Abstract
Objective:
The aim of this study was to compare the antiplaque and antigingivitis effects of a mouthwash containing tea tree oil (TTO) with a cetylpyridinium chloride (CPC) mouthwash.
Materials and Methods:
This was a randomized 4 × 4, controlled, cross-over, involving 20 healthy volunteers in a 5-day plaque re-growth model. Test mouthwashes were TTO (Tebodont®) and a mouthwash containing CPC 0.05% (Aquafresh®). A 0.12% chlorhexidine (CHX) mouthwash (Oro-Clense®) was used as positive and colored water (placebo [PLB]) as negative controls. Gingival bleeding index (GBI) and plaque index (PI) scores were recorded before and after each test period. Test periods were separated with 2 weeks washout period.
Results:
All four mouthwashes significantly (P < 0.001) reduced the GBI scores when compared to the baseline GBI scores. There was no significant difference between PLB and active mouthwashes in the GBI scores. CHX and CPC mouthwashes were found more effective in reducing the PI scores than TTO and PLB mouthwashes. There was no significant difference in PI scores of CHX and CPC mouthwashes.
Conclusion:
0.05% CPC mouthwash can be an alternative to CHX mouthwash since it is alcohol free and found as efficient as CHX in dental plaque reduction with lesser side effects. More studies are needed to test antigingivitis effects of the mouthwashes used in this study, preferably without initial scaling and polishing.
Keywords: Antigingivitis, antiplaque, cetylepyridinium chloride, mouthwash, tea tree oil
Introduction
It is well established that the microbial plaque is an important etiological factor in the development of gingivitis.[1,2] Microbial plaque accumulation can be controlled by daily usage of mechanical means such as toothbrush, dental floss, inter-dental brushes, wooden picks, etc., Effectiveness of plaque removal may be improved by additional use of chemical plaque control agents.[3] The use of antimicrobial mouthwashes as an adjunct to mechanical oral hygiene has become well established in dental practice for the control of supra-gingival plaque and gingivitis.[4] A broad variety of antiseptic mouthwashes is offered for patient selection. Chlorhexidine (CHX), a bisbiguanide, considered to date the most effective antiplaque agent, is not easily formulated into toothpastes, and local side effects, especially reversible brown staining of teeth, tongue and resin restorations, and transient impairment of taste perception have limited the long term use of CHX as a mouthwash.[5,6,7,8,9,10] The problem associated with side effects stimulated the search for alternative antiplaque, antigingivitis agents.
Tea tree oil (TTO) derived from the paperbark tea tree is being used in medicinal and dental products because of its antibacterial and antiinflammatory activities. These activities were shown to be related to the active ingredients such as 1,8-cineole and terpinen-4 ol.[11,12] There are very few studies regarding the effects of TTO on periodontal tissues.
In a double-blind, longitudinal, noncrossover study, the effects of topically applied TTO-containing gel on dental plaque and chronic gingivitis were compared with CHX gel. Since TTO significantly reduced the gingival inflammation, however without reducing the plaque scores, its mechanism of activity could have been antiinflammatory rather than antibacterial.[13]
Cetylpyridinium chloride (CPC) is a cationic antiseptic and commonly found as an active ingredient in many mouthwashes. Its inhibitory effect on plaque formation was shown in several clinical studies.[14,15]
There has been a continuous search for a better mouthwash in terms of efficacy in reduction of plaque and gingivitis, patient's tolerance and having minimal side effects.
The objective of this study was to assess and compare the antiplaque and antigingivitis effects of commercial mouthwash containing TTO (Melaleuca alternifolia 1.5%) to a mouthwash containing a quaternary ammonium compound (CPC 0.05%). CHX was used as a positive control mouthwash and colored water (placebo [PLB]) was used as a negative control mouthwash.
