Abstract
Background:
Aggressive periodontitis is a characterized by rapid attachment loss, bone destruction and familial aggregation. Platelet-rich plasma (PRP) has been proposed to promote regeneration of the lost periodontal tissues. The aim of this study was to evaluate and compare the efficacy of PRP combined with hydroxyapatite (HA) graft in the treatment of intra-bony defects in localized aggressive periodontitis (L-AgP) patients.
Materials and Methods:
Ten L-AgP patients having bilateral intra-bony defect ≥2 mm and probing depth (PD) ≥6 mm were randomly treated either with the PRP/HA graft or HA graft alone. The clinical (plaque control record, bleeding on probing index, PD, and relative attachment level [RAL]), and radiographic parameters (size of the bone defect) were recorded pre- and post-operatively at 3, 6, and 12 months.
Results:
After 12 months, for both maxillary and mandibular arches, the mean PD decrease was significantly more (P < 0.05) for the test group than the control group (3.2 mm vs. 1.9 mm and 3.6 mm vs. 1.9 mm, respectively). Furthermore, the mean RAL decrease in both maxillary and mandibular arches was significantly more (P < 0.05) for the test group than the control group (3.0 mm vs. 1.2 mm and 3.1 mm vs. 1.4 mm, respectively). Radiographically, the test group showed significantly more defect fill as compared with the control group.
Conclusion:
Both treatments provided significant improvements in clinical and radiographic parameters in a 12-month postoperative period. PRP/HA group presented superior results regarding PD reduction, clinical attachment gain and radiographic bone fill than HA group.
Keywords: Aggressive periodontitis, intra-bony defects, regeneration
Introduction
Localized aggressive periodontitis (L-AgP) is characterized by deep vertical osseous defects that affect the first molars and incisors of young adults. Long-term stabilization of periodontal health in such patients usually requires comprehensive mechanical/surgical and antimicrobial therapy. However, conservative periodontal therapy may result in reparative wound healing with limited regeneration of the lost tissues, which has always been a challenge in clinical periodontics.
Regeneration is defined as reproduction or reconstitution of a lost or injured part.[1] It is, therefore, a biologic process by which the architecture and function of the lost tissues are completely restored. The main approaches for periodontal regeneration requires introduction of a “filler” material into the periodontal defect in the hope of inducing bone regeneration.[2] For decades, various types of synthetic artificial bone substitutes have been utilized for regenerative periodontal treatment in intra-bony defects.[3] Porous hydroxyapatite (HA) bone grafting material has been used to fill periodontal intra-bony defects, which has resulted in clinically acceptable responses.[4] However, true periodontal regeneration is not achieved because the healing, which occurs is a connective tissue encapsulation of the graft with a long junctional epithelium.[5]
Alternatively, techniques are being developed to guide and instruct the specialized cellular components of the periodontium to participate in the regenerative process.[2] This latter approach to reconstruction makes use of understanding of the development of the periodontium and the cellular processes that are involved. Polypeptide growth factors are biological mediators that have the ability to regulate cell proliferation, chemotaxis, differentiation, and extracellular matrix synthesis.[6] Unfortunately, these important wound healing proteins have not been available to clinicians for frequent use. However, the use of autologous platelet-rich plasma (PRP) to make platelet gel allows clinician to concentrate naturally occurring growth factors for immediate clinical use.[7] The processing of PRP involves the sequestration and concentration of platelets through centrifugation and subsequent polymerization.[7] Platelets play a crucial role wound healing because they promote the initial coagulation at wound site and also release many wound healing growth factors.[8] Thrombin and calcium are usually added to platelet concentrate (PC) to induce platelet aggregation and degranulation, release of growth factors, and better handling of the graft material. The basic strategy is to amplify and accelerate the effects of growth factors contained in the platelets (platelet derived growth factor [PDGF], transforming growth factor-β, insulin-like growth factor, fibroblast growth factor, vascular endothelial growth factor, epithelial growth factor), which are universal initiators of almost all wound healing.[7] The use of PRP as an adjunct to bone grafting procedures has become an attractive option since its introduction by Whitman et al.[9] and Marx et al.[10]
Platelet-rich plasma also contains three proteins (fibrinogen, fibronectin, vitronectin) in blood known to act as cell adhesion molecules for osteoconduction and as a matrix for bone and connective tissue.[11] Due to its high fibrin content, PRP presents with a “sticky” characteristic which works as a hemostatic and stabilizing agent and may aid the blood clot and bone graft immobilization in the defect area.[12] Fibrin component of PRP also adheres to the root surface and so may impede the apical migration of epithelial cells and connective tissue cells from the flap.[13]
The aim of this study was to evaluate and compare efficacy of PRP combined with HA graft, and HA graft alone in the treatment of intra-bony defects in L-AgP patients.
