Abstract
Background:
Diabetes Mellitus is a metabolic syndrome, affecting the oral health in various ways with dental caries being one of the most common problems encountered. Saliva is one of the most abundant secretions in the human body with a variety of natural protective and defence molecules bathing the oral cavity maintaining equilibrium. Its collection is easy and non-invasive.
Aims:
To compare and evaluate salivary alkaline phosphatase levels and calcium ion levels between caries active type II diabetes mellitus patients and non-diabetics.
Materials and Methods:
This study was carried out on caries-active age and gender matched 60 non-diabetic and 60 patients with known Type II diabetes mellitus subjects of age group 25-50 years with DMFT index >10. Saliva sample was collected to analyse for alkaline phosphatase enzyme and concentration of calcium ions using Agappe kits.
Statistical Analysis:
Student ‘t’ test was used to correlate the salivary electrolyte concentration in non- diabetic and diabetic patients with dental caries. A ‘P’ value of 0.05 or less was considered significant. Results are presented as mean ± standard deviation (X ± SD).
Results:
The alkaline phosphatase (ALP) activity in saliva was higher in diabetic patients when compared to that of non-diabetic patients with salivary calcium ions were significantly higher in non-diabetic individuals.
Conclusion:
Diabetes Mellitus patients are more prone to dental caries, hence require intervention to improve the quality of saliva.
Keywords: Alkaline phosphatase, calcium, diabetes mellitus, saliva
Introduction
Diabetes mellitus is a clinical syndrome characterized by hyperglycemia, due to deficiency or diminished effectiveness of insulin. The disease is chronic and affects the metabolism of carbohydrate, protein, fat, water, and electrolytes. Diabetes is characterized by high levels of blood glucose resulting from defects in insulin production, insulin action, or both. The effects of diabetes mellitus include long-term damage, dysfunction and failure of various organs.[1]
Dental caries has been more prevalent and even severe in diabetic patients than nondiabetics.[2,3] Oral complications such as periodontal disease (gingivitis, periodontitis), dental caries, salivary dysfunction, dry mouth, mucosal diseases, infections like candidiasis, salivary gland hypofunction are all more prone in diabetes mellitus condition.[4,5] Epidemiological study by Moore et al. states that 5% of all patients seen in dental clinics are reported to have diabetes.[6] The ambiguity of various aspects of dental caries still exists, and hence every effort of prevention and recurrence has partially been practiced and successful.
Saliva is essential for a lifelong conservation of dentition. Various functions of saliva have been implicated in the maintenance of oral health and protection of teeth.[7] The potential of saliva for diagnosis purpose has attracted more attention in recent years.[8,9] Saliva has enormous defense potential comprising both immunologic and enzymatic mechanisms.[10] According to recent data by Nagler et al. and Chiappelli et al. saliva mirrors general health condition thus reflecting various systemic changes in the body.[11,12,13] This sync with blood may help in the assessment of various parameters, which are found in blood and saliva may become alternate sampling media.[14] This has been tested by Hegde et al. where serum and salivary molecules were compared and evaluated in order to prove their correlation especially with dental caries.[15,16,17] Shahrabi et al. documented that many constituents of serum and saliva, both organic and inorganic have potentially protective role, which include calcium, phosphate, fluoride ions, and bicarbonate buffer systems.[18]
Alkaline phosphatase (ALP) is a calcium and phosphate binding protein and a phosphor-hydrolytic enzyme.[19] ALP, a salivary protein, may increase the concentration of salivary phosphate and the balance of demineralization to remineralization process of enamel. It seems that the function of this protein relatively depends on the salivary pH and buffering capacity.[20]
Calcium is an important ion present in the body. The balance between demineralization remineralization processes depends on the concentrations of the salivary parameters - calcium, phosphate and the pH. In alkaline pH, calcium plays an important role in remineralization of the enamel surface via formation of hydroxyapatite crystals, while in acidic pH salivary calcium plays a role in preventing the dissolution of enamel.[21]
A limited amount of studies is undertaken on salivary ALP activity and calcium levels in diabetic patients with dental caries. Considering this, the present study aims to evaluate and compare the salivary ALP and calcium levels in nondiabetic and diabetic patients with dental caries.