Materials and Methods
The study was designed as a randomized, double-blind (assessors and volunteers), 4 × 4 controlled crossover study. It involved 20 healthy volunteers, 16 females and four males (mean age 22.55 ± 1.79), in a 5-day plaque re-growth model. Test mouthwashes were: (1) a mouthwash containing TTO (Tebodont, DrWild and Co AG, Switzerland®) and (2) a mouthwash containing CPC 0.05% and sodium fluoride 0.05% (Aquafresh, GlaxoSmithKline Consumer Healthcare, UK®). A 0.12% CHX mouthwash (Oro-Clense, Germiphene Corporation, Canada®) was used as positive and colored water (PLB) as negative controls. During the fall semester of 2011, volunteers were recruited from the dental students at the University of Sharjah who responded positively to classroom announcement about the study and from the patients seeking dental treatment in the dental teaching clinics at the College of Dentistry, University of Sharjah, after obtaining informed consent from them. Selection criteria had a dentition with minimum 20 teeth, no periodontitis, no orthodontic appliances, no known allergy to any of the components of mouthwashes and no antibiotic use for the last 3 months, no known systemic diseases, not pregnant or lactating. The study was conducted in accordance with the ethical principals in the declaration of Helsinki and consistent with good clinical practice. Ethical approval was also granted by Ethics and Research Committee at the University of Sharjah.
At the beginning of the study, all mouthwashes were placed in the same kind of containers and labeled with numbers from 1 to 4. No. 1 was used for CPC, 2 for TTO, 3 for CHX and 4 for colored water. Each volunteer picked four numbers from a box which determined his/her sequence of use of mouthwashes to randomize the order of mouthwash use.
All clinical examinations were conducted by two calibrated dentists, who were blinded of the types of mouthwash used. A brief examination of the oral mucosa, gingiva, hard and soft palate and teeth of the patients at baseline and after each mouthwash use was done. Plaque index (PI) and gingival bleeding index (GBI) scores, any changes from baseline in soft tissues and presence of extrinsic tooth stains were recorded, with stain recorded dichotomously as present or absent.
On day 1 of each test period, all participants received an oral soft and hard tissue examination and professional prophylaxis in order to get the PI to 0. The presence or absence of gingival bleeding (GBI) was determined at baseline and after each mouthwash use by gentle probing of the gingival crevice with a periodontal probe.[16] The appearance of bleeding within 10 s indicated a positive score that was expressed as a percentage of the total number of gingival margins examined. For each tooth one buccal and one lingual/palatal gingival margins were examined.[16]
Plaque index was scored according to Turesky's modified Quigley-Hein PI at baseline after using erythrosin disclosing solution.[17] Then volunteers suspended their oral hygiene for 5-day and used the mouthwash allocated randomly to them, twice daily according to the manufacturer's dosage instructions. Use of mouthwashes was at home and unsupervised. On day 6, participants were examined again for disclosed plaque and GBI. An oral examination was done to assess the side effects. Each test period was separated by a 2 weeks washout period during which subjects resumed their normal oral hygiene habits. Figure 1 summarizes the outline of the clinical trial.
Figure 1.
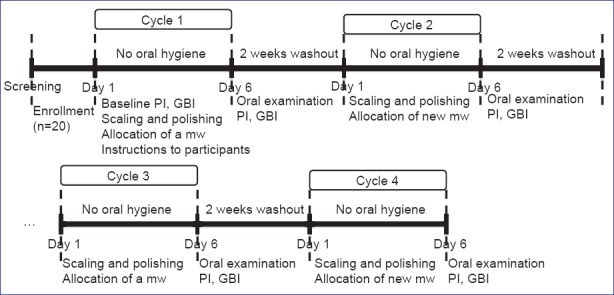
Outline of the clinical trial
Statistical analysis
The data retrieved from the clinical examination were entered and statistically analyzed using a computer program Predictive Analysis Software version 18.0 (IBM, Chicago, United States). Analysis of variance (ANOVA) with repeated measures was used to test if any differences between mouthwashes existed. Ninety-five percent confidence intervals were used to determine differences between mouthwashes. The student paired t-test was conducted to test if there were any significant differences between the baseline measures of PI, GBI and following the use of control and test mouthwashes, after the distribution of the data proved to be a normal distribution. The level of significance was determined to be at P = 0.05.
