Josephs et al. use a multi-modal approach to assess neuroanatomical and clinical changes over time in primary progressive apraxia of speech. They demonstrate that progressive atrophy of cortex, basal ganglia and midbrain accompanies the clinical progression, including the emergence of progressive supranuclear palsy five years post-onset in some subjects.
Keywords: non-fluent speech, parkinsonism, progressive supranuclear palsy, disease progression, magnetic resonance imaging
Abstract
Primary progressive apraxia of speech is a recently described neurodegenerative disorder in which patients present with an isolated apraxia of speech and show focal degeneration of superior premotor cortex. Little is known about how these individuals progress over time, making it difficult to provide prognostic estimates. Thirteen subjects with primary progressive apraxia of speech underwent two serial comprehensive clinical and neuroimaging evaluations 2.4 years apart [median age of onset = 67 years (range: 49–76), seven females]. All underwent detailed speech and language, neurological and neuropsychological assessments, and magnetic resonance imaging, diffusion tensor imaging and 18F-fluorodeoxyglucose positron emission tomography at both baseline and follow-up. Rates of change of whole brain, ventricle, and midbrain volumes were calculated using the boundary-shift integral and atlas-based parcellation, and rates of regional grey matter atrophy were assessed using tensor-based morphometry. White matter tract degeneration was assessed on diffusion-tensor imaging at each time-point. Patterns of hypometabolism were assessed at the single subject-level. Neuroimaging findings were compared with a cohort of 20 age, gender, and scan-interval matched healthy controls. All subjects developed extrapyramidal signs. In eight subjects the apraxia of speech remained the predominant feature. In the other five there was a striking progression of symptoms that had evolved into a progressive supranuclear palsy-like syndrome; they showed a combination of severe parkinsonism, near mutism, dysphagia with choking, vertical supranuclear gaze palsy or slowing, balance difficulties with falls and urinary incontinence, and one was wheelchair bound. Rates of whole brain atrophy (1.5% per year; controls = 0.4% per year), ventricular expansion (8.0% per year; controls = 3.3% per year) and midbrain atrophy (1.5% per year; controls = 0.1% per year) were elevated (P ≤ 0.001) in all 13, compared to controls. Increased rates of brain atrophy over time were observed throughout the premotor cortex, as well as prefrontal cortex, motor cortex, basal ganglia and midbrain, while white matter tract degeneration spread into the splenium of the corpus callosum and motor cortex white matter. Hypometabolism progressed over time in almost all subjects. These findings demonstrate that some subjects with primary progressive apraxia of speech will rapidly evolve and develop a devastating progressive supranuclear palsy-like syndrome ∼ 5 years after onset, perhaps related to progressive involvement of neocortex, basal ganglia and midbrain. These findings help improve our understanding of primary progressive apraxia of speech and provide some important prognostic guidelines.
Introduction
We recently described detailed clinical and neuroimaging characteristics of 12 subjects who had presented with a progressive, and isolated, motor speech disorder that we referred to as primary progressive apraxia of speech (PPAOS) (Josephs et al., 2012). These 12 subjects were unique given the absence of any other neurological features, including aphasia, memory loss, ideomotor limb apraxia, pyramidal signs, or parkinsonism, at the time of presentation. Group analysis of the neuroimaging characteristics of PPAOS revealed very focal and specific involvement of the lateral superior premotor cortex and supplementary motor area. These focal areas of involvement were identified both on structural MRI and 18F-fluorodeoxyglucose (FDG) PET analyses. We also assessed white matter tract degeneration with diffusion tensor imaging (DTI) analysis and showed focal white matter tract degeneration beneath the areas of grey matter atrophy, as well as in the body of the corpus callosum. The fact that these clinical and neuroimaging characteristics had not been previously reported in any other neurodegenerative disease led us to designate PPOAS as a novel degenerative syndrome. While we were the first to fully characterize and describe this entity, others have described a few cases with isolated apraxia of speech, although different terminologies were used to describe such patients (Cohen et al., 1993; Broussolle et al., 1996; Josephs et al., 2005, 2006; Duffy, 2006; Wambaugh et al., 2006; Ricci et al., 2008; Deramecourt et al., 2010). Primary progressive apraxia of speech is not an uncommon entity and accounts for ∼20% of all types of higher level progressive speech and language disorders (Wicklund et al., 2014).
We have recognized (Josephs et al., 2012, 2013a) that, by loosely interpreting the consensus criteria for the diagnosis of primary progressive aphasia and its subtypes (Gorno-Tempini et al., 2011), patients we have diagnosed as PPAOS could be considered by others to meet criteria for the agrammatic variant of primary progressive aphasia as apraxia of speech and agrammatism are designated as the two core features of the disorder, only one of which must be present for the diagnosis. However, before applying the subclassification criteria, the consensus recommendations first require patients to meet criteria for primary progressive aphasia where it is explicitly stated that ‘patients who display speech impairments without aphasia, do not qualify for the diagnosis of primary progressive aphasia’ (Mesulam, 2013 p. 456) and ‘deficits cannot be due to disruption of the formation of words’ (Mesulam, 2003 p. 1536). Furthermore, impaired grammaticality should be the single core feature of agrammatic primary progressive aphasia (Mesulam et al., 2012). Due to the logical inconsistency of diagnosing patients as having a variant of primary progressive aphasia without actual evidence of aphasia, and because the distinction between ‘agrammatic primary progressive aphasia with aphasia’ versus ‘agrammatic primary progressive aphasia without aphasia’ is relevant to localization, underlying pathology and, particularly germane to the current study of disease evolution, the recognition of PPAOS as a distinct disorder should be considered important until proven otherwise.
One of the central questions that has arisen regarding PPAOS relates to the progression of the syndrome over time because of its importance for determining prognosis and ultimately to designing optimal pharmacological and behavioural interventions. It is unclear whether subjects with PPAOS develop memory loss with evolving features akin to Alzheimer’s dementia (McKhann et al., 2011), aphasia with features more akin to primary progressive aphasia (Mesulam, 2003), behavioural dyscontrol with features more akin to a frontotemporal dementia (Neary et al., 1998), extra-pyramidal signs with features more akin to Parkinson’s disease (Gibb and Lees, 1988), pyramidal signs with features more akin to amyotrophic lateral sclerosis (Brooks et al., 2000) or remain with an isolated apraxia of speech.
Over the past 4 years, we have encountered a relatively large cohort of subjects meeting criteria for PPAOS. To date, a number of them have returned for follow-up and have been enrolled in a National Institute of Health funded study that aims to track the progression of clinical and neuroimaging characteristics over time. Therefore, in this study we aimed to determine how the clinical characteristics of people with PPAOS evolve over time and to report on the corresponding brain imaging changes that occur with MRI, FDG-PET, and DTI analyses. Our hypothesis was that PPAOS would progress very slowly over time and that the apraxia of speech would continue to dominate the clinical phenotype for up to the first 5 years of the illness (Josephs et al., 2012).