Materials and Methods
Study design
This study was a randomized controlled clinical trial, carried out after obtaining the ethical clearance from Institutional Review Board. Ten patients with L-AgP[14] showing clinical and radiographic evidence of bilateral angular defects were recruited for the study. Patients selected were in good general health, having an intra-bony defect ≥ 2 mm with a probing depth (PD) ≥6 mm. Patients with abnormal platelet count, smokers and pregnant women were excluded from the study. The patients selected on the above criteria were then explained about the treatment procedure, and their written consent was obtained.
A split-mouth design was adopted and therefore n = 10 for both groups (PRP/HA and HA).
Control group
Ten intra-bony defects (five in the maxillary arch and five in the mandibular arch), subjected to open flap debridement with HA graft material placed in the defects.
Test group
Ten intra-bony defects (five in the maxillary arch and five in the mandibular arch), subjected to open flap debridement and HA graft material mixed with PRP placed in the defect.
Initial therapy consisted of oral hygiene instructions with thorough full mouth scaling and root planing. Four weeks following Phase I therapy, a periodontal re-evaluation was performed to confirm the suitability of the sites for this periodontal surgical study. Patients were properly oriented in home care, and only those patients who had attained the satisfactory level of oral hygiene status were included in the study.
Clinical measurements
Plaque control record,[15] bleeding on probing (BOP) index,[16] PD and relative attachment level (RAL) were recorded. Occlusal stents for positioning the UNC-15 probe were fabricated with self-cured acrylic resin on a cast obtained from an alginate impression.[17] The occlusal stent was made to cover the occlusal surfaces and were also extended apically on the buccal and lingual surfaces to cover the coronal third of the teeth involved. A wire was placed on the bucco-occlusal line angle of teeth during fabrication. This wire was then exposed by making grooves in the inter-dental regions, such that the probe when inserted in the inter-proximal area was in contact with the wire; hence, the probe position and angulation remained the same for all pre- and post-operative measurements. Using the groove and wire as a guide, the periodontal probe was inserted into the gingival sulcus and pocket depth (using the gingival margin as reference), and RAL (using the stent wire as a reference) were recorded.
Radiographic measurements
Radiographs were taken using the RINN XCP system® (Dentsply, USA) by the standardized paralleling technique with the digital radiovisuography (RVG) (Suni Ray® Suni Imaging Microsystem Inc., San Jose, CA, USA). The size of the defect was calculated using the RVG software X-ray Vision® Apetryx. In order to calibrate measurements digitally, the distance from the cusp tip to the cementoenamel junction was measured clinically after flap reflection and recorded, the same was measured radiographically and all further radiographic measurements were calibrated according to this scale. The landmarks on the radiographs were identified according to the study of Eickholz et al.[18] which were then used in the current study to calculate the size of the defect (size of defect = INFRA × BDW × AB).
All clinical and radiographic parameters were recorded preoperatively [Figures 1a and 2a] and postoperatively at 3, 6, and 12 months.
Figure 1.
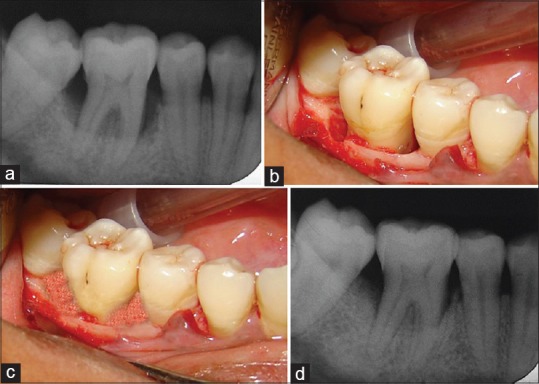
Control group (a) Baseline radiograph showing infrabony defect, (b) 6 months postoperative radiograph showing bone fill, (c) Infrabony defect after debridement, (d) Hydroxyapatite graft packed into the defect
Figure 2.