Subjects and Methods
This study was initiated only after being approved by the Institutional Ethical Committee. Approximately 1,300 healthy adult patients are coming to the outpatient departments of Department of Conservative Dentistry and Endodontics, A. B. Shetty Memorial Institute of Dental Sciences were screened and 120 patients, age and gender matched under the age group of 25–50 years who fulfilled the inclusion criteria were selected, after signing an informed consent form explaining the aims and objectives of the study and their participatory role in it.
Subjects
A total of 60 diabetic individuals (fasting blood glucose >125 mg/dl) with dental caries were selected as the study group and 60 nondiabetic individuals with caries were selected as a control group. Their caries status was assessed according to World Health Organization “(WHO) recommendations 1997” to calculate dental caries index.[22] Before collecting the saliva samples, the oral hygiene status and periodontium status were assessed.
All patients underwent scaling and curettage from the Department of Periodontics of the same institution and were enrolled into the study only after the reversal of their periodontal condition.
All patients were instructed to refrain from eating, drinking, smoking, and performing oral hygiene practices for 2 h before their saliva collection. The saliva sample was collected using WHO standard precautions laid down for health-care workers, over 5 min, between 9:00 a.m. and 11:00 a.m.[23] The vials were labeled and packaged immediately to the Central Research Laboratory for the required biochemical tests to be carried out.
Inclusion criteria
Type II diabetes mellitus patients under the age group of 25–50 years with fasting blood glucose >125 mg/dl
Patients with active dental caries, decayed, missing, and filled teeth index with decayed teeth >10.
Exclusion criteria
All the subjects were free from any vascular complication of diabetes as well as other systemic illness
Patients are taking any caries preventive regimen such as fluoride toothpaste, fluoride rinses, or NaF/calcium tablets
Pregnant women
Patients on radiotherapy
Patients who smoked in last 24 h or consumed alcohol.
Patients on calcium supplements
Patient under any reported xerostomia, antipsychotic, anticholinergic, antihypertensives, antisecretogogues and thyroid medication.
Collection of saliva for salivary analysis
All patients were asked to rinse the mouth with 15 ml of distilled water to wash out exfoliated cells. Ong stated the importance of accuracy in saliva measurements. Presence of circadian rhythm and fasting has been reported to influence salivary flow rate, which makes time-point of the test critical. Hence, saliva was collected between 9:00 a.m. and 11:00 a.m.[23]
Patients were asked to sit in an upright position with their head slightly inclined forward so that saliva was collected in the floor of the mouth. Unstimulated whole saliva was expectorated into sterile tubes collecting 5 ml of saliva from the patient who, was centrifuged, and the supernatant obtained was stored at 4°C for subsequent analysis.
Estimation of alkaline phosphatase activity
Alkaline phosphatase activity was determined using kit procedures (Agappe, India).
Kinetic determination of ALP according to the following reaction:
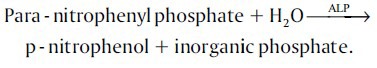
Estimation of calcium levels
Salivary calcium level was estimated using Calcium ChemKit (Agappe Diagnostics Pvt. Ltd., India). This allows an endpoint colorimetric analysis by mixing reagent 1 and reagent 2 in ratio 1:1 and incubating for 5 min at room temperature. The absorbance value was read using the following formula;

Statistical analysis
Student's t-test was used to correlate the salivary electrolyte concentration in nondiabetic and diabetic patients with dental caries. P = 0.05 or less was considered as significant. Results are presented as mean ± standard deviation (SD) (X ± SD).
Results
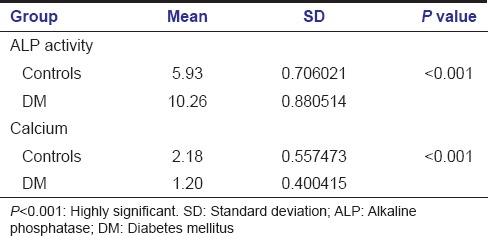
The ALP activity in saliva was higher in diabetic patients when compared to that of nondiabetic patients with dental caries. The mean activity of ALP in saliva of nondiabetic was 5.94 ± 0.7 and in diabetic patients was found to be 10.26 ± 0.89. ‘P’ value was statistically significant for both parameters studied (P < 0.001) [Table 1]. The salivary calcium ions were significantly higher in nondiabetic individuals with a mean level of 2.12 ± 0.42 than type II diabetics who had a mean level of 1.24 ± 0.58 [Graph 1].