Materials and methods
Recruitment
Between 1 April 2013 and 31 March 2014, we enrolled all subjects who (i) had been previously enrolled into another National Institute of Health funded grant and had been given a diagnosis of PPAOS; and (ii) were willing to return for a follow-up evaluation that included the identical clinical and neuroimaging protocol that was administered for the baseline study. Detailed information regarding the baseline study protocol has been published previously (Josephs et al., 2012). In brief, for the baseline study we attempted to recruit all patients presenting to the Neurology Department with a higher-level speech and language disorder suspected to be secondary to a degenerative process. Only subjects over 18 years, with an informant to provide independent evaluation of functioning, and who spoke English as their primary language, were included. All subjects underwent detailed neurological evaluation, speech and language examination, neuropsychological testing, and neuroimaging analysis that included a volumetric head MRI, DTI and FDG-PET scan, over a span of 48–72 h. For the baseline study, subjects were diagnosed with PPAOS if the dominant presenting sign was that of apraxia of speech and any other neurological and aphasia characteristics were considered no more than equivocal. Diagnosis was made after review of video and audio recordings and speech and language test scores by two speech-language pathologists (J.R.D. and E.A.S.). All subjects enrolled in the follow-up study were brought back at least 1.5 years after the baseline assessment and underwent the identical protocol he/she had completed for the baseline study. Hence, all subjects in this study had the identical protocol completed at two time-points.
The study was approved by the Mayo Clinic Institutional Review Board and all subjects were consented for enrolment into the study.
Speech and language assessment
The Western Aphasia Battery aphasia quotient (WAB-AQ) (Kertesz, 2007), Part 1, served as the primary measure of global language ability. The WAB Part 1 included assessments of spontaneous speech (including the fluency, grammatical competence and paraphasias score and information content score), auditory verbal comprehension, repetition, and naming and word finding. Writing was assessed with the Writing Output subtest of the WAB, Part 2. Reading irregular and non-words were also assessed on the WAB, Part 2. The Token Test, Part V (De Renzi and Vignolo, 1962), served as a sensitive measure of verbal comprehension and, more specifically, grammatic/syntactic comprehension, the 15-item Boston Naming Test (Lansing et al., 1999) as a sensitive measure of confrontation-naming ability, and the Pyramids and Palm Trees Test (Howard and Patterson, 1992) as a sensitive measure of semantic knowledge. Action fluency (verbs) and semantic (animal) fluency scores were obtained. A score >2 standard deviations (SD) below the control mean on the WAB–AQ and Token Test was considered abnormal. A score of <6 on the WAB fluency rating was considered abnormal (i.e. agrammatic). The Northwestern Anagram Test (10-item version) (Weintraub et al., 2009) was completed only at time point 2, and served as a sensitive measure of grammatic expression that was independent of speech production. A score of <9 was considered abnormal. The writing sample from the WAB picture description was also reviewed for the presence or absence of agrammatism. Judgements about motor speech abilities were based on all spoken language tasks of the WAB plus additional speech tasks that included vowel prolongation, speech alternating motion rates (e.g. rapid repetition of ‘puhpuhpuh…’), speech sequential motion rates (e.g. rapid repetition of ‘puhtuhkuh’), word and sentence repetition tasks, and a conversational speech sample. Sixteen speech characteristics, consistent with current criteria for apraxia of speech diagnosis (Duffy, 2005; Wambaugh et al., 2006; McNeil et al., 2009), or observations of characteristics of apraxia of speech associated with neurodegenerative disease (Duffy, 2006) were rated on the Apraxia of Speech Rating Scale, which provided a quantitative index of apraxia of speech characteristics and severity (Josephs et al., 2012). The same speech tasks were also judged for the presence or absence of dysarthria, which was rated on a 0–4 severity scale. An eight-item measure of non-verbal oral praxis, with responses to each item rated on a 0–4 scale (with 4 representing best/normal performance), served as a quantitative index of non-verbal oral apraxia (Botha et al., 2014a). A global judgement about the presence or absence of non-verbal oral apraxia was also made.
For this study, quantitative scores and video recordings of crucial aspects of the test protocol were reviewed for all subjects by two of the co-authors (J.R.D and E.A.S.) who made independent judgements about the presence or absence of aphasia, apraxia of speech, dysarthria, and non-verbal oral apraxia, and the severity of each disorder. To reduce any testing biases, no subject was evaluated by the same speech/language pathologist at both baseline and at follow-up.
Neurological examination
All subjects underwent detailed neurological examination by a behavioural and movement disorders specialist (K.A.J.) for the baseline study and the identical protocol for the follow-up evaluation. Testing of general cognitive function included the Mini-Mental State Examination (Folstein et al., 1975); executive function with the Frontal Assessment Battery (FAB) (Dubois et al., 2000); praxis with the limb apraxia subscale of the WAB (Kertesz, 2007); functional performance with the Clinical Dementia Rating Scale sum of boxes (CDR-SB) (Hughes et al., 1982); neuropsychiatric features with the brief questionnaire form of the Neuropsychiatric Inventory (NPI-Q) (Kaufer et al., 2000), motor and non-motor function with the Movement Disorders Society sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Parts I–III (Goetz et al., 2008); eye movement abnormality with the progressive supranuclear palsy (PSP) Saccadic Impairment Scale (Whitwell et al., 2011) and gait/midline function and falls severity on subtests of the progressive supranuclear palsy rating scale (Golbe and Ohman-Strickland, 2007). On all tests, a z-score of >2 SD below the mean using published or derived means and standard deviations were considered abnormal.
In addition, documentation of the presence or absence of the following clinical signs or symptoms was prospectively performed: dysphagia, rapid eye movement sleep behavioural disorder, photosensitivity, urinary incontinence, aphasia, yes–no reversal, dysarthria, masked facie, axial rigidity, appendicular rigidity, decreased arm swing, postural stability, appendicular tremor, myoclonus, dystonia, alien limb phenomenon, mirror movements, ideomotor apraxia, vertical supranuclear gaze palsy or slowing, apraxia of eyelid opening, pseudobulbar affect, snout reflex, palmomental, spasticity, limb weakness and other involuntary movements.
Other cognitive testing
A clinical neuropsychologist (M.M.M.) oversaw test administration, scoring accuracy and quality control at baseline and follow-up. Motor speed was assessed using the Trail Making Test A (Spreen and Strauss, 1998); executive function using the Delis-Kaplan Executive Function System (DKEFS) Card Sort (Delis et al., 2001); episodic memory using the Auditory Verbal Learning Tests (Rey, 1964) and visuospatial and visuo-perceptual function using the Cube Analysis and Incomplete Letters, respectively, from the Visual Object and Space Perception Battery (Warrington and James, 1991). Mayo Older American Normative Studies age-adjusted scaled scores (Ivnik et al., 1992) were used for the Trail Making Test A and Auditory Verbal Learning Tests; these scores are constructed to have a mean of 10 and SD 3 in cognitively healthy participants. Scores <7 are generally considered abnormal. For the cube analysis and fragmented letters tests scores were converted to z-scores with a z-score of >2 SD below the mean considered abnormal.