Results
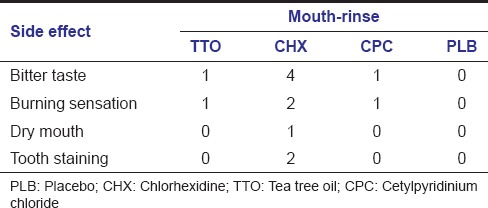
All 20 subjects completed the trial. Although some of them complained of disturbances due to the use of mouthwashes [Table 1], the compliance of the subjects was not affected. Tooth staining was observed in 2 of the subjects after the use of CHX mouthwash.
Table 1.
Number of subjects with side effects
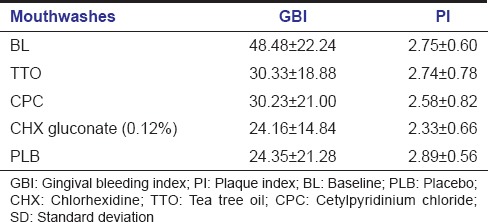
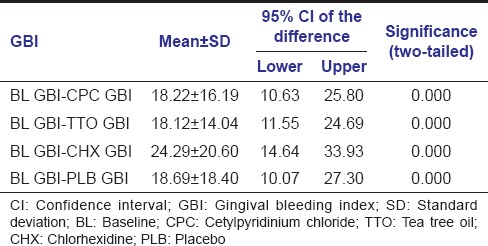
Mean and standard deviations of PI and GBI scores of subjects at baseline and after the use of TTO, CPC, CHX and PLB are shown in Table 2. Lowest PI and GBI scores were observed after the use of CHX mouthwash.
Table 2.
Mean and SD of GBI and PI scores at BL and after the use of different mouthwashes
Gingival bleeding index results for different mouthwashes inbox whisker plots were shown in Figure 2. There were no statistically significant differences among the mouthwashes when GBI scores after the 5-day use of CHX, CPC, TTO and PLB mouthwashes were compared. However GBI scores, after the use of all four mouthwashes when compared to baseline GBI scores were highly significant (P < 0.001) [Table 3].
Figure 2.
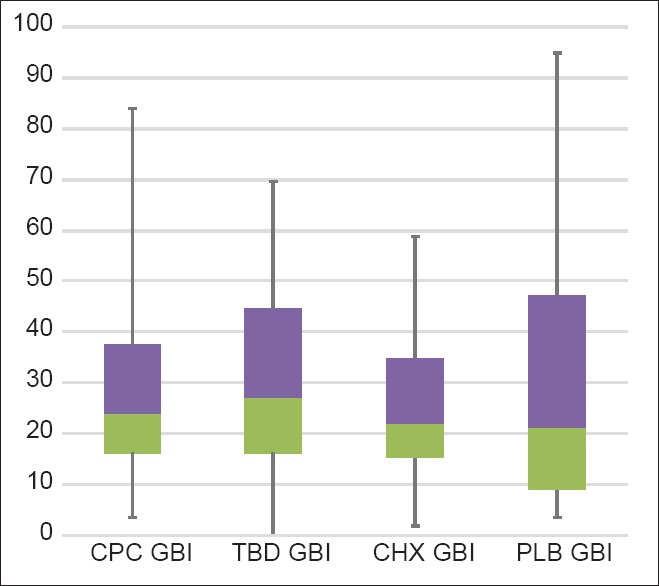
Gingival bleeding index results for different mouth-rinses in box whisker plots
Table 3.
Mean reduction in GBI from BL GBI after the use of different mouthwashes
Plaque index results from ANOVA for the different mouthwashes inbox whisker plots are shown in Figure 3. There was a statistically significant difference in PI scores for the CPC and CHX when compared to PLB. TTO mouthwash had a reduction effect, but it was not statistically significant. Largest reduction effect compared with PLB was with CHX mouthwash [Table 4].
Figure 3.