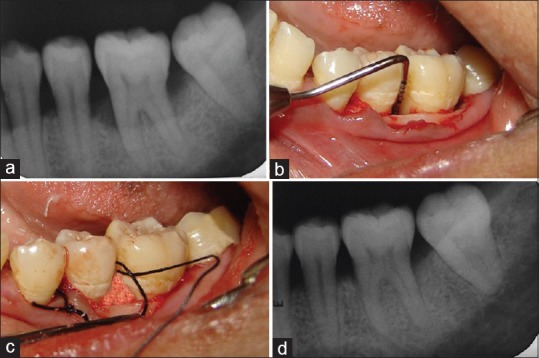
Test group (a) Baseline radiograph showing infrabony defect, (b) 6 months postoperative radiograph showing bone fill, (c) Infrabony defect after debridement, (d) Platelet-rich plasma/hydroxyapatite graft packed into the defect
Platelet-rich plasma procurement
One hour before the surgery, 10 ml of blood was withdrawn from the anticubital vein of the patient and collected into vacutainer tube containing sodium citrate as an anticoagulant. It was then placed in an automated centrifuge. First spin of 1200 rpm for 20 min separated the whole blood into three fractions that is, platelet poor plasma (PPP), PRP and red blood cells (RBC). After discarding the RBC fraction a second spin of 2000 rpm for 15 min was given that separated the components into PPP and concentrated PRP (cPRP).[19] After discarding the PPP, the remaining cPRP was activated by clot initiator (human thrombin) and 10% calcium chloride to obtain a sticky gel consistency. Then HA granules were mixed with coagulated PRP gel in a proportion of 1:1. HA, granules plus saline will serve as a control treatment. Platelet counts were performed on each sample, including a peripheral blood count and PRP count by hemocytometer.[20] Minimum 3 times increase in PC from baseline platelet counts were considered adequate for use.
Surgical procedure
The surgical procedure was performed by local infiltration of 2% xylocaine hydrochloride with adrenaline 1:80,000. Buccal and lingual/palatal sulcular incisions were used, and full-thickness mucoperiosteal flaps were elevated with a blunt dissection using a periosteal elevator. Care was excised to preserve as much inter-proximal tissue as possible. After meticulous defect debridement and removal of granulation tissue [Figures 1b and 2b], graft material was placed up to the vertical height of the corresponding adjacent bone level [Figures 1c and 2c]. Surgical flap was repositioned and sutured with 3-0 silk and covered with a periodontal dressing. Patients were given analgesics (ibuprofen 400 mg every 8 h) and antibiotics (amoxicillin 500 mg every 6 h) for 5 postoperative days and were advised to use 0.12% chlorhexidine mouth rinse twice daily for 14 days. Subjects were recalled after 1 week for suture removal, thereafter every 15th day for oral hygiene follow-up and oral prophylaxis for 3 months.
Statistical analysis
Descriptive data that included mean and standard deviations were determined for each clinical and radiographic parameter in each group at all the time intervals and were used for analysis. Student's paired t-test was used to compare data from the baseline to that of 3 and 12 months for each treatment group. Comparison between the test and the control group (inter-group) at baseline, 3 and 12 months were accomplished using Student's unpaired t-test. The statistical analysis was done using SPSS software 15.0.1 version (SPSS Inc., Chicago, IL, USA).
Results
Reduction in plaque [Table 1] and gingival BOP [Table 2] scores were observed, from their preprophylactic levels to preoperative levels and up to 12 months postoperatively for both control and test groups for both arches. There was no significant difference between the plaque scores as well as gingival BOP scores of both treatment groups at all time periods in both arches.
Table 1.