Neuroimaging
Control subjects
A cohort of 20 healthy subjects was selected as a control cohort for the imaging analyses. They had all undergone serial MRI performed at two time-points using the identical acquisition parameters to the PPAOS cohort, and were selected to match the PPAOS cohort in age at baseline MRI, gender and scan interval. Nine (45%) of the control cohort were male, with age at baseline of 72.2 years (range 60–78), and scan interval of 2.3 years (range 1.1–3.2).
MRI and PET image acquisition
All subjects underwent a standardized MRI imaging protocol at 3.0 T, that included a 3D MP-RAGE sequence and a single-shot echo-planar DTI pulse sequence with 41 diffusion encoding steps and five non-diffusion weighted T2 images, as previously detailed (Josephs et al., 2012).
All FDG-PET scans were acquired using a PET/CT scanner (GE Healthcare) operating in 3D mode. Subjects were injected with an average, 459 MBq (range, 367–576 MBq) of FDG in a dimly lit room with minimal auditory stimulation. After a 30-min uptake period, an 8-min FDG scan was performed consisting of four 2-min dynamic frames following a low dose CT transmission scan. Individual frames of the FDG were realigned if motion was detected and then a mean image was created.
Whole brain, ventricular and midbrain volume analyses
All MP-RAGE images underwent preprocessing correction for gradient non-linearity (Jovicich et al., 2006) and intensity non-uniformity (Sled et al., 1998). Rates of whole brain atrophy and ventricular expansion were calculated for each PPAOS subject. Each follow-up scan was spatially registered to the baseline scan using a nine degrees of freedom rigid-body registration. A differential bias correction (Lewis and Fox, 2004; Gunter et al., 2012) was then performed in order to balance image intensities within each scan-pair. Change in brain and ventricle volume was then calculated automatically from each registered scan-pair using the boundary shift integral (Freeborough and Fox, 1997; Gunter et al., 2003). Midbrain volumes were also calculated for each scan using atlas-based parcellation. A manually traced midbrain mask was propagated onto each native-space MP-RAGE, as previously described (Botha et al., 2014b).
Voxel-based morphometry analysis
Voxel-based morphometry (Ashburner and Friston, 2000) using SPM5 was used to assess regional patterns of grey matter volume loss in PPAOS compared to controls at baseline. All MP-RAGE scans were normalized to a customized template and segmented using unified segmentation (Ashburner and Friston, 2005), followed by a clean-up step that uses a hidden Markov random field model. Grey matter images were modulated and smoothed at 8 mm full-width at half-maximum. Two-sided t-tests were used to assess patterns of loss in the PPAOS subjects compared to controls. Results were assessed first at a lenient statistical threshold of P < 0.001 with correction for multiple comparisons using the family-wise error correction at the cluster-level, and then also at a more stringent threshold corrected for multiple comparisons at the voxel-level using the family-wise error correction at P < 0.05.
Tensor-based morphometry analysis
Longitudinal patterns of regional atrophy over time were also assessed directly using an in-house developed version of tensor-based morphometry using symmetric normalization (TBM-SyN) (Jack et al., 2014). The SyN algorithm (Avants et al., 2008) was used to compute a non-linear deformation required to transform the later image to the earlier image for each pair of scans, producing an image of the log of the Jacobian determinants for each deformation. The log Jacobian image for each subject was scaled by the inter-scan interval, producing annualized log Jacobian images. A two-sided t-test was used to compare the annualized log Jacobians in template space between PPAOS and controls. Results were assessed using the same statistical thresholds described in the voxel-based morphometry section.
DTI analysis
Each of the 41 diffusion-weighted DTI images was registered to the non-diffusion weighted b0 volumes using affine transformations. Images were brain-extracted (Smith et al., 2004) and fractional anisotropy and mean diffusivity maps were generated (Behrens et al., 2003). A whole-brain voxel-based analysis was performed as previously described (Schwarz et al., 2014), comparing PPAOS and controls, at both the baseline and follow-up time-points, using the fractional anisotropy and mean diffusivity images. Two-sided t-tests in SPM5 were used to assess fractional anisotropy and mean diffusivity in the PPAOS subjects compared with controls. Results were assessed using the same statistical thresholds described in the voxel-based morphometry section. Exclusive masking was used to identify regions with fractional anisotropy or mean diffusivity abnormalities in PPAOS at follow-up that were not present at baseline.
FDG-PET analysis
Individual patterns of hypometabolism were assessed using the clinical tool of 3D stereotactic surface projections (SSP) (Minoshima et al., 1995). The activity in each subject’s PET data set was normalized to the pons and compared with an age-segmented normative database, yielding a 3D SSP z-score image. The image produced by this analysis produces a metabolic map using the z-scores as calculated for each surface pixel. The software package used to perform these analyses was CortexID (GE Healthcare).
Statistical analysis
Fisher’s exact and Mann-Whitney U tests were used to compare all binary and all continuous data, respectively. Statistical analyses were performed using the JMP software (JMP Software, version 10.0.0; SAS Institute Inc.) with significance set at P < 0.05.
Results
Demographic features
Thirteen subjects (seven females), met diagnostic criteria for PPAOS and completed baseline and follow-up evaluations. Demographic features are shown in Table 1. The median education was 15 years (range 10–20). Nine subjects were right-handed, three left-handed and one was ambidextrous. The median age at onset was 67 years (49–76). Baseline evaluation occurred at 71 years (53–80) and follow-up evaluations occurred 2.4 years (1.6–3.0) later. The median disease duration from onset to follow-up was 6.9 years (3.7–11.7).
Table 1.
Demographic features
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Median |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | F | F | M | F | F | M | M | F | M | M | M | F | F | NA |
| Education (years) | 15 | 15 | 16 | 12 | 17 | 18 | 12 | 15 | 10 | 12 | 20 | 18 | 15 | 15 |
| Handedness | R | A | L | R | R | R | L | R | R | R | L | R | R | NA |
| Age at onset (years) | 76 | 74 | 63 | 67 | 70 | 73 | 71 | 67 | 49 | 60 | 62 | 51 | 74 | 67.0 |
| Age at baseline evaluation (years) | 79 | 80 | 68 | 69 | 73 | 78 | 77 | 71 | 53 | 62 | 65 | 61 | 75 | 71.1 |
| Age at follow-up evaluation (years) | 82 | 82 | 70 | 71 | 74 | 79 | 79 | 74 | 56 | 65 | 67 | 62 | 77 | 74.1 |
| Time from baseline to follow-up (years) | 3.0 | 2.4 | 1.5 | 2.5 | 1.8 | 1.6 | 2.0 | 3.0 | 2.8 | 2.6 | 2.5 | 1.7 | 1.8 | 2.4 |
| Time from onset to last follow-up (years) | 6.9 | 8.3 | 7.3 | 4.8 | 5.4 | 6.6 | 8.0 | 7.1 | 7.6 | 5.2 | 5.7 | 11.7 | 3.7 | 6.9 |
A = ambidextrous; N/A = not applicable, R = right-handed; L = left-handed.