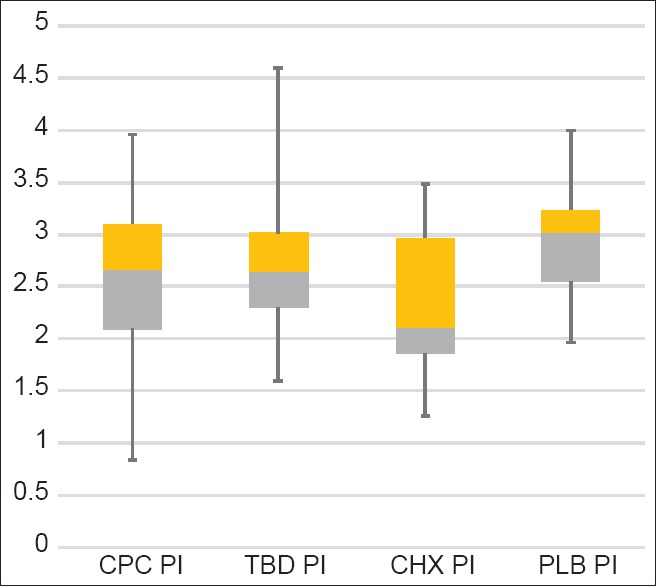
Plaque index results from analysis of variance for the different mouthwashes in box whisker plots
Table 4.
Comparison of CPC, TTO and CHX mouthwashes to the PLB in PI reduction
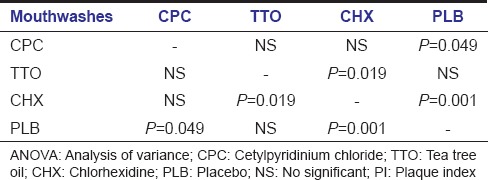
When all the mouthwashes were compared to CHX, we found that effect of CHX in reducing the PI was better than the rest. However it was statistically significant only when compared to PLB and TTO mouthwashes. Therefore, the results indicate that CHX is more effective than PLB and TTO, yet there were no significant differences between PI scores of CHX and CPC mouthwashes.
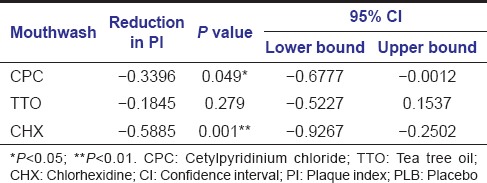
When comparing all mouthwashes in reference to CPC, we found that CPC was only significantly better in PI reduction when compared to PLB (P < 0.05) [Table 5].
Table 5.
Differences between the active mouthwashes and PLB in PI reduction (ANOVA)
Discussion
Mechanical plaque control by tooth brushing methods and inter-dental aids may not be enough to completely remove dental plaque from tooth surfaces. Antimicrobial mouthwashes may help to improve plaque control and gingival health by reducing dental plaque from difficult to reach areas.
Chlorhexidine has been considered as the gold standard among mouthwashes due to its long-term effectiveness in reducing dental plaque.[7] However, it has well known drawbacks of causing teeth staining, alcohol content and altering taste perceptions. These drawbacks limits its use to short term basis.[8,9]
In the search of alternative alcohol free mouthwashes to CHX, in our study we compared antiplaque and antigingivitis effects of TTO mouthwash (M. alternifolia 1.5%) with CPC (0.05%) mouthwash. A 0.12% CHX mouthwash (containing 10% alcohol) and PLB were used as positive and negative controls respectively.
The results of this study have shown that all the four mouthwashes highly and significantly reduced (P < 0.001) gingival bleeding scores when compared to baseline scores. There were no significant differences in GBI scores among the active mouthwashes and between the PLB and active mouthwashes. We anticipate that since all the participants received scaling and polishing at baseline, eliminating calculus and plaque at the beginning of the study might have affected this finding. Reduced gingival bleeding scores might be because of the combined effect of mouthwashes and mechanical cleaning. In a noncrossover study which did not include baseline scaling, Soukoulis and Hirsch tested the effect of TTO gel on gingivitis and plaque.[13] They reported significant reductions in gingival inflammation with TTO gel when compared to PLB and positive control gels (CHX). However, TTO gel did not reduce the plaque scores. Hence they concluded that TTO has no plaque inhibitory effect, but antiinflammatory effect on gingivitis and can be a useful adjunct to chemotherapeutic periodontal therapy. Moreover, in a crossover 4-day plaque re-growth study comparing TTO mouthwash with CHX, Arweiler et al. stated that TTO had no positive effect on the quantity or quality of supra-gingival plaque.[18] However, in our study TTO mouthwash slightly reduced the plaque when compared to PLB but this effect was not statistically significant. Since the antibacterial effects of TTO were shown in vitro, it is reasonable to think that the inferior antiplaque activity of TTO might be due to poor substantivity or loss of its antibacterial properties once bounded to oral tissues.[11,12] More studies are needed to study this matter. Concerning the antigingivitis effect of TTO, our study found it neither superior than other test mouthwashes nor the PLB. Thus, we cannot say that we have shown clear antigingivitis effect of TTO in this study. Our results are indecisive about the antigingivitis effect of TTO.