Inter- and intra-arch comparison of plaque control record for control and test groups
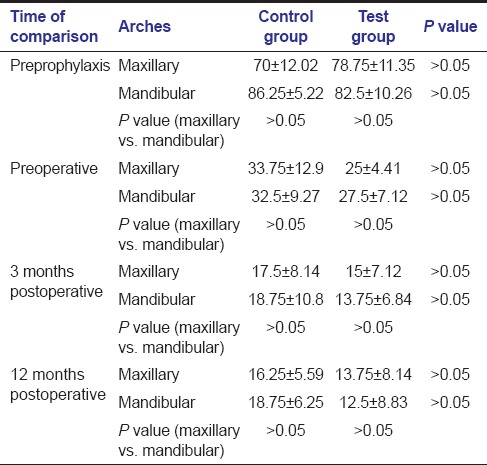
Table 2.
Inter- and intra-arch comparison of gingival bleeding on probing for control and test groups
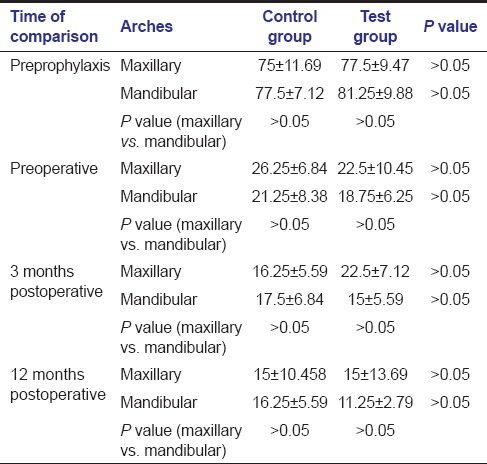
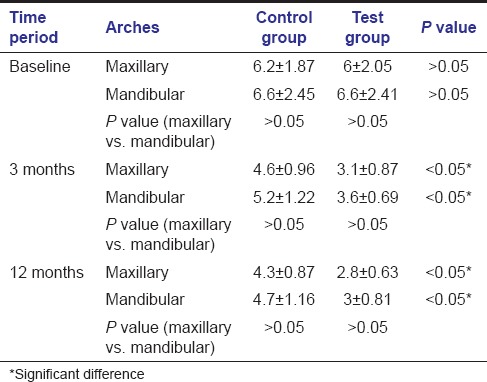
For the maxillary arch, the mean PD decrease [Table 3] was more for the test group in comparison to the control group (3.2 mm vs 1.9mm). Furthermore, for the mandibular arch, the mean PD decrease was more for the test group in comparison to the control group (3.6 vs 1.9mm). This difference in mean PDs by the two surgical procedures was significant at all times in both the arches i.e., at 3 and 12 months postoperatively (P < 0.05). Comparison of PDs between different arches at different time periods revealed that there was no significant difference between the two arches at different time periods for both control and test groups (P > 0.05).
Table 3.
Inter- and intra-arch comparison of probing depth for control and test sites
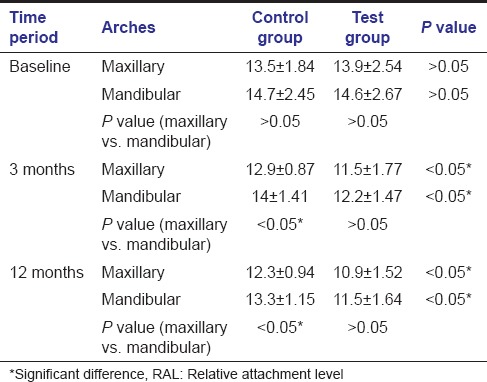
In the maxillary arch, the mean RAL decrease [Table 4] was more for the test group in comparison to the control group (3.0 mm vs 1.2mm). In the mandibular arch also the mean RAL decrease was more for the test group in comparison to the control group (3.1 mm vs 1.4mm). This difference in mean PDs by the two surgical procedures was significant at all times in both arches i.e., at 3 and 12 months postoperatively (P < 0.05). Comparison of RAL between different arches at different time periods for both groups indicate that there was a significant difference between the two arches at all time periods for the control group (P < 0.05). However, no significant difference between the two arches at different time periods was found for the test group (P > 0.05).
Table 4.