Clinical signs and symptoms
At baseline, all PPAOS subjects complained of primary difficulty with their speech and none reported any other symptoms, or were found to have any additional neurological signs on examination, except for a spastic dysarthria in two subjects, non-verbal oral apraxia in four and vertical supranuclear gaze palsy in one, as previously reported (Josephs et al., 2012). None had gait or balance problems or falls. At the time of follow-up, all subjects reported additional symptoms and were found to exhibit additional signs (Table 2). Approximately half reported swallowing difficulties with choking episodes, particularly while drinking liquids; some had prolonged coughing spells triggered by simply swallowing saliva. Urinary incontinence requiring the usage of adult diapers developed in four subjects and two had noted prominent photosensitivity requiring constant use of dark sunglasses.
Table 2.
Clinical signs and symptoms that were observed at follow-up
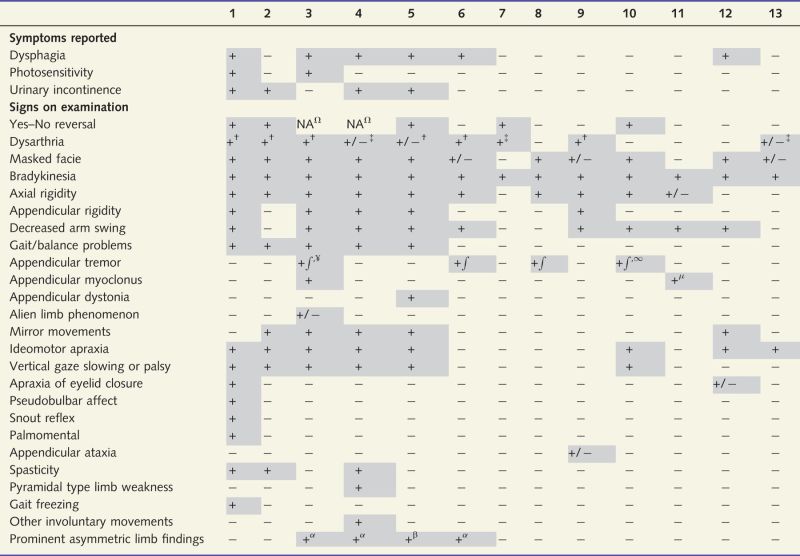 |
† = spastic; ‡ = hypokinetic; ¥ = Lip/jaw tremor present; ∫ = kinetic tremor; ∞ = postural tremor; Ω = subject anarthric or almost anarthric; + = present; − = absent; + /− = equivocal; µ = mini-myoclonus; α = left side affected more than right; β = right side affected more than left; NA = not able. Patient 10 also had Bruxism.
Shaded cells represent positive signs or symptoms.
On neurological examination at follow-up less than half of the subjects were noted to have a yes/no reversal (i.e. subject would nod or say yes when they meant no, or would shake their head left and right or say no, when they meant yes) (Frattali et al., 2003). All subjects had parkinsonian signs, the most common being bradykinesia and a masked facie. Axial rigidity was observed in all but three subjects and was more common than appendicular rigidity. None of them had a rest tremor of the limbs, although one had a lip/jaw tremor. Postural and kinetic tremors were present but infrequent. Pyramidal signs, such as limb spasticity, were infrequent although almost half of the subjects had a spastic dysarthria at follow-up; hypokinetic dysarthria was less frequent. Cortical abnormalities, including ideomotor apraxia and mirror movements, were present in more than half of the group with one subject having equivocal alien limb phenomenon. Six subjects had developed eye movement abnormalities; five of them were also posturally unstable on the pull test. Other signs were less common including myoclonus, dystonia, and apraxia of eye-lid closure. Only one subject had gait freezing. No subject reported features consistent with REM sleep behaviour disorder and none had definite ataxia, chorea, or myorhythmia, or lower motor neuron signs. In four subjects, the observed appendicular abnormalities were asymmetric.
Quantitative clinical measures of progression
The most striking neurological changes over time were observed on the UPDRS part III and the FAB, with almost all subjects showing significant decline from baseline (Table 3). Similarly, although less frequent, the WAB ideomotor apraxia scale demonstrated decline in praxis over time in more than half of the subjects. Five of the 13 subjects developed balance problems with falls. Slowing of vertical eye movements were noted in three subjects (PSIS = 2), whereas vertical supranuclear gaze palsy was present in an additional three subjects (PSIS > 2). Generalized cognitive impairment declined over time in two subjects, although five had evidence of functional decline. Behavioural features did not progress and were absent-mild at baseline and follow-up.
Table 3.
Quantitative neurological, speech and language, and other cognitive test scores at baseline/follow-up
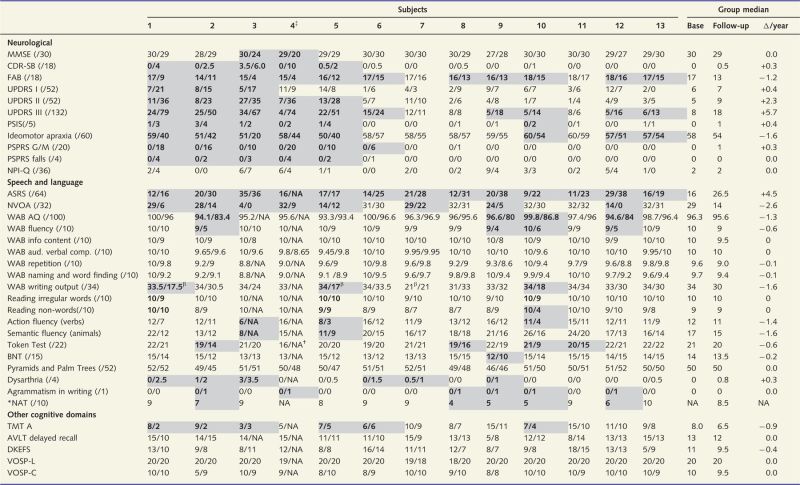 |
AQ= aphasia quotient; ASRS= Apraxia of Speech Rating Scale; AVLT= Auditory Verbal Learning Test; BNT= Boston Naming Test; FR= fluency rating; G/M= gait/midline; MMSE= Mini-Mental State Examination, N/A= not able; NAT= Northwestern Anagram Test; TMT= Trail Making Test; VOSP –L= Visual Object and Space Perception-letters; VOSP-C= Visual object and Space Perception-cube.
*Only completed at follow-up.
†Could not manipulate tokens due to severe limb immobility.
‡Subject with near absence of speech but did produce some interpretable whispered responses. Shaded cells represent clinically significant change from baseline to follow-up and/or with follow-up scores considered clearly abnormal.
βLower scores during this 3 min task reflect limited output due to motor slowing, not agrammatism.
All subjects had significant worsening of apraxia of speech (Table 3). Eight developed or showed progression of non-verbal oral apraxia. As noted above, unequivocal dysarthria developed or worsened in six subjects, with two additional subjects showing equivocal signs of dysarthria. Five subjects developed agrammatism in writing, or on the Northwestern Anagram Test, at follow-up, with an additional subject (Subject 4) showing agrammatism in writing but was untestable in verbal tasks due to reduced speech intelligibility. Four of these five subjects also had a WAB-AQ score in the abnormal range at follow-up, and four performed in the abnormal range on the Token Test at follow-up. Only one subject each exhibited naming difficulty on the Boston Naming Test, and reading non-words, and none performed in the abnormal range on the Pyramids and Palm Trees or reading irregular words test, at follow-up. Three subjects showed a decline of more than four words on action or semantic fluency tests at follow-up compared to baseline.