When the effects on gingivitis were compared, we did not find any difference between CPC mouthwash and the positive control (CHX). Similarly in two noncrossover, parallel groups, positively controlled studies comparing antiplaque and antigingivitis effects of CPC (0.07%) mouthwash with a positive control essential oil rinse (Listerine [Johnson and Johnson Health Care Products by McNeil, Pennsylvania, USA] -a recognized antiplaque and antigingivitis mouthwash containing alcohol), no significant differences were found between treatment and control groups when used as an adjunct to tooth brushing.[19,20] In our study CPC and CHX mouthwash also showed no significant difference in their plaque reduction properties, however they were superior to the PLB mouthwash. Similar to our results, Rawlinson et al. in a crossover study showed less plaque accumulation when comparing PLB with the use of two different concentrations of CPC mouthwashes (0.1% and 0.05%). They did not find any difference in plaque reduction between the two concentrations of CPC.[14] In another study, Stookey et al. compared 0.075% and 0.1% CPC mouthwashes to a control rinse for a 6 month period on the development of gingivitis and plaque inhibition. No statistically significant differences were found between the two different concentrations of CPC, but they were superior to the control mouthwash.[21]
The essential oil of M. alternifolia (TTO) may have some side effects such as being toxic if ingested in higher doses and may cause skin irritation and allergic reaction.[22] When used as a mouthwash solution Groppo et al. in a study (involved 30 subjects) comparing activity of TTO, garlic and CHX mouthwash against oral microorganisms reported unpleasant taste 30% with TTO and 40% with CHX, burning sensation 60% with TTO and 40% with CHX.[23] On the other hand, CPC mouthwash has been reported to have lesser side effects then CHX mouthwash.[24] Blenman et al. found less staining with CPC mouthwash compared to CHX mouthwash. They suggested that patients can use longer rinsing time with CPC mouth-rinse assuming that there will be no burning sensation and bitter taste since it doesn’t contain alcohol.[24]
Regarding the side effects of mouthwashes in our study, four subjects reported bitter taste with CHX, one with TTO and one with CPC mouthwash. Two subjects complained from burning sensation with CHX, one subject with CPC and one subject with TTO mouthwashes. Tooth staining was observed only in two subjects after the use of CHX.
In spite of the limitations of our study, we can say that 0.05% alcohol free CPC mouthwash can be considered as an alternative to CHX mouthwash given that it was found as efficient as CHX in dental plaque reduction with much lesser side effects. However, more clinical studies with longer durations are needed to be able to comment on the antiplaque and antigingivitis effects of TTO mouthwash.
Acknowledgments
This study was supported by University of Sharjah, grant no. (101006).
Footnotes
Source of Support: This study was supported by University of Sharjah, grant no. (101006).
Conflict of Interest: None declared.