Inter- and intra-arch comparison of RAL measurements for control and test sites
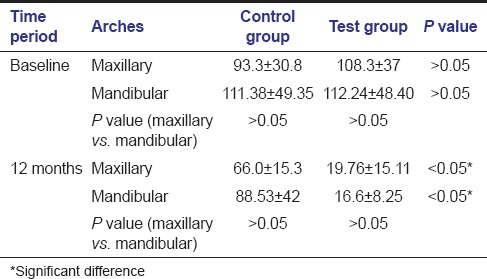
On comparing control and test groups at baseline, it was found that at 5% level of significance, there was no significant difference for radiographic parameter [Table 5] and the defects being treated in both groups were similar in maxillary as well as mandibular arches. At 12 month inter-group comparison in both maxillary and mandibular arch, there was a significant difference in both surgical modalities for the size of defect, test group showing more defect fill as compared to the control group. [Figures 1d and 2d] Comparison of radiographic parameters between different arches at different time periods for both groups showed that there was no significant difference between the two arches (P > 0.05).
Table 5.
Inter- and intra-arch comparison of radiographic measurement for control and test sites
Discussion
A major objective of periodontal therapy continues to be regeneration of the attachment structures of teeth destroyed by periodontal disease. Biologic molecules, such as growth factors, could enhance regeneration. PRP, which is an autologous volume of plasma with a high PC, has been shown to contain several growth factors that may enhance wound healing and shorten the healing period.
Very few studies have analyzed periodontal regenerative treatment in patients affected by L-AgP. The clinical results of this study demonstrated that the use of PRP and HA in intra-bony defects may lead to statistically significant periodontal probing depth reduction and clinical attachment loss (CAL) gain for patients with L-AgP. In this study, the comparison was done between the maxillary and mandibular sites as there are anatomical differences between the bone and the teeth of the two arches.
In this study, reduction in plaque scores and BOP was observed, from their preprophylactic levels to preoperative levels and then up to 12 months postoperatively. The improvement in scores support the observed treatment effects obtained under a strict oral hygiene regimen. These results are in accordance with the results of Hanna et al.[21] who reported that all patients enrolled for the study maintained very low mean plaque index scores of 0.98 at baseline and 0.43 at 6 months and also very low mean gingival index scores of 0.40 at baseline and 0.22 at 6 months. Okuda et al.[22] reported no significant difference in plaque index scores, gingival index and BOP scores at baseline and 12 months between the PRP + HA and HA + saline groups. Results of this study are also in conformity with the studies with triple combination therapy including PRP, bovine porous bone mineral (BPBM), and guided tissue regeneration (GTR) carried out by Camargo et al.,[23] Lekovic et al.,[24] Lekovic et al.,[25] Camargo et al.[17] in which they reported slight decrease in plaque index and scores sulcus bleeding index from baseline to 6 months postoperatively, the difference was not statistically significant between the test and the control groups.
Results of this study show that the decrease in PD and gain in RAL was more where defects were filled with PRP + HA graft. These results are in conformity with the results of Hanna et al.[21] who compared bovine-derived xenograft (BDX) with and without PRP in the treatment of human intra-bony defects. The mean PD of the deepest sites in the group treated with BDX showed a reduction from 7.0 to 4.46, while in the PRP/BDX treated group, the mean PD reduction was from 7.3 to 3.76. This change amounts to 2.54 and 3.54 mm in BDX and PRP/BDX treated groups respectively, and were comparable to our study. Okuda et al.[22] compared a combination of PRP and HA with a mixture of HA and saline in a 1-year study. The mean PD difference from baseline to 12 months was 4.7 in the PRP + HA sites and 3.7 in the HA sites. They also reported 3.14 mm of CAL gain in the intra-bony defects treated with PRP + HA graft in comparison to 2.0 mm in the HA graft sites. They concluded that the test group exhibited statistically significant changes compared to the control sites in PD reduction and CAL gain. Ouyang and Qiao[26] evaluated the effectiveness of PRP as an adjunct to BPBM graft in the treatment of human intra-bony defects and reported that the test group exhibited statistically significant changes compared to the control sites in PD reduction. They also reported that the group receiving PRP + BPBM exhibited 4.52 mm of mean CAL gain as compared with 2.85 mm in the group receiving BPBM alone. The triple combination therapy including PRP, BPBM, and GTR was found clinically more effective in reducing the pocket depth and CAL than open flap debridement[17,25] and GTR,[23] but no significant differences with a combination of PRP + BPBM.[24] Lekovic et al.[24] have reported that GTR adds no clinical benefit to PRP + BPBM, which means the combination of PRP + BPBM might prove the same clinical benefit as the triple combination. In a similar study conducted on 60 patients of chronic periodontitis, the efficacy of PRP/HA bone graft combination with a mixture of HA/saline was evaluated. Results showed a significant PD reduction (5.4 ± 0.49 mm vs. 4.0 ± 0.45 mm) and clinical attachment gain (5.4 ± 1.2 mm vs. 3.1 ± 1.1 mm).[27] Whereas, in both the studies of Döri et al.[28,29] no significant difference in pocket reduction and CAL gain between the two groups were reported. The difference could be attributed to the reason that no blood parameters were evaluated, which may entail the risk of production of PRP volumes with low PCs leading to disappointing results.