The only test assessing other cognitive domains that showed abnormalities was the Trail Making Test-A at follow-up, demonstrating cognitive slowing in six subjects (Table 3). None of the 13 subjects showed any deficits in memory, executive function or visuospatial or perceptual function.
Structural MRI analysis
The subjects showed median rates of whole brain atrophy of −1.5% per year (range −0.6 to −3.0), rates of ventricular expansion of 8.0% per year (5.5 to 16.9) and rates of midbrain atrophy of −1.5% per year (0.5 to −4.9). Rates of whole brain atrophy, ventricular expansion and midbrain atrophy were significantly faster than those observed in controls (Fig. 1). All subjects showed rates of whole brain atrophy, ventricular expansion and midbrain atrophy greater than the median of the control cohort (Fig. 1).
Figure 1.
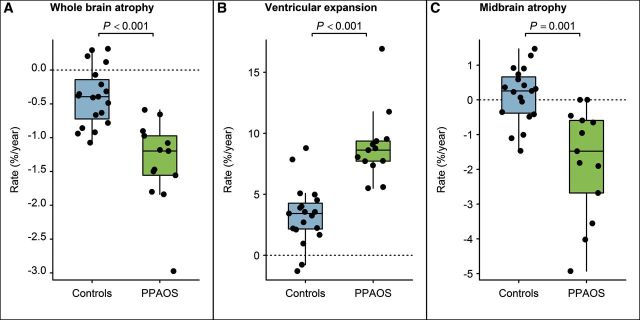
Box-plots showing rates of whole brain atrophy, ventricular expansion and midbrain atrophy over time in PPAOS subjects and controls. Boxes represent 25th, 50th and 75th percentiles. Individual subject points are shown.
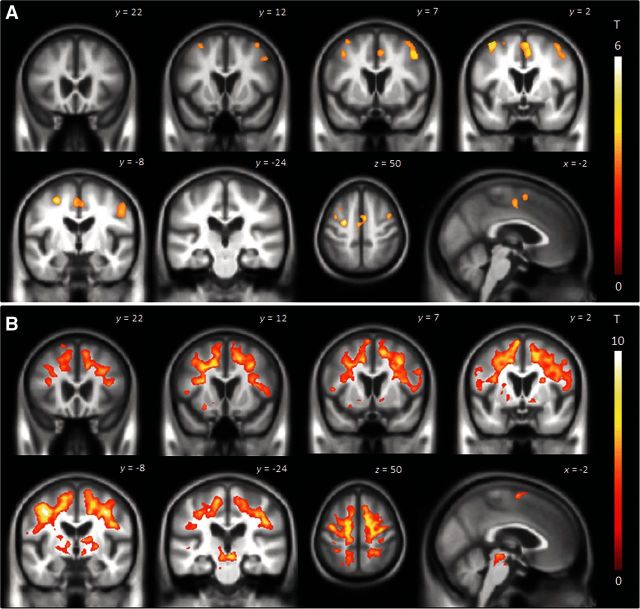
The voxel-level group analysis showed reduced grey matter volume bilaterally in the medial and lateral superior premotor cortex at baseline, compared to controls (Fig. 2A). These findings did not survive a voxel-level correction for multiple comparisons. Tensor-based morphometry revealed increased rates of regional atrophy over the 2.4-year interval in PPAOS compared to controls (Fig. 2B). Increased rates were observed throughout the medial and lateral premotor cortex bilaterally, and extending forward into prefrontal cortex and backwards into primary motor cortex. Increased rates of atrophy were also observed in the basal ganglia, including globus pallidus, putamen and caudate, and in midbrain (Fig. 2B). After a stringent correction for multiple comparisons at the voxel-level, increased rates of atrophy were observed in superior and middle premotor cortex and midbrain.
Figure 2.
Patterns of grey matter loss in PPAOS compared to controls at baseline (A) and regions of brain tissue loss over time on tensor-based morphometry in PPAOS subjects compared to controls (B). Results are shown on coronal, axial and sagittal slices of the brain corrected for multiple comparisons at the cluster level using P < 0.05.
DTI analysis
The PPAOS subjects showed reduced fractional anisotropy bilaterally in the body of the corpus callosum, callosal fibres spreading into superior premotor cortex, and inferior and middle premotor white matter at baseline, compared to controls (Fig. 3). At follow-up, fractional anisotropy reductions in these regions were more extensive, with additional involvement of the splenium of the corpus callosum, superior motor white matter and the fornix (Fig. 3). After a stringent correction for multiple comparisons at the voxel-level, reduced fractional anisotropy at baseline and follow-up was observed in inferior, middle and superior premotor white matter and body of the corpus callosum.
Figure 3.
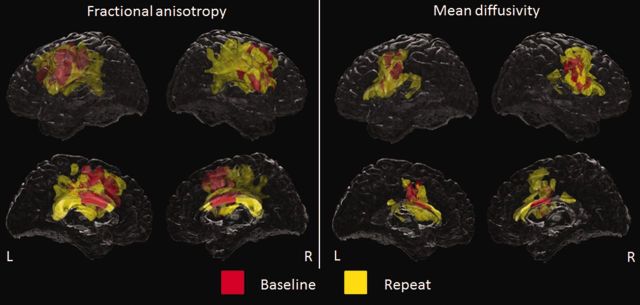
Patterns of fractional anisotropy and mean diffusivity abnormalities in PPAOS compared to controls at the baseline and repeat time-points. Results are shown on lateral and medial transparent renderings of the brain corrected for multiple comparisons at the cluster level using P < 0.05. Baseline findings are shown in red and additional findings at follow-up are shown in yellow. L = left; R = right.
The PPAOS subjects showed increased mean diffusivity in the body of the corpus callosum and inferior and middle premotor white matter at baseline, compared to controls (Fig. 3). At follow-up, mean diffusivity increases in these regions were more extensive with additional involvement of more anterior and posterior aspects of the corpus callosum (Fig. 3). After a stringent correction for multiple comparisons at the voxel-level, increased mean diffusivity at baseline and follow-up was observed in the left inferior and middle premotor white matter.