References
- 1.Listgarten MA. The role of dental plaque in gingivitis and periodontitis. J Clin Periodontol. 1988;15:485–7. doi: 10.1111/j.1600-051x.1988.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 2.Tatakis DN, Trombelli L. Modulation of clinical expression of plaque-induced gingivitis. I. Background review and rationale. J Clin Periodontol. 2004;31:229–38. doi: 10.1111/j.1600-051x.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 3.Paraskevas S. Randomized controlled clinical trials on agents used for chemical plaque control. Int J Dent Hyg. 2005;3:162–78. doi: 10.1111/j.1601-5037.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 4.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–98. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 5.Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9(Suppl 1):23–9. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 6.Keijser JA, Verkade H, Timmerman MF, Van der Weijden FA. Comparison of 2 commercially available chlorhexidine mouthrinses. J Periodontol. 2003;74:214–8. doi: 10.1902/jop.2003.74.2.214. [DOI] [PubMed] [Google Scholar]
- 7.Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol. 2012;39:1042–55. doi: 10.1111/j.1600-051X.2012.01883.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanz M, Vallcorba N, Fabregues S, Müller I, Herkströter F. The effect of a dentifrice containing chlorhexidine and zinc on plaque, gingivitis, calculus and tooth staining. J Clin Periodontol. 1994;21:431–7. doi: 10.1111/j.1600-051x.1994.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosin M, Welk A, Kocher T, Majic-Todt A, Kramer A, Pitten FA. The effect of a polyhexamethylene biguanide mouthrinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque regrowth. J Clin Periodontol. 2002;29:392–9. doi: 10.1034/j.1600-051x.2002.290503.x. [DOI] [PubMed] [Google Scholar]
- 11.Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78:264–9. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 12.May J, Chan CH, King A, Williams L, French GL. Time-kill studies of tea tree oils on clinical isolates. J Antimicrob Chemother. 2000;45:639–43. doi: 10.1093/jac/45.5.639. [DOI] [PubMed] [Google Scholar]
- 13.Soukoulis S, Hirsch R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Aust Dent J. 2004;49:78–83. doi: 10.1111/j.1834-7819.2004.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 14.Mankodi S, Bauroth K, Witt JJ, Bsoul S, He T, Gibb R, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent. 2005;18 Spec No:9A–14. [PubMed] [Google Scholar]
- 15.Rawlinson A, Pollington S, Walsh TF, Lamb DJ, Marlow I, Haywood J, et al. Efficacy of two alcohol-free cetylpyridinium chloride mouthwashes: A randomized double-blind crossover study. J Clin Periodontol. 2008;35:230–5. doi: 10.1111/j.1600-051X.2007.01187.x. [DOI] [PubMed] [Google Scholar]
- 16.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 17.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 18.Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A. Clinical and antibacterial effect of tea tree oil: A pilot study. Clin Oral Investig. 2000;4:70–3. doi: 10.1007/s007840050118. [DOI] [PubMed] [Google Scholar]
- 19.Witt JJ, Walters P, Bsoul S, Gibb R, Dunavent J, Putt M. Comparative clinical trial of two antigingivitis mouthrinses. Am J Dent. 2005;18 Spec No:15A–7. [PubMed] [Google Scholar]
- 20.Albert-Kiszely A, Pjetursson BE, Salvi GE, Witt J, Hamilton A, Persson GR, et al. Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis: A six-month randomized controlled clinical trial. J Clin Periodontol. 2007;34:658–67. doi: 10.1111/j.1600-051X.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 21.Stookey GK, Beiswanger B, Mau M, Isaacs RL, Witt JJ, Gibb R. A 6-month clinical study assessing the safety and efficacy of two cetylpyridinium chloride mouthrinses. Am J Dent. 2005;18 Spec No:24A–8. [PubMed] [Google Scholar]
- 22.Hammer KA, Carson CF, Riley TV, Nielsen JB. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem Toxicol. 2006;44:616–25. doi: 10.1016/j.fct.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Groppo FC, Ramacciato JC, Simões RP, Flório FM, Sartoratto A. Antimicrobial activity of garlic, tea tree oil, and chlorhexidine against oral microorganisms. Int Dent J. 2002;52:433–7. doi: 10.1111/j.1875-595x.2002.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 24.Blenman TV, Morrison KL, Tsau GJ, Medina AL, Gerlach RW. Practice implications with an alcohol-free, 0.07% cetylpyridinium chloride mouthrinse. Am J Dent. 2005;18 Spec No:29A–34. [PubMed] [Google Scholar]