Table 1.
Student t-test comparing ALP activity and calcium ion concentration between diabetics and nondiabetics (control)
Graph 1.
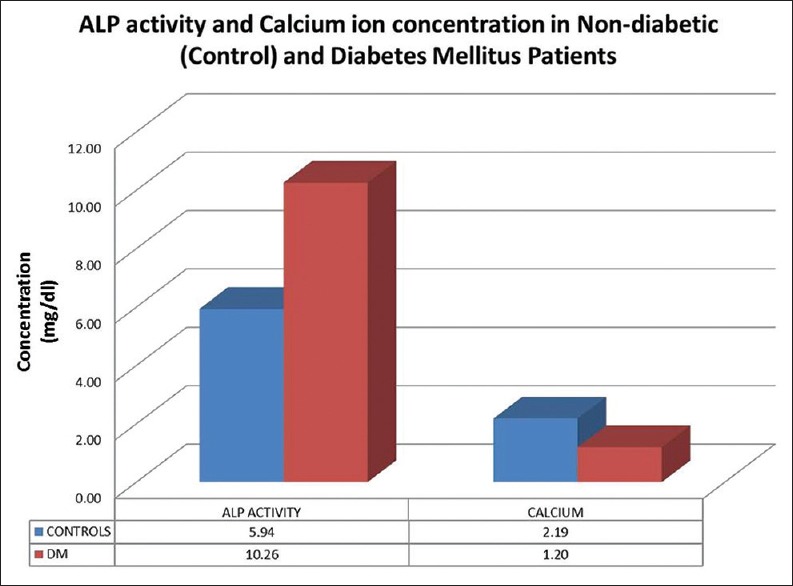
Alkaline phosphatase activity and calcium ion concentration in nondiabetic (control) and diabetes mellitus patients
Discussion
Dental caries has a multifactorial etiology and diabetes mellitus patients due to their frequent snacking and/or high-carbohydrate diet are prone to have a high caries index.[24] The oral milieu is in a constant dynamic equilibrium maintaining demineralization-remineralization process at all times. A breach in this equilibrium, initiates the caries process and the prime factor to govern the caries process is the salivary pH. Thus, all patients were refrained from eating, drinking, rinsing their mouth for 2 h prior to saliva sample collection.
Calcium in unstimulated whole saliva is typically present at 1.4 mmol/l[25] and is one of the efficient buffers for regulating the body fluids unlike phosphates which are more resistant to depression of plaque pH towards the critical pH.[26] Hence this study focused on comparing salivary calcium level in caries-active type II diabetics and nondiabetics; and diabetics in comparison to nondiabetics had a significantly lower salivary calcium ion level, exhibiting their lower remineralization potential than normal individuals and hence being categorized as high caries risk individuals.
Several studies have shown that diabetes mellitus patients have presented a decrease in salivary flow and pH.[3,27,28] Therefore maintaining a high-calcium level is of utmost importance to avoid carious attacks and supersaturate the saliva favoring remineralization. Several sugar free remineralizing agents can be prescribed to diabetes mellitus patients, which will not only increase the salivary flow, but also maintain a neutral pH in the oral environment, giving calcium ions the right zone to function in. A study reported by Karshan, stated that the calcium content of saliva is low in caries-active persons, confirming our finding that the state of calcium in saliva may be related to dental caries risk.[29]
Alkaline phosphatase enzyme is the prime enzyme balancing remineralization-demineralization cycle. This study showed significantly higher levels of ALP in caries-active type II diabetes mellitus individuals than in nondiabetics. ALP is known to be associated with inflammatory state and chronic periodontitis.[30] Not avoiding this aspect; all patients’ periodontium status was assessed and recruited into the study only after scaling and curettage, which resulted in the reversal of their periodontal condition in order to evaluate its role with dental caries.