FDG-PET analysis
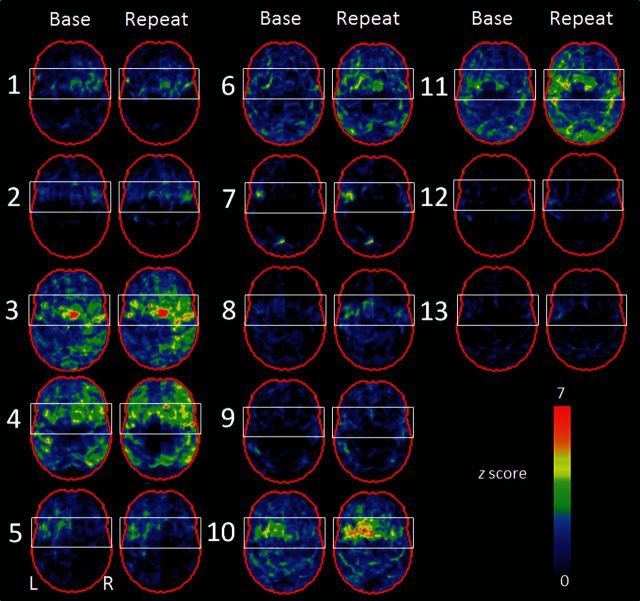
The severity of hypometabolism varied somewhat across subjects, although all subjects with PPAOS showed abnormalities in medial and lateral premotor cortex. In the majority of these, the hypometabolism was most striking in the superior view (white boxes, Fig. 4). The temporal and parietal lobes were only involved in Subjects 3, 5, 7 and 11. The severity of hypometabolism progressed over time in all PPAOS subjects (Fig. 4).
Figure 4.
Statistical stereotactic surface projection maps showing patterns of FDG-PET hypometabolism at baseline and repeat time-points in each of the 13 PPAOS subjects. Subject numbers are shown to the left of each set of images. Z-score values are colour coded as indicated in the colour scale (0 = normal; 7 = most abnormal).
Discussion
In this comprehensive clinico-neuroimaging study of progression over time in PPAOS, we demonstrate that PPAOS either remains predominantly PPAOS, albeit with the development of mild parkinsonism, or progresses fairly rapidly into a devastating condition with features consistent with an atypical parkinsonian disorder.
The fact that these individuals exhibited parkinsonian features at follow-up, ∼7 years from onset, yet did not exhibit these features at baseline, suggests that most people with PPAOS are likely to develop extrapyramidal features after ∼5 years. This finding has important implications for prognosis, showing that extrapyramidal features are a late characteristic of PPAOS. In fact, in some individuals the extrapyramidal features became the most debilitating aspect of the disorder and, although none of the subjects in this study have yet died, many have swallowing difficulties with choking episodes which can lead to aspiration pneumonia and subsequent death. Another feature that developed in a third of the group was urinary incontinence that required adult diapers. Urinary incontinence is a risk factor for urinary tract infections (Eriksson et al., 2010), which is another cause for increased mortality in older people in general (Gee, 1993), as well as those with a neurodegenerative disorder (Gambassi et al., 1999).
It is clear that all subjects had progressive worsening of their apraxia of speech and all developed parkinsonian features. Five subjects (Subjects 1–5) showed a rapid evolution, developing vertical supranuclear gaze palsy or slowing and prominent gait and balance problems with many falls reminiscent of the PSP syndrome. None of these five would have met the current National Institute of Neurological Disease and Stroke and Society of PSP criteria for possible or probable PSP (Litvan et al., 1996) for 5 years, given that none had gait/postural instability or falls within the first 5 years after onset. All five also had ideomotor apraxia, four had urinary incontinence, and three had prominent asymmetric features, which are somewhat atypical for PSP (Litvan et al., 1996). Regardless, the evolution of these five subjects demonstrates that PPAOS can be a prodromal syndrome to a PSP-like syndrome. Hence, we suggest referring to such subjects as having PSP-AOS when PPAOS has evolved into a PSP-like syndrome. The finding that PPAOS can be the presenting symptom 5 years before evolving into a PSP-like syndrome may have important implications for PSP therapeutic trials that seek to enrol subjects at the earliest stages of the disease. The remaining eight subjects, on the other hand, continued to have apraxia of speech as the most prominent feature of the disease with no other features present that would suggest an alternative diagnosis that would trump a diagnosis of PPAOS. Significant differences in progression were noted between the five PSP-AOS subjects and the eight remaining PPAOS subjects (Table 4). The former group had faster rates of whole brain and midbrain changes over time, and greater rates of clinical decline of extrapyramidal features, frontal lobe function, eye movement abnormalities and gait and balance compared to the remaining eight subjects (Table 4). These clinical and imaging features are all consistent with the development of a PSP-like syndrome at follow-up (Paviour et al., 2005, 2006; Whitwell et al., 2007, 2012; Litvan and Kong, 2014). Conversely, the latter group had greater rates of decline of speech apraxia as measured by the Apraxia of Speech Rating Scale compared to the PSP-AOS group (Table 4). These differences suggest that both groups may have a different trajectory and hence different prognosis over time. The fact that speech apraxia progressed more in the latter group argues against the notion that the PSP-AOS group was just progressing faster in general. Unfortunately, after examination of the data for baseline clinical predictors regarding future progression, none were found. Hence, this issue is ripe for further investigation in larger cohorts because of its potential prognostic value.
Table 4.
A comparison of the annualized rates of clinical and imaging change over time between subjects that evolved into PSP-AOS and those that remained PPAOS
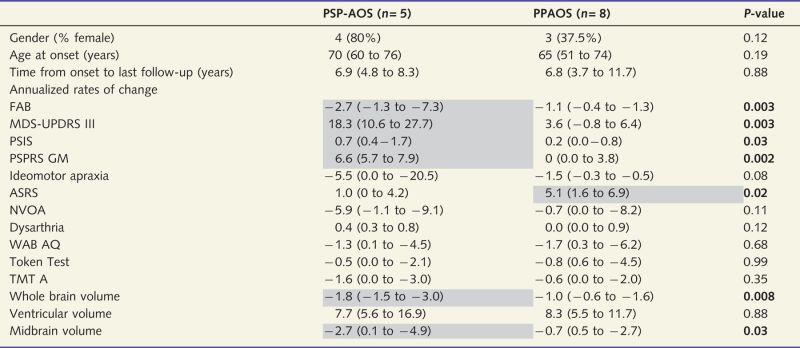 |
Data shown as median (range); ASRS = Apraxia of Speech Rating Scale; NVOA = non-verbal oral apraxia; TME = Trail Making Test.
Shaded areas represent more severe change over time.
Importantly, none of the 13 subjects in this study developed features suggestive of Alzheimer’s disease (McKhann et al., 1984), dementia with Lewy bodies (McKeith et al., 2005) or Parkinson’s disease (Gibb and Lees, 1988). None of them had lower motor neuron findings and hence none could be reclassified as having amyotrophic lateral sclerosis (Brooks et al., 2000). Some of our PPAOS subjects did show decline on the FAB, which is a test of frontal lobe function. Although impaired frontal lobe function can be a feature of the behavioural variant of frontotemporal dementia (Neary et al., 1998), no subject developed significant behavioural dyscontrol which is the key feature for the diagnosis (Rascovsky et al., 2011). In fact, a review of the individual FAB subtests revealed that the drop in scores was a result of poorer performance on the lexical fluency and motor series components of the FAB (Dubois et al., 2000); the latter being a test of ideational apraxia. Decline in the FAB has been associated with PSP (Paviour et al., 2005; Josephs et al., 2013b). Only the Trail Making Test-A test (Spreen and Strauss, 1998), a test of cognitive speed, was abnormal on neuropsychological testing. All other neuropsychological tests assessing episodic memory, visuospatial, and visuoperceptual functions were in the normal range, again arguing against a diagnosis of another neurodegenerative disorder.
