Abstract
Introduction:
Rapid maxillary expansion (RME) is an orthopedic treatment procedure routinely used to treat constricted maxillary arches and also a potential additional treatment in children presenting with sleep-disordered breathing (SDB).
Aims and Objectives:
The main objective of this study was to evaluate the effects of RME on sleep characteristics in children.
Materials and Methods:
Polysomnography was done on children of 8-13 years of age before expansion (T0), after expansion (T1) and after a period of 3 months after retention (T2). Bonded rapid maxillary expander was cemented in all children. Inter-molar distance was also measured at T0 and T2.
Statistical Analysis:
Nonparametric Friedman test was used for comparing the averages of sleep parameters at different time period (T0, T1, T2). Wilcoxon signed ranks test was used for comparing the averages of inter-molar width (T0-T2). P < 0.05 were considered as significant.
Results:
All children showed an improvement in sleep parameters with an increase in sleep efficiency, decreased in arousal and desaturation index after expansion. Total sleep time showed a statistically significant increase after expansion. A statistically significant increase in inter-molar distance was obtained after expansion.
Conclusions:
Rapid maxillary expansion is a useful treatment option for improving quality of sleep even in normal children without SDB. It also induces widening of the maxilla, corrects posterior crossbites and improves maxillary and mandibular dental arch coordination.
Keywords: Polysomnography, rapid maxillary expansion, sleep-disordered breathing
Introduction
Rapid maxillary expansion (RME) occupies a unique niche in dentofacial therapy which increases the transverse dimensions of the maxilla by separating the two maxillary halves from the midpalatal suture in a short period in turn treats posterior cross bites, crowding, and nasal stenosis.[1] It is also considered as a potential treatment in children presenting with obstructive sleep apnea syndrome (OSAS). Children with OSAS may have narrow and long face, with large tonsils, a narrow upper airway, maxillary constriction, and some degree of mandibular retrusion. Increased nasal resistance may lead to unfavourable facial growth and to the development of dental malocclusion.[2] When these conditions appear in early life, they can cause deformation of maxilla affecting its cross-sectional development. Obstructive sleep apnea (OSA) is a life-threatening condition in which patient suffers periodic cessation of breathing during sleep, which impairs the quality of life.
Normal sleep architecture[3] is characterized by two forms. These forms are referred to as nonrapid eye movement and rapid eye movement. These sleep states alternate throughout the sleep cycle. Most adults in the absence of disease or other abnormal environmental conditions, sleep between 7.5 and 8.5 h/sleep cycle. Arousal from sleep increases with age. These arousals can be the result of many factors, including respiratory disturbances. Sleep is associated with specific neurological events that can be quantified in the sleep laboratory. Standard laboratory tests for OSA are nocturnal polysomnograms (PSG). PSG[4] is a complex procedure that should be performed by a trained technologist.
The severity of the accompanying oxygen desaturation and sleep fragmentation during PSG are combined with the clinical symptoms to assess the immediate consequences to the individual from the sleep-disordered breathing (SDB). It is generally believed that adeno-tonsillectomy (AT) is the most performed as the first line treatment in childhood -SDB. Recent studies have documented the persistence of SDB indices on PSG in 47% children after AT. Studies have shown that RME, while producing orthopedic and orthodontic corrections, may also promote an increase in width of the nasal cavity, improving mouth breathing, and reducing airway resistance.[5] Improvement of nasal breathing after RME occurs due to the anterior and inferior dislocation of the maxilla after maxillary expansion.[6] With RME there is a significant decrease in the apnea-hypopnea index (AHI), and arousal index, even in patients with mild or severe tonsillar hypertrophy. Orthodontic treatments[1] such as RME has been shown to modify craniofacial anomalies in children with dental malocclusion and also to normalize sleep architecture and improve sleep respiratory disturbances.
This study aims to evaluate the effects of RME on sleep characteristics in children by
Dental evaluation of inter-molar distance before and after expansion with RME
Comparing the following sleep parameters before expansion, after expansion and after retention period of 3 months.
