Abstract
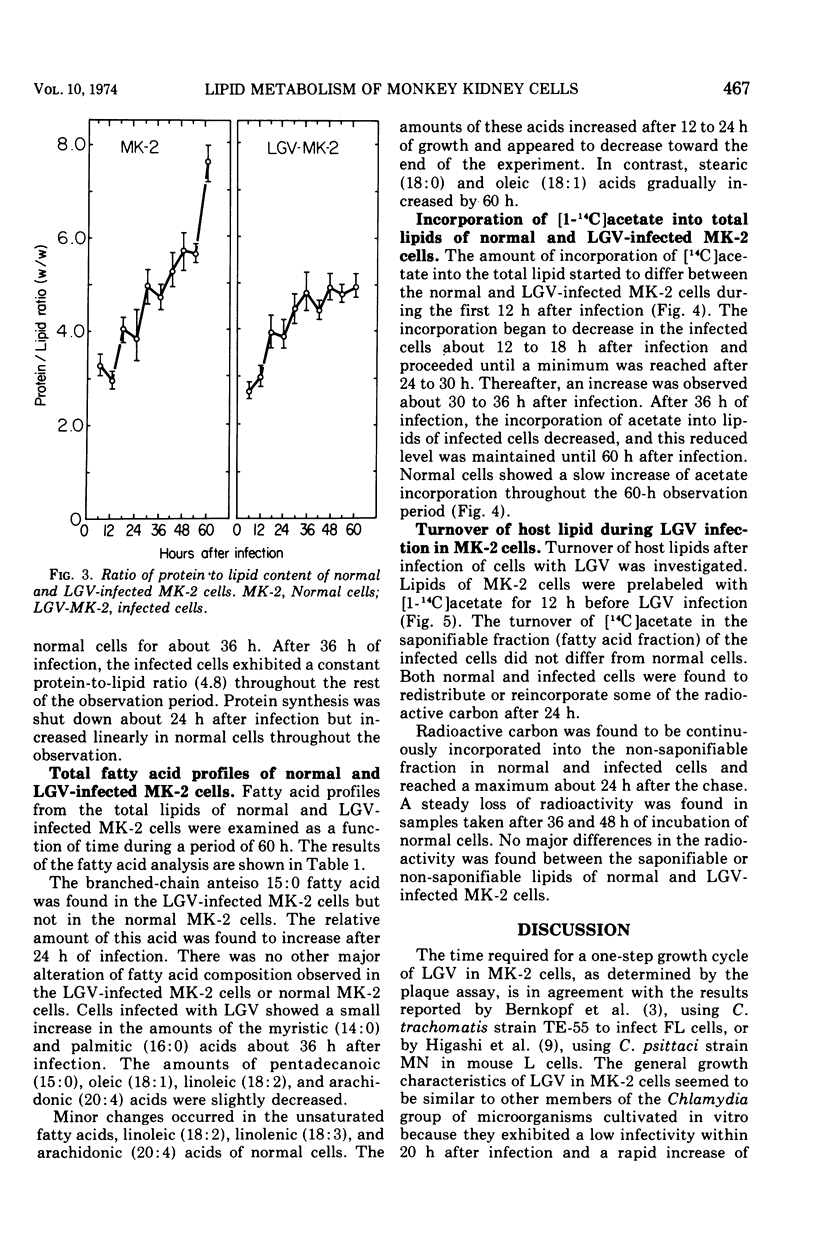