All subjects in this cohort presented with apraxia of speech as the most prominent clinical feature and none had more than equivocal evidence of aphasia in spoken or written language at baseline; hence, our diagnosis of PPAOS (Josephs et al., 2012) was justified. Although one could quibble about the sensitivity of our tests to detect subtle aphasia, a diagnosis of primary progressive aphasia requires prominent and early aphasia (Mesulam, 1982). Therefore, none would have met strict criteria for primary progressive aphasia at baseline. At follow-up, five of the 13 PPAOS subjects had developed unequivocal evidence of aphasia; a sixth subject also had agrammatism in writing. Although it is possible that we were not able to detect aphasia in two subjects due to their speech being sparse and reduced in intelligibility, we did have writing samples. The aphasia characteristics were predominantly agrammatic in all these subjects, similar to the aphasic characteristics of the agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2011). However, the aphasia was never in isolation, never the most prominent feature of the syndrome, never the main patient complaint or the main cause for functional impairment, and was always overshadowed by the apraxia of speech and other parkinsonian and motor features at follow-up. Hence, it would be inappropriate to label such patients as having the agrammatic variant of primary progressive aphasia at follow-up. In fact, even at follow-up, when compared to other studies of the agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2004; Catani et al., 2013), our subjects still performed strikingly better on tests of language.
We also investigated the neuroanatomical substrate for the changes we observed in PPAOS. As we previously demonstrated, PPAOS is associated with focal superior premotor and supplementary motor grey matter abnormalities (Josephs et al., 2013a). We extend these findings in the current study by using the technique of tensor-based morphometry to assess regional atrophic changes in PPAOS. We showed, firstly, that involvement of the superior premotor cortex, including the supplementary motor area, worsens over time. As these regions correlate with apraxia of speech severity (Josephs et al., 2013a; Whitwell et al., 2013a), it was not surprising that all 13 subjects showed worsening of their apraxia of speech over time. In addition, a number of regions that were not significantly abnormal at baseline showed significant atrophy. The focal superior premotor abnormality present at baseline extended down into the lateral inferior premotor region, which encompasses Broca’s area, at follow-up. It is therefore no surprise that some of our PPAOS subjects developed agrammatic aphasia (Amici et al., 2007; Peelle et al., 2008; Josephs et al., 2013a). More interesting and novel, however, is the explicit finding of atrophy in subcortical grey matter structures and brainstem across all 13 PPAOS subjects, consistent with these regions transitioning from normal to abnormal over a 2-year interval. Firstly, we observed greater atrophy in the globus pallidus, putamen and caudate nuclei in PPAOS compared to controls. The involvement of these structures may account for the development of the parkinsonian features, although future studies that directly assess the correlation between rates of basal ganglia atrophy and worsening of Parkinsonism, ideally in a larger number of subjects, would be required to test this hypothesis. Atrophy of the putamen may also be playing a role in the development of the urinary incontinence, as is the medial prefrontal cortex (Perneczky et al., 2008). Increased midbrain atrophy in PPAOS compared to controls was observed both in the tensor-based morphometry and atlas-based parcellation analyses. This finding is in-keeping with our previous study showing that the midbrain area is reduced in PPAOS subjects compared to controls (Josephs et al., 2013a, b). Midbrain volume loss is a feature of pathologically confirmed PSP when the clinical presentation is the classic PSP syndrome (Massey et al., 2013; Whitwell et al., 2013b). Hence, we hypothesize that the development of midbrain atrophy in PPAOS accounted for the development of some of the PSP-like features in many of our subjects. Indeed, as discussed above, there was evidence that midbrain atrophy was greater in the five PSP-AOS subjects. In addition to observing progressive involvement of subcortical and brainstem grey matter structures, we also observed increased atrophy extending posteriorly into the primary motor cortex, which could account for the development of ideomotor apraxia, spasticity, pseudobulbar affect and spastic dysarthria. Spastic dysarthria and pseudobulbar affect have been linked to involvement of primary motor cortex and descending corticospinal and corticobular tracts (Clark et al., 2014).
Similar to the cortical grey matter findings, DTI analysis demonstrated more widespread white matter tract degeneration beneath the premotor cortex extending into motor cortices at follow-up. Therefore, PPAOS is associated with progressive grey and white matter degeneration. We also showed greater involvement of the splenium of the corpus callosum at follow-up compared to baseline, in-keeping with the fact that grey matter atrophy also extended to involve more posterior regions at follow-up. Involvement of the corpus callosum could explain the observation of mirror movements in some of our PPAOS subjects (Nass, 1985; Danek et al., 1992), although some studies have suggested that mirror movements are due to involvement of the ipsilateral corticospinal tract and not the corpus callosum (Krams et al., 1999). Further studies are needed to determine the relative extent to which degeneration of the grey matter, as opposed to the white matter, may be involved in the progression of clinical features in PPAOS.
While the anatomical imaging analyses described above reflect group-level analyses, the FDG-PET scans demonstrate that progression can be observed at the single-subject level. Subtle, yet obvious, progression of hypometabolism over time was observed in most, if not all, of the 13 subjects. FDG-PET scans could, therefore, be a useful clinical tool to demonstrate progression and solidify a PPAOS diagnosis, particularly in situations in which it is unclear whether the speech abnormalities are neurological in origin. We also measured whole brain, ventricular and midbrain volumes at the single-subject level and observed increased rates of change in all three measures compared to the control cohort, with increased rates observed in the majority of the PPAOS subjects. Hence, whole brain, ventricular and midbrain volumes could also be useful in PPAOS to demonstrate a progressive degenerative process as opposed to a functional or psychogenic speech disorder (Lang et al., 1989; Dafotakis et al., 2010). These findings also suggest that these rate measurements could be useful disease biomarkers if PPAOS subjects were included in future treatment trials.
Conclusion
In this study, we show that PPAOS, a motor speech planning/programming disorder, will either maintain a course dominated by apraxia of speech after 5 years, or rapidly evolve into a devastating PSP-like syndrome. These findings offer further insight into the nature of PPAOS and provide some prognostic guidelines. Future studies will need to identify the pathological process or processes underlying PPAOS.
Acknowledgements
The authors would like to thank Drs Ahlskog, Boeve, Bower, Drubach, Knopman and Petersen for subject referral and Mrs Sarah Boland, Mayo Clinic Rochester, MN, for performing the neuropsychometric testing and organizing all subject’s test schedules.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- FAB
Frontal Assessment Battery
- FDG
fluorodeoxyglucose
- PPAOS
primary progressive apraxia of speech
- PSP
progressive supranuclear palsy
- WAB
Western Aphasia Battery
Funding
This study was funded by R01 DC012519 (PI Whitwell) and R01 DC010367 (PI Josephs) from the National Institute on Deafness and Communication Disorders.