Following were the parameters evaluated
Sleep efficiency
Arousal index
Apnea hypopnea index
Desaturation index
Periodic leg movements
Total sleep time.
Materials and Methods
Fifteen children (9 boys and 6 girls) between the age group of 8-13 years were included in the study. All the participants were selected from the outpatient Department of Orthodontics, Amrita School of Dentistry, Kochi from 2011 to 2013. All the patients and parents were informed about the purpose of study. Ethical Committee approval was obtained before expansion. Children with any one or more of the following criteria were selected; Narrow maxillary arch, high narrow palate, unilateral or bilateral posterior cross bite, class III malocclusion with anterior cross bite, s igns and symptoms of apnea and restless sleep as witnessed by parents. Children with obesity, cardio-respiratory or neuromuscular diseases, craniofacial abnormalities or associated chromosomal syndromes, presence of open bite, mentally retarded children were excluded from this study.
A detailed personal and family history was obtained for all participants and the general clinical examination was performed. This included detailed case history, occlusal radiographs, lateral cephalograms, photographs and study models. Before the RME was cemented, all the children underwent an oto-rhinolaryngologic examination and an orthodontic assessment to detect possible skeletal or dental malocclusion, possible jaw deviation from normal occlusion. The selected children had undergone overnight PSG (T0) before RME cementation. The RME device (Leone Hyrax Screw 11 mm) was then cemented in all children (bonded RME) [Figure 1]. The screw was turned 2 turns a day until palatal cusp of the upper molar came into contact with the buccal cusp of lower molar [Figure 2]. All these patients were advised about oral hygiene measures to be followed. Both the parents and patients were instructed about the activation method. Patients were recalled after 2 weeks for clinical evaluation. After initial treatment, when maxillary arch was sufficiently over-expanded, the screw was fixed to prevent further activation for next 3 months as retention phase. All children had undergone PSG immediately after expansion of the arch (T1) and after a period of 3 months when the appliance was removed (T2). Inter-molar distance (mesial pit of permanent first molar to the mesial pit of opposite first molar) was measured in all patients before expansion and 3 months after expansion. At follow-up patient were re-evaluated by orthodontist, an otolaryngologist and sleep physician.
Figure 1.
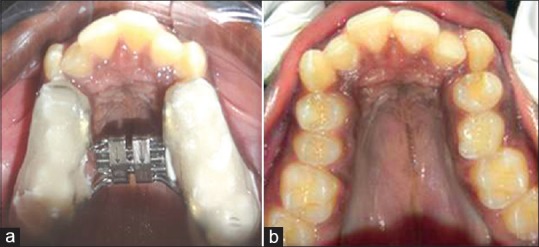
(a and b) Before expansion
Figure 2.
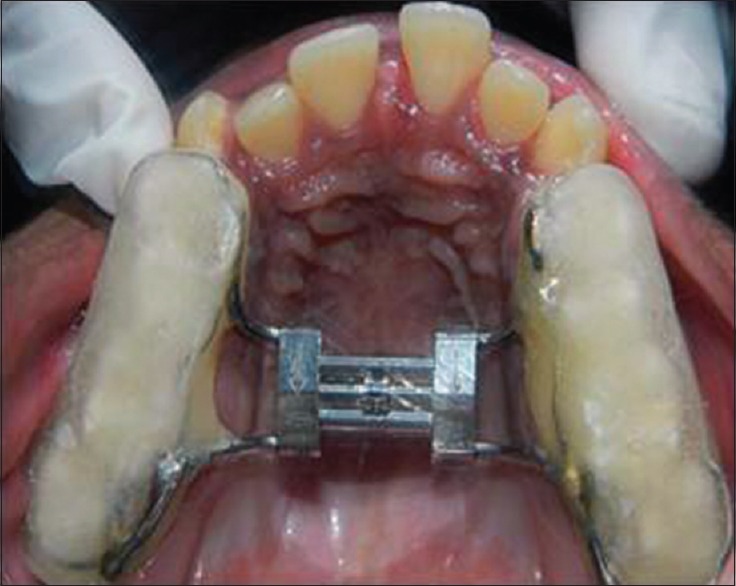
After expansion
Standard overnight PSG recordings were obtained with a Grass Heritage polygraph. Oronasal airflow was recorded with a thermocouple. Arterial oxygen saturation was monitored with a pulse oximeter. Stages were scored according to the standard criteria by Rechtschaffen and Kales.[1] Wires for each channel of recorded data lead from the patient and converge into a central box, which in turn was connected to a computer system for recording, storing and displaying the data [Figure 3]. During sleep, the computer monitor can display multiple channels continuously.