Results of this study indicated a greater amount of bone gain with PRP + HA graft. The bone gain by PRP + HA observed in this study is in accordance with the study by Marx et al.[10] who reported that the addition of PRP to grafts evidenced a radiographic maturation rate 1.62–2.16 times that of grafts without PRP. The histomorphometric assessment provided evidence of greater trabecular bone density in grafts in which PRP was added than in grafts in which PRP was not added. Nevins et al.[30] observed that bone height increased from a mean of 7.38 to 9.52 mm in defects treated by rhPDGF + allogeneic bone. This change amounts to 2.14 mm. McGuire et al.[31] observed a substantial increase in linear bone gain and percent bone fill in sites treated with rhPDGF-BB + β-tricalcium phosphate (β-TCP) as compared with sites treated with β-TCP + buffer. Menezes and Rao[27] also reported a significantly increased defect fill in PRP/HA treated defects as compared with HA/saline cases (3.2 ± 0.8 mm vs. 2.1 ± 0.6 mm). However, Tamura et al.[32] have reported that the addition of PRP to porous β-TCP for onlay bone grafting produced no significant difference in the relative amounts of mineralized bone generated in the blocks. This difference may be attributed to the density of platelets in PRP that increased only 3.5-fold relative to peripheral blood, whereas in this study, there was a 5-10 fold increase in PC relative to peripheral blood. This can be explained by differences in the procedure for blood centrifugation.
Finally, it can be suggested that the successful treatment of intra-bony defects may not be related only to the addition of PRP to HA graft. Other factors may play a more significant role in affecting the regenerative responses to the graft materials. Any or all of the following factors may influence periodontal regeneration: Depth and width of the defect and attachment level prior to treatment, thickness of the gingival flap, plaque accumulation, quality of the recall maintenance program, periodontal history of the affected tooth, healing response of the subject and clinicians’ surgical skill. The decision to treat a defect with a regenerative technique must be based on consideration of these factors, which in turn will determine the predictability of a successful result. Additionally, it is important to point to the practical aspects related to PRP preparation, which involves an additional step to the surgical procedure. The many steps involved in PRP procurement makes the procedure technique sensitive, which may lead to different growth factor concentration.