It is well-established that initial caries lesion formation seen clinically as white spot lesion is marked by a subsurface demineralization with an intact surface layer[31,32] which can be remineralized, re-establishing the demineralization-remineralization equilibrium. Studies documented by Vijayaprasad et al.,[33] Gandhy and Damle[34] have shown a positive and direct correlation between salivary ALP level and dental caries. However, diabetes mellitus patients in this study demonstrated a remarkably higher level of ALP level than nondiabetes. This could be attributed to a higher tendency of calculus formation and salivary changes like reduced pH and flow rate seen in diabetic individuals making them more prone for dental caries.[3,27,28]
Conclusion
Saliva, which is often looked upon as the “bloodstream of tooth”[33] has various enzymes, micro-nutrients and macronutrients influencing dental caries. This study made an attempt to compare the same factors between normal and type II diabetes mellitus patients as not much research has been documented for the same. Within the limitations of the study, we conclude that salivary status of diabetes mellitus patients fluctuates the demineralization-remineralization equilibrium to a greater extent, posing them at a higher risk of developing dental caries. Hence making it imperative for dental practitioners to use saliva as a diagnostic or monitoring tool identifying this risk at earlier stages and intervene, ultimately improving the oral health status.
Clinical significance
Salivary substitutes, such as remineralization tooth mousse, cremes and chewing gums which would stabilize the oral environment can be introduced in the oral health-care regime for caries prone patients and especially diabetic patients as they have frequent short meals to maintain their blood glucose levels, which prolong the acidic oral environment for dental caries.
Acknowledgments
The authors are grateful and express their gratitude to the Central Research Lab of Nitte University, Mangalore, India to provide us with their facilities and technical assistance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawed M, Khan RN, Shahid SM, Azhar A. Protective effects of salivary factors in dental caries in diabetic patients of Pakistan. Exp Diabetes Res. 2012;2012:947304. doi: 10.1155/2012/947304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal S, Kazmi F, Asad S, Mumtaz M, Khan A. Dental caries and diabetes mellitus. Pak Oral Dent J. 2011;31:60–3. [Google Scholar]
- 4.Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. J Am Dent Assoc. 2003;134 Spec No:24S–33S. doi: 10.14219/jada.archive.2003.0366. [DOI] [PubMed] [Google Scholar]
- 5.Bastos AS, Leite AR, Spin-Neto R, Nassar PO, Massucato EM, Orrico SR. Diabetes mellitus and oral mucosa alterations: Prevalence and risk factors. Diabetes Res Clin Pract. 2011;92:100–5. doi: 10.1016/j.diabres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Moore PA, Zgibor JC, Dasanayake AP. Diabetes: A growing epidemic of all ages. J Am Dent Assoc. 2003;134 Spec No:11S–5S. [PubMed] [Google Scholar]
- 7.Castagnola M, Picciotti PM, Messana I, Fanali C, Fiorita A, Cabras T, et al. Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngol Ital. 2011;31:347–57. [PMC free article] [PubMed] [Google Scholar]
- 8.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 10.Zussman E, Yarin AL, Nagler RM. Age- and flow-dependency of salivary viscoelasticity. J Dent Res. 2007;86:281–5. doi: 10.1177/154405910708600316. [DOI] [PubMed] [Google Scholar]
- 11.Nagler RM, Hershkovich O, Lischinsky S, Diamond E, Reznick AZ. Saliva analysis in the clinical setting: Revisiting an underused diagnostic tool. J Investig Med. 2002;50:214–25. doi: 10.2310/6650.2002.33436. [DOI] [PubMed] [Google Scholar]
- 12.Nagler RM. Saliva analysis for monitoring dialysis and renal function. Clin Chem. 2008;54:1415–7. doi: 10.1373/clinchem.2008.112136. [DOI] [PubMed] [Google Scholar]