Figure 3.
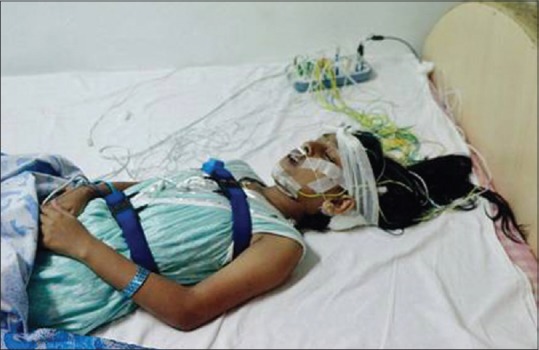
Patient undergoing polysomnography
Results
Statistical analysis was done using IBM SPSS statistics 20. Continuous variables were presented as mean ± standard deviation. Nonparametric Friedman test was used for comparing the averages of sleep parameters at different time period (T0, T1, T2). Wilcoxon signed ranks test was used for comparing the averages of inter-molar width (T0-T2). P <0.05 were considered as significant.
Fifteen patients with the mean age of 10.83 years (8-13 years) were included in this study of RME and PSG evaluation. These patients had an average inter-molar distance of 44.03 mm before expansion. After expansion, there was a significant increase in inter-molar distance to an average value of 52.16 mm [Figure 4 and Table 1].
Figure 4.
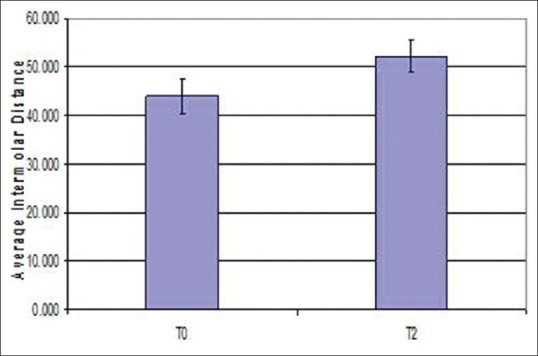
Error bar diagram of inter-molar distance
Table 1.
Mean value of inter-molar distance

Polysomnographic data before (T0), after expansion with RME (T1) and after retention phase (T2) are shown in Table 2. There was an increase in sleep efficiency on average after expansion when compared between different time periods T0-T1-T2. Sleep efficiency improved in all patients except for 1 patient who showed a decrease in sleep efficiency after expansion [Figure 5]. Though there was a gradual increase in sleep efficiency after expansion, average sleep efficiency showed a P = 0.470 (>0.05) so the results obtained were not statistically significant. Desaturation index is the number of times per hour of sleep that the blood's oxygen level drops by 3% or more from baseline. Desaturation index reduced after expansion when compared between different time periods [Figure 6]. Since the P value for desaturation index is 0.946 (>0.05), results obtained were not statistically significant.
Table 2.
Mean values of polysomnographic parameters
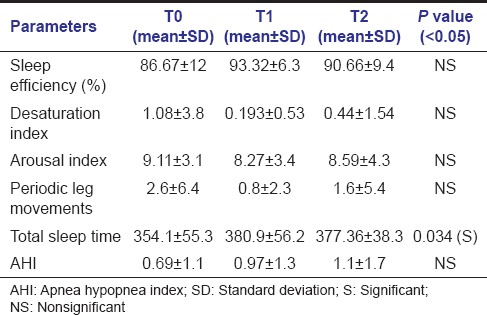
Figure 5.
