O'Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, Goldberg RM, Mayer RJ, Schilsky RL, Bertagnolli MM, Blanke CD. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) [Abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 51.Joulain F, Proskorovsky I, Allegra C, Tabernero J, Hoyle M, Iqbal SU, van Cutsem E. Mean overall survival gain with aflibercept plus FOLFIRI vs placebo plus FOLFIRI in patients with previously treated metastatic colorectal cancer. Br J Cancer. 2013;109:1735–43. doi: 10.1038/bjc.2013.523. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718114814
- 52.Falcone A, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Trenta P, Tomasello G, Ronzoni M, Ciuffreda L, Zaniboni A, Tonini G, Buonadonna A, Valsuani C, Chiara S, Carlomagno C, Boni C, Marcucci L, Boni L, Loupakis L. FOLFOXIRI/bevacizumab (bev) versus FOLFIRI/bev as first-line treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): Results of the phase III TRIBE trial by GONO group [Abstract]. Presented at 2013 ASCO Annual Meeting; 31 May-4 June 2013; Chicago, IL. [Google Scholar]
- 53.Loupakis F, Cremolini C, Salvatore L, Masi G, Sensi E, Schirripa M, Michelucci A, Pfanner E, Brunetti I, Lupi C, Antoniotti C, Bergamo F, Lonardi S, Zagonel V, Simi P, Fontanini G, Falcone A. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718149639
- 54.Stintzing S, Jung A, Rossius L, Modest DP, von Weikersthal LF, Decker T, Kiani A, Al-Batran S, Vehling-Kaiser U, Heintges T, Moehler M, Scheithauer W, Kirchner T, Heinemann V. Mutations within the EGFR signaling pathway: Influence on efficacy in FIRE-3—A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients [Abstract]. Presented at 2014 Gastrointestinal Cancers Symposium; 16-18 June 2014; San Francisco, CA. [Google Scholar]
- 55.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 56.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 57.Gramont A de, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, Braud F de, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 58.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/719779750
- 59.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel, Guy A, Robinson B, Broad A, Ganju V, Ackland SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y, Zalcberg JR, Simes RJ, Price TJ. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–8. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720139980
- 60.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D, Saunders MP. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–85. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718106825
- 61.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, Braud F de, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 62.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 63.Sartore-Bianchi A, Moroni M, Veronese S, Carnaghi C, Bajetta E, Luppi G, Sobrero A, Barone C, Cascinu S, Colucci G, Cortesi E, Nichelatti M, Gambacorta M, Siena S. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol. 2007;25:3238–45. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/719779751
- 64.Ciardiello F, Lenz H, Kohne C, Heinemann V, Tejpar S, Melezinek I, Beier F, Stroh C, Van Cutsem E. Treatment outcome according to tumor RAS mutation status in CRYSTAL study patients with metastatic colorectal cancer (mCRC) randomized to FOLFIRI with/without cetuximab [Abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 65.Douillard J, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718107013
- 66.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon J, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J Clin Oncol. 2014;32:2240–7. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718333626
- 67.Bokemeyer C, Kohne C, Ciardiello F, Lenz H, Heinemann V, Klinkhardt U, Beier F, Duecker K, Tejpar S. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab [Abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 68.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689–90. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 69.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmueller J, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Mueller S, Schaefer B, Modest DP, Jung A, Stintzing S. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3) [Abstract]. Presented at 2013 ASCO Annual Meeting; 31 May-4 June 2013; Chicago, IL. [Google Scholar]
- 70.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon J, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J Clin Oncol. 2014;32:2240–7. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 71.Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, André T, Bennamoun M, Mabro M, Artru P, Carola E, Flesch M, Dupuis O, Colin P, Larsen AK, Afchain P, Tournigand C, Louvet C, Gramont A de. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–33. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1166969
- 72.Arnold D, Graeven U, Lerchenmuller CA, Killing B, Depenbusch R, Steffens C, Al-Batran SE, Lange T, Dietrich G, Stoehlmacher J, Tannapfel A, Schmoll H, Reinacher-Schick A, Hegewisch-Becker S. Maintenance strategy with fluoropyrimidines (FP) plus Bevacizumab (Bev), Bev alone, or no treatment, following a standard combination of FP, oxaliplatin (Ox), and Bev as first-line treatment for patients with metastatic colorectal cancer (mCRC): A phase III non-inferiority trial (AIO KRK 0207) [Abstract]. Presented at 2014 ASCO Annual Meeting; 30 May-3 June 2014; Chicago, IL. [Google Scholar]
- 73.Koopman M, Simkens LHJ, Tije AJT, Creemers G, Loosveld OJL, de Jongh FE, Erdkamp F, Erjavec Z, van der Torren AME, Van der Hoeven JJM, Nieboer P, Braun JJ, Jansen RL, Haasjes JG, Cats A, Wals JJ, Mol L, Dalesio O, van Tinteren H, Punt CJA. Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC): The phase III CAIRO3 study of the Dutch Colorectal Cancer Group (DCCG) [Abstract]. Presented at 2013 ASCO Annual Meeting; 31 May-4 June 2013; Chicago, IL. [Google Scholar]
- 74.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 75.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, van Cutsem E, Moos R von, Viéitez JM, Bouché O, Borg C, Steffens C, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717964859
- 76.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJA, Strickland AH, Wilson G, Ciuleanu TE, Roman L, van Cutsem E, Tian Y, Sidhu R. Final results from a randomized phase 3 study of FOLFIRI {+/-} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:107–16. doi: 10.1093/annonc/mdt523. [DOI] [PubMed] [Google Scholar]
- 77.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz H, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 78.Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N, Chau I, Hill M, Dawson L, Falk S, O'Callaghan A, Benstead K, Chambers P, Oliver A, Marshall H, Napp V, Quirke P. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–59. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 80.van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon J, van Laethem J, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 81.Grothey A, van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz H, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717965145
- 82.Yoshino T, Mayer R, Falcone A, Ohtsu A, Garcia-Carbonero R, Mizunuma A, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni, A, Hochster H, Benedetti F, Aivado M, Makris L, Ito M, Van Cutsem E. Results of a multicenter, randomized, double.-blind, phase III study of TAS-102 vs. placebo, with best supportive care (BSC), in patients (pts) with metastatic colorectal cancer (mCRC) refractory to standard therapies (RECOURSE) Ann Oncol. 2014;25(suppl 2):ii114. doi: 10.1093/annonc/mdu193.22. [DOI] [Google Scholar]