Conclusion
This study showed that both treatments (PRP/HA and HA) provided significant improvements in clinical and radiographic parameters for intra-bony periodontal defects in patients with L-AgP in a 12-month postoperative period. PRP/HA group presented superior results regarding PD reduction, clinical attachment gain and radiographic bone fill than HA group.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.American Academy of Periodontology. 4th ed. Chicago: American Academy of Periodontology; 2001. Glossary of Periodontal Terms. [Google Scholar]
- 2.Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 3.Yukna RA. Synthetic bone grafts in periodontics. Periodontol 2000. 1993;1:92–9. [PubMed] [Google Scholar]
- 4.Kenney EB, Lekovic V, Han T, Carranza FA, Jr, Dimitrijevic B. The use of a porous hydroxylapatite implant in periodontal defects. I. Clinical results after six months. J Periodontol. 1985;56:82–8. doi: 10.1902/jop.1985.56.2.82. [DOI] [PubMed] [Google Scholar]
- 5.Meffert RM, Thomas JR, Hamilton KM, Brownstein CN. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J Periodontol. 1985;56:63–73. doi: 10.1902/jop.1985.56.2.63. [DOI] [PubMed] [Google Scholar]
- 6.Graves DT, Cochran DL. Mesenchymal cell growth factors. Crit Rev Oral Biol Med. 1990;1:17–36. doi: 10.1177/10454411900010010301. [DOI] [PubMed] [Google Scholar]
- 7.Lynch SE. Introduction. In: Lynch SE, Genco RJ, Marx RE, editors. Tissue Engineering Applications in Maxillofacial Surgery and Periodontics. Chicago: Quintessence Publishing Company, Inc; 1999. pp. xi–xviii. [Google Scholar]
- 8.Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998;13(Suppl):11–45. [PubMed] [Google Scholar]
- 9.Whitman DH, Berry RL, Green DM. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 10.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 11.Kawase T, Okuda K, Saito Y, Yoshie H. In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol. 2005;76:760–7. doi: 10.1902/jop.2005.76.5.760. [DOI] [PubMed] [Google Scholar]
- 12.Hood AG, Hill AG, Reeder GD, Potter PS, Iverson L, Keating RF, et al. Peri-operative autologous sequestration III. A new physiologic glue with wound healing properties. Proc Am Cardiovasc Perfusion. 1993;14:126–9. [Google Scholar]
- 13.Gottlow J. Periodontal regeneration. In: Lang NP, Karring T, editors. Proceedings of the 1st European workshop in periodontology. London: Quintessence publishing company; 1994. pp. 172–92. [Google Scholar]
- 14.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 17.Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. A reentry study on the use of bovine porous bone mineral, GTR, and platelet-rich plasma in the regenerative treatment of intrabony defects in humans. Int J Periodontics Restorative Dent. 2005;25:49–59. [PubMed] [Google Scholar]
- 18.Eickholz P, Hörr T, Klein F, Hassfeld S, Kim TS. Radiographic parameters for prognosis of periodontal healing of infrabony defects: Two different definitions of defect depth. J Periodontol. 2004;75:399–407. doi: 10.1902/jop.2004.75.3.399. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 20.Nagata MJ, Messora MR, Furlaneto FA, Fucini SE, Bosco AF, Garcia VG, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: An experimental study in rabbits. Eur J Dent. 2010;4:395–402. [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna R, Trejo PM, Weltman RL. Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet-rich plasma: A randomized clinical trial. J Periodontol. 2004;75:1668–77. doi: 10.1902/jop.2004.75.12.1668. [DOI] [PubMed] [Google Scholar]
- 22.Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, et al. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: A comparative controlled clinical study. J Periodontol. 2005;76:890–8. doi: 10.1902/jop.2005.76.6.890. [DOI] [PubMed] [Google Scholar]
- 23.Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37:300–6. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 24.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral in the treatment of intrabony defects: A re-entry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 25.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Aleksic Z, Kenney EB. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J Clin Periodontol. 2003;30:746–51. doi: 10.1034/j.1600-051x.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang XY, Qiao J. Effect of platelet-rich plasma in the treatment of periodontal intrabony defects in humans. Chin Med J (Engl) 2006;119:1511–21. [PubMed] [Google Scholar]
- 27.Menezes LM, Rao J. Long-term clinical evaluation of platelet-rich plasma in the treatment of human periodontal intraosseous defects: A comparative clinical trial. Quintessence Int. 2012;43:571–82. [PubMed] [Google Scholar]
- 28.Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol. 2007;34:254–61. doi: 10.1111/j.1600-051X.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- 29.Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral and expanded polytetrafluoroethylene membranes. J Periodontol. 2007;78:983–90. doi: 10.1902/jop.2007.060349. [DOI] [PubMed] [Google Scholar]
- 30.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–15. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 31.McGuire MK, Kao RT, Nevins M, Lynch SE. rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int J Periodontics Restorative Dent. 2006;26:223–31. [PubMed] [Google Scholar]
- 32.Tamura K, Sato S, Kishida M, Asano S, Murai M, Ito K. The use of porous beta-tricalcium phosphate blocks with platelet-rich plasma as an onlay bone graft biomaterial. J Periodontol. 2007;78:315–21. doi: 10.1902/jop.2007.060228. [DOI] [PubMed] [Google Scholar]