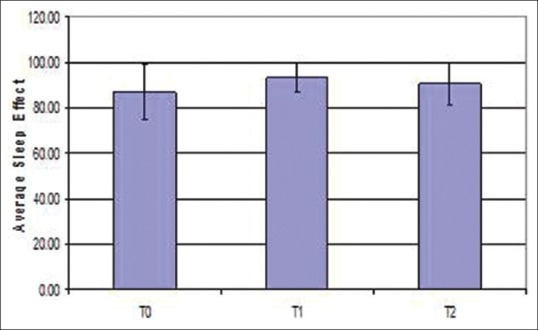
Error bar diagram of sleep efficiency
Figure 6.
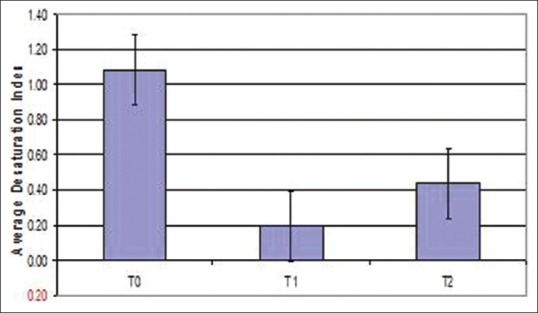
Error bar diagram of desaturation index
Arousal index reduced after expansion when compared between different time periods, but was not statistically significant (P = 0.886) [Figure 7]. PSG indicated that periodic leg movements were reduced on an average after expansion [Figure 8]. Of the 15 patients; only 1 patient showed an increased periodic leg movement after expansion. All the patients had AHI below 5; which is considered normal. Total sleep time showed a statistically significant increase after expansion when compared between different time periods (T0-T1-T2) (P = 0.034) [Figure 9]. There was an improvement in sleep parameters evaluated after expansion since there is increase in sleep efficiency and total sleep time. Arousal index and desaturation index also reduced following expansion. Average inter-molar distance also showed a statistically significant increase from T0 to T2. Results obtained were almost stable at T2.
Figure 7.
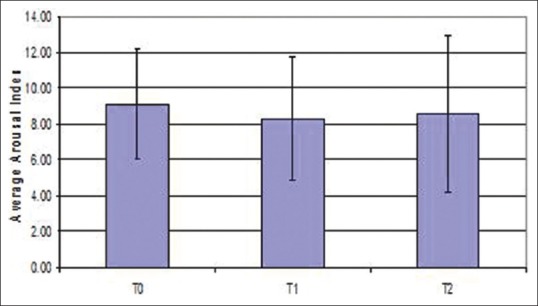
Error bar diagram of arousal index
Figure 8.
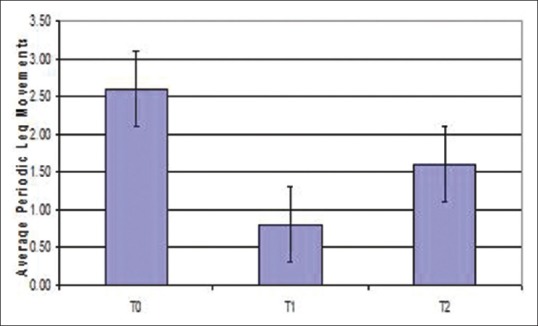
Error bar diagram of periodic leg movements
Figure 9.