References
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–90. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014a;82:1729–35. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Whitwell JL, Madhaven A, Senjem ML, Lowe V, Josephs KA. The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat Disord. 2014b;20:180–5. doi: 10.1016/j.parkreldis.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Broussolle E, Bakchine S, Tommasi M, Laurent B, Bazin B, Cinotti L, et al. Slowly progressive anarthria with late anterior opercular syndrome: a variant form of frontal cortical atrophy syndromes. J Neurol Sci. 1996;144:44–58. doi: 10.1016/s0022-510x(96)00096-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(Pt 8):2619–28. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson EJ, Josephs KA. Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol. 2014;21:368–76. doi: 10.1111/ene.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Benoit N, Van Eeckhout P, Ducarne B, Brunet P. Pure progressive aphemia. J Neurol Neurosurg Psychiatry. 1993;56:923–4. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafotakis M, Schmidt M, Meister IG, Fink GR, Dennin A, Nowak DA. Degenerative anterior biopercular syndrome initially misdiagnosed as a psychogenic disorder. J Neurol. 2010;257:1576–8. doi: 10.1007/s00415-010-5559-7. [DOI] [PubMed] [Google Scholar]
- Danek A, Heye B, Schroedter R. Cortically evoked motor responses in patients with Xp22.3-linked Kallmann's syndrome and in female gene carriers. Ann Neurol. 1992;31:299–304. doi: 10.1002/ana.410310312. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–78. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS): examiner's manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–9. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differetial diagnois, and management. 2nd edn. St Louis, MI: Mosby; 2005. [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–27. [Google Scholar]
- Eriksson I, Gustafson Y, Fagerstrom L, Olofsson B. Prevalence and factors associated with urinary tract infections (UTIs) in very old women. Arch Gerontol Geriatr. 2010;50:132–5. doi: 10.1016/j.archger.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frattali C, Duffy JR, Litvan I, Patsalides AD, Grafman J. Yes/no reversals as neurobehavioral sequela: a disorder of language, praxis, or inhibitory control? Eur J Neurol. 2003;10:103–6. doi: 10.1046/j.1468-1331.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imag. 1997;16:623–9. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer's disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67:59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee WM. Causes of death in a hospitalized geriatric population: an autopsy study of 3000 patients. Virchows Arch A Pathol Anat Histopathol. 1993;423:343–9. doi: 10.1007/BF01607146. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130(Pt 6):1552–65. doi: 10.1093/brain/awm032. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter JL, Senjem ML, vemuri P, Jack CR., Jr Wang L, Yushkevich P, Ourselin S, editors. Comparison of mask-based differences, boundary shift integral and symmetric normalization jacobian integration. MICCAI 2012 workshop on novel imaging biomarkers for Alzheimer's disease and related disorders (NIBAD'12); 2012. p. 239–46. [Google Scholar]
- Gunter JL, Shiung MM, Manduca A, Jack CR., Jr Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson K. The pyramids and palm trees test: a test of semantic access from words and picture. Bury St Edmonds: Thames Valley Test Company. 1992 [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec J, Smith GE, Tangalos EG, Petersen RC, Kokmen E. Mayo's older american normative studies: WAIS-R, WMS-R and AVLT norms for ages 56-97. Clin Neuropsychol. 1992;(Suppl 6):1–104. [Google Scholar]
- Jack CR, Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–12. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–96. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013a;81:337–45. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Xia R, Mandrekar J, Gunter JL, Senjem ML, Jack CR, Jr, et al. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 2013b;28:1117–24. doi: 10.1002/mds.25437. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–36. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery (Revised) San Antonio, TX: PsychCorp; 2007. [Google Scholar]
- Krams M, Quinton R, Ashburner J, Friston KJ, Frackowiak RS, Bouloux PM, et al. Kallmann's syndrome: mirror movements associated with bilateral corticospinal tract hypertrophy. Neurology. 1999;52:816–22. doi: 10.1212/wnl.52.4.816. [DOI] [PubMed] [Google Scholar]
- Lang C, Reichwein J, Iro H, Treig T. Foix-Chavany-Marie-syndrome–neurological, neuropsychological, CT, MRI, and SPECT findings in a case progressive for more than 10 years. Eur Arch Psychiatry Neurol Sci. 1989;239:188–93. doi: 10.1007/BF01739653. [DOI] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999;14:481–7. [PubMed] [Google Scholar]
- Lewis EB, Fox NC. Correction of differential intensity inhomogeneity in longitudinal MR images. Neuroimage. 2004;23:75–83. doi: 10.1016/j.neuroimage.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Litvan I, Kong M. Rate of decline in progressive supranuclear palsy. Mov Disord. 2014;29:463–8. doi: 10.1002/mds.25843. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Massey LA, Jager HR, Paviour DC, O'Sullivan SS, Ling H, Williams DR, et al. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856–61. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MR, Robin RA, Schmidt RA. Apraxia of speech: definition and differential diagnosis. In: McNeil MRE, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 2009. [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia–a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia and the language network: the 2013 H. Houston Merritt Lecture. Neurology. 2013;81:456–62. doi: 10.1212/WNL.0b013e31829d87df. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(Pt 5):1537–53. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48. [PubMed] [Google Scholar]
- Nass R. Mirror movement asymmetries in congenital hemiparesis: the inhibition hypothesis revisited. Neurology. 1985;35:1059–62. doi: 10.1212/wnl.35.7.1059. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain. 2006;129(Pt 4):1040–9. doi: 10.1093/brain/awl021. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Winterburn D, Simmonds S, Burgess G, Wilkinson L, Fox NC, et al. Can the frontal assessment battery (FAB) differentiate bradykinetic rigid syndromes? Relation of the FAB to formal neuropsychological testing. Neurocase. 2005;11:274–82. doi: 10.1080/13554790590962933. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;21:418–32. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Diehl-Schmid J, Forstl H, Drzezga A, May F, Kurz A. Urinary incontinence and its functional anatomy in frontotemporal lobar degenerations. Eur J Nucl Med Mol Imaging. 2008;35:605–10. doi: 10.1007/s00259-007-0626-8. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Ricci M, Magarelli M, Todino V, Bianchini A, Calandriello E, Tramutoli R. Progressive apraxia of speech presenting as isolated disorder of speech articulation and prosody: a case report. Neurocase. 2008;14:162–8. doi: 10.1080/13554790802060839. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- Wambaugh JL, Duffy JR, McNeil MR, Robin DA, Rogers MA. Treatment guidelines for acquired apraxia of speech: a synthesis and evaluation of the evidence. J Med Speech Lang Pathol. 2006;14:35–67. [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Dement. 2009;24:408–16. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013a;125:245–52. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, Gunter JL, Weigand SD, Boeve BF, et al. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol. 2013b;20:1417–22. doi: 10.1111/ene.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, Knopman DS, Boeve BF, Petersen RC, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130(Pt 4):1148–58. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Master AV, Avula R, Kantarci K, Eggers SD, Edmonson HA, et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol. 2011;68:753–60. doi: 10.1001/archneurol.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Xu J, Mandrekar JN, Gunter JL, Jack CR, Jr, Josephs KA. Rates of brain atrophy and clinical decline over 6 and 12-month intervals in PSP: determining sample size for treatment trials. Parkinsonism Relat Disord. 2012;18:252–6. doi: 10.1016/j.parkreldis.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82:1119–26. doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]