- 13.Chiappelli F, Iribarren FJ, Prolo P. Salivary biomarkers in psychobiological medicine. Bioinformation. 2006;29(1):331–4. doi: 10.6026/97320630001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDevitt JT. Saliva as the next best diagnostic tool. J Biochem. 2006;45:23–5. [Google Scholar]
- 15.Hegde MN, Kumari S, Hegde ND, Shetty S. Myeloperoxidase and glutathione peroxidase activity of saliva and serum in adults with dental caries: A comparative study. Journal of Free Radic Antioxid Photon. 2013;139:175–80. [Google Scholar]
- 16.Hegde MN, Kumari S, Hegde ND, Shetty S, Kumari N. Evaluation of the status of salivary nitric oxide in patients with dental caries. Nitte Univ J Health Sci. 2012;2:6–9. [Google Scholar]
- 17.Hegde ND, Hegde MN, Shetty S, Kumari S. Evaluation of salivary total antioxidants, superoxide dismutase activity and glutathione levels in oral cancer patients. J Oral Maxillofac Surg Photon. 2013;116:150–5. [Google Scholar]
- 18.Shahrabi M, Nikfarjam J, Alikhani A, Akhoundi N, Ashtiani M, Seraj B. A comparison of salivary calcium, phosphate, and alkaline phosphatase in children with severe, moderate caries, and caries free in Tehran's kindergartens. J Indian Soc Pedod Prev Dent. 2008;26:74–7. doi: 10.4103/0970-4388.41621. [DOI] [PubMed] [Google Scholar]
- 19.Sanikop S, Patil S, Agrawal P. Gingival crevicular fluid alkaline phosphatase as a potential diagnostic marker of periodontal disease. J Indian Soc Periodontol. 2012;16:513–8. doi: 10.4103/0972-124X.106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahedi M, Davoodi P, Goodarzi MT, Rezaei-Soufi L, Jazaeri M, Mortazavi H, et al. Comparison of salivary ion activity product for hydroxyapatite (IPHA), alkaline phosphatase and buffering capacity of adults according to age and caries severity. J Dent Shiraz Univ Med Sci. 2012;13:139–45. [Google Scholar]
- 21.Hassan SA, Al-Sandook TA. Salivary calcium concentration is seen in patients with high incidence of calculus formation. Al Rafidain Dent J. 2005;5:88–90. [Google Scholar]
- 22.World Health Organization. Basic methods. 3rd Ed. Geneva: 1997. Oral Health Survey. [Google Scholar]
- 23.Ong DC. Circardian rhythyms in the flow rate and proportional contribution of parotid to whole saliva and its composition in the humans. Mol Cell Biochem. 2001;261:137–42. [Google Scholar]
- 24.Al-Attas S, Oda S. Caries experience and selected caries-risk factors among a group of adult diabetics. Saudi Dent J. 2008;20:129–39. [Google Scholar]
- 25.Patrick P. Calcium. Report of London Laboratory Services Group. 2003:1–2. [Google Scholar]
- 26.Buche J. Herbs, Minerals and Goods web site. Montreal, Canada: 2004. The Calcium Connection; pp. 1–5. [Google Scholar]
- 27.Goyal D, Kaur H, Jawanfa M, Verma S, Parhar S. Salivary pH and dental caries in diabetes mellitus. J Oral Maxillofac Pathol. 2012;3:13–6. [Google Scholar]
- 28.Bernardi MJ, Reis A, Loguercio AD, Kehrig R, Leite MF, Nicolau J. Study of the buffering capacity, pH and salivary flow rate in type 2 well-controlled and poorly controlled diabetic patients. Oral Health Prev Dent. 2007;5:73–8. [PubMed] [Google Scholar]
- 29.Karshan M. Factors in saliva correlated with dental caries. J Dent Res. 1939;18:395–407. [Google Scholar]
- 30.Kumar R, Sharma G. Salivary alkaline phosphatase level as diagnostic marker for periodontal disease. J Int Oral Health. 2011;3:81–5. [Google Scholar]
- 31.Darling AI. Studies on the early lesion of enamel caries. Br Dent J. 1956;101:289. [Google Scholar]
- 32.Silverstone LM. Structure of carious enamel, including the early lesion. Oral Sci Rev. 1973;3:100–60. [PubMed] [Google Scholar]
- 33.Vijayaprasad KE, Ravichandra KS, Vasa AA, Suzan S. Relation of salivary calcium, phosphorus and alkaline phosphatase with the incidence of dental caries in children. J Indian Soc Pedod Prev Dent. 2010;28:156–61. doi: 10.4103/0970-4388.73789. [DOI] [PubMed] [Google Scholar]
- 34.Gandhy M, Damle SG. Relation of salivary inorganic phosphorus and alkaline phosphatase to the dental caries status in children. J Indian Soc Pedod Prev Dent. 2003;21:135–8. [PubMed] [Google Scholar]