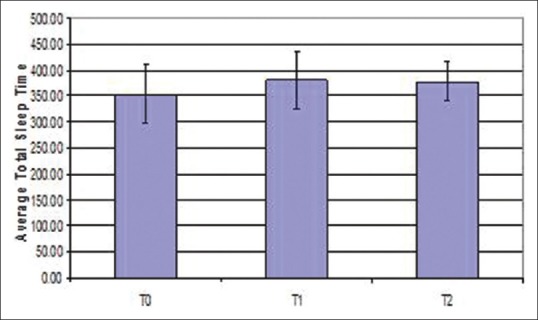
Error bar diagram of total sleep time
Discussion
There is a growing recognition that craniofacial alterations can lead to obstructive sleep apnea.[7] Among the commonly identified abnormalities, maxillary constriction plays a role in pathophysiology of OSA. Maxillary constriction is one of the features that characterize the so-called skeletal developmental syndrome, represented by bilateral dental maxillary crossbite, high palatal vault, nasal obstruction derived from elevation of the nasal floor and mouth breathing. constriction[8] is also associated with lowered tongue posture, which could result in retroglossal airway narrowing. An increased nasal resistance has been described in subjects with maxillary constriction. Katz et al.[4] found an association between OSAS and maxillofacial malformation, and malocclusion. The recognition of the role of craniofacial abnormalities in the development of OSA has led to a number of treatment strategies aimed at correcting or improving craniofacial structure. RME is one such well-known procedure that has been used for many years in children, with orthodontic problems[9] with maxillary constriction.
Starnbach et al.[10] in their study showed that RME brings about skeletal changes by maxillary sutural expansion and widening of the nasal cavity. Maxillary expansion[11] has been shown to mainly induce widening of the anterior part of oro-pharyngeal and nasal spaces; hence the function of the nasopharyngeal space would also be positively influenced. In a study by Smith et al.[12] showed RME resulted in a significant increase in nasal cavity volume, nasopharynx volume, anterior and posterior facial heights, palatal and mandibular planes and also enlarges pharyngeal airway and oro-pharyngeal space. According to Buccheri et al.,[13] RME increases nasopharyngeal space, thus improving the position of the tongue and increasing nasopharynx space. This suggests that orthodontic treatment like RME[14] improves or prevents SDB by enlarging nasal passages or airway. This study was undertaken to identify the effects of RME on sleep characteristics in children with craniofacial alterations of maxilla.
Sleep-disordered breathing has an important impact on the quality of life. Gonçalves et al.[15] assessed the quality of life of children with sleep-disordered breathing before and after RME and concluded that quality of life of these children improved significantly after expansion, regardless of the degree of airway obstruction. Untreated SDB has been shown to have neurocognitive complications, as well as disturbances of growth.[16] So in this study expansion was done on children with maxillary constriction or craniofacial alterations but without SDB. If these patients were left untreated there would be chances of them developing alterations in normal sleep architecture which may lead to OSA or SDB later on in life. According to O’Brien et al.[17] children with so-called primary snoring, but with normal PSG suffer at times from the same significant consequences as children with PSG-diagnosed SDB.
A child can withstand up to 1 mm of expansion daily.[3] The total expansion effect consists of a downward and forward movement of the maxillary complex with a resulting increase in the nasal canal with an improvement in nasal airflow. In this study also, 0.5 mm expansion twice daily was done until palatal cusp of the upper molar coincides with the buccal cusps of lower molars. Villa et al.[18] in their study on RME, did 0.5 mm expansion twice a day for the first 10 days until the palatal cusp of the upper molar came into contact with the buccal cusp of the lower molar. Side effects of RME include downward displacement of the maxilla, dental extrusion, dental tipping, lateral rotations of maxillary segments, opening of bite.[19] Bonded expanders are used to counteract the negative effects of expansion. Pangrazio-Kulbersh et al.[20] evaluated skeletal and dental effects of banded versus bonded expanders using cone beam computed tomography and concluded that banded expanders produce more dental tipping and alveolar bending at the level of first molars. There is a better vertical dimensional control with bonded expanders when compared to banded expanders. To get a better vertical dimensional control, bonded expanders were used in this study.
According to Pirelli et al.[21] RME has the potential to play an important role as preventive treatment in children with OSA, particularly during the prepubertal growth period. In this study, all patients included were in their prepubertal stage that is, between 8 and 13 years. It has also been shown that early application of RME could be of absolute importance because it precedes the sutural age of the young patients in whom the effect is two-thirds skeletal and one-third dental, contrary to adolescents in whom the dental component would prevail, making a greater obstacle in the repair of bony suture dislocation and a greater likelihood of periodontal problems.
Pirelli et al.[1] also evaluated the effect of RME on children with nasal breathing and OSAS and found that -AHI and arousal index reduced after expansion. In the present study, RME was capable of reducing the frequency of arousals associated with sleep at different periods of time from T0-T1-T2. Arousal index reduced from 9.1 to 8.7 events/h. Total sleep time also showed a statistically significant increase from T0-T1-T2. Villa et al.[22] in their study on 14 children treated with RME also found a statistically significant increase in total sleep time, longer duration of time in bed after expansion. An increase in sleep efficiency from 86.67% at T0 to 93.32% at T1 was found in this study. The results of the sleep architecture revealed some differences between T0 and T1. The increase in sleep efficiency, and total sleep time after expansion may be a marker of sleep quality alterations. This study shows that there is an improvement in sleep parameters evaluated after expansion on normal children. Though there was an increase in sleep efficiency, differences between the groups were not statistically significant. This might be because of smaller sample size of 15 subjects included in this study.
This study has a number of limitations, and therefore it would be premature to make definitive conclusions about the benefit of RME on sleep characteristics in normal children, even though all patients showed an improvement in sleep parameters. Moreover, all patients included in this sample had normal AHI. So decrease or increase in this AHI value could not be taken into consideration. However, other than small sample size, another common limitation of these studies is the lack of the control group due to ethical reasons. It could be argued that it is ethically difficult to refrain from treating children with dental malocclusion. Villa et al.[23] in their study showed that by repositioning the jaws, there is an improvement in daytime symptoms and PSG indexes in children with OSAS. Since craniofacial morphology[24] like maxillary constriction and maxillary retrognathia has genetic influence on the development of obstructive sleep apnea, it is essential to treat them early in life with RME.
Conclusions
Rapid maxillary expansion is an orthopedic treatment procedure routinely used to treat constricted maxillary arches and also a potential additional treatment in children presenting with SDB. OSA may evolve during childhood before becoming clinically evident later in life. The importance of these observations lies not only in the potential to treat the underlying craniofacial abnormalities, but more importantly raises the possibility that early detection and treatment of children at high risk of developing OSA may prevent the disorder. Since maxillary constriction is a feature of chronic naso-respiratory obstruction,[25] RME has the potential to play an important role in such a preventative strategy. The quality of sleep of these children improves after RME, regardless of the severity of their respiratory obstruction. The early detection and treatment of children at risk of developing OSA may prevent the sequelae of the disease. With RME, there is an increase in sleep efficiency and total sleep time in children without SDB.
Rapid maxillary expansion treatment also induces widening of the maxilla and corrects posterior crossbites, improving maxillary and mandibular dental arch coordination of class II and III malocclusions. Orthodontists who perform a comprehensive head-neck examination should be in a unique position to identify children with malocclusion that can lead to development of SDB and refer them to sleep specialist for further evaluation. RME is a useful treatment option for improving quality of sleep even in normal children without SDB but who are at higher risk of developing SDB due to their craniofacial morphology.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004;27:761–6. doi: 10.1093/sleep/27.4.761. [DOI] [PubMed] [Google Scholar]
- 2.Melsen B. Histological analysis of the postnatal development of the nasal septum. Angle Orthod. 1977;47:83–96. doi: 10.1043/0003-3219(1977)047<0083:HAOTPD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Joseph R, Deatherage R, Roden D, Zouhary K. Normal sleep architecture. Semin Orthod. 2009;15:86–7. [Google Scholar]
- 4.Katz ES, D’Ambrosio CM. Pediatric obstructive sleep apnea syndrome. Clin Chest Med. 2010;31:221–34. doi: 10.1016/j.ccm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Garib DG, Henriques JF, Carvalho PE, Gomes SC. Longitudinal effects of rapid maxillary expansion. Angle Orthod. 2007;77:442–8. doi: 10.2319/0003-3219(2007)077[0442:LEORME]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Hershey HG, Stewart BL, Warren DW. Changes in nasal airway resistance associated with rapid maxillary expansion. Am J Orthod. 1976;69:274–84. doi: 10.1016/0002-9416(76)90076-2. [DOI] [PubMed] [Google Scholar]
- 7.Cistulli PA. Craniofacial abnormalities in obstructive sleep apnoea: Implications for treatment. Respirology. 1996;1:167–74. doi: 10.1111/j.1440-1843.1996.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 8.Subtelny JD. The significance of adenoid tissue in orthodontia. Angle Orthod. 1954;24:59–69. [Google Scholar]
- 9.Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998;21:831–5. doi: 10.1093/sleep/21.8.831. [DOI] [PubMed] [Google Scholar]
- 10.Starnbach H, Bayne D, Cleall J, Subtelny JD. Facioskeletal and dental changes resulting from rapid maxillary expansion. Angle Orthod. 1966;36:152–64. doi: 10.1043/0003-3219(1966)036<0152:FADCRF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Monini S, Malagola C, Villa MP, Tripodi C, Tarentini S, Malagnino I, et al. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol Head Neck Surg. 2009;135:22–7. doi: 10.1001/archoto.2008.521. [DOI] [PubMed] [Google Scholar]
- 12.Smith T, Ghoneima A, Stewart K, Liu S, Eckert G, Halum S, et al. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2012;141:618–26. doi: 10.1016/j.ajodo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Buccheri A, Dilella G, Stella R. Rapid palatal expansion and pharyngeal space. Cephalometric evaluation. Prog Orthod. 2004;5:160–71. [PubMed] [Google Scholar]
- 14.Praud JP, Dorion D. Obstructive sleep disordered breathing in children: Beyond adenotonsillectomy. Pediatr Pulmonol. 2008;43:837–43. doi: 10.1002/ppul.20888. [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves LPV, Filho C, Araujo F, Filipe, Barra R. Quality of life of children with sleep-disordered breathing after rapid maxillary expansion: Assessment by Osa-18. Rev Gaúha Odontol. 2013;61:235–43. [Google Scholar]
- 16.Sinha D, Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131:311–20. [PubMed] [Google Scholar]
- 17.O’Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–9. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Villa MP, Rizzoli A, Miano S, Malagola C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011;15:179–84. doi: 10.1007/s11325-011-0505-1. [DOI] [PubMed] [Google Scholar]
- 19.Garib DG, Henriques JF, Janson G, de Freitas MR, Fernandes AY. Periodontal effects of rapid maxillary expansion with tooth-tissue-borne and tooth-borne expanders: A computed tomography evaluation. Am J Orthod Dentofacial Orthop. 2006;129:749–58. doi: 10.1016/j.ajodo.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Pangrazio-Kulbersh V, Wine P, Haughey M, Pajtas B, Kaczynski R. Cone beam computed tomography evaluation of changes in the naso-maxillary complex associated with two types of maxillary expanders. Angle Orthod. 2012;82:448–57. doi: 10.2319/072211-464.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirelli P, Saponara M, Attanasio G. Obstructive Sleep apnoea syndrome (OSAS) and rhino-tubaric disfunction in children: Therapeutic effects of RME therapy. Prog Orthod. 2005;6:48–61. [PubMed] [Google Scholar]
- 22.Villa MP, Malagola C, Pagani J, Montesano M, Rizzoli A, Guilleminault C, et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8:128–34. doi: 10.1016/j.sleep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Villa MP, Bernkopf E, Pagani J, Broia V, Montesano M, Ronchetti R. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med. 2002;165:123–7. doi: 10.1164/ajrccm.165.1.2011031. [DOI] [PubMed] [Google Scholar]
- 24.Casale M, Pappacena M, Rinaldi V, Bressi F, Baptista P, Salvinelli F. Obstructive sleep apnea syndrome: From phenotype to genetic basis. Curr Genomics. 2009;10:119–26. doi: 10.2174/138920209787846998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jureyda S, Shucard DW. Obstructive sleep apnea - An overview of the disorder and its consequences. Semin Orthod. 2004;10:63–72. [Google Scholar]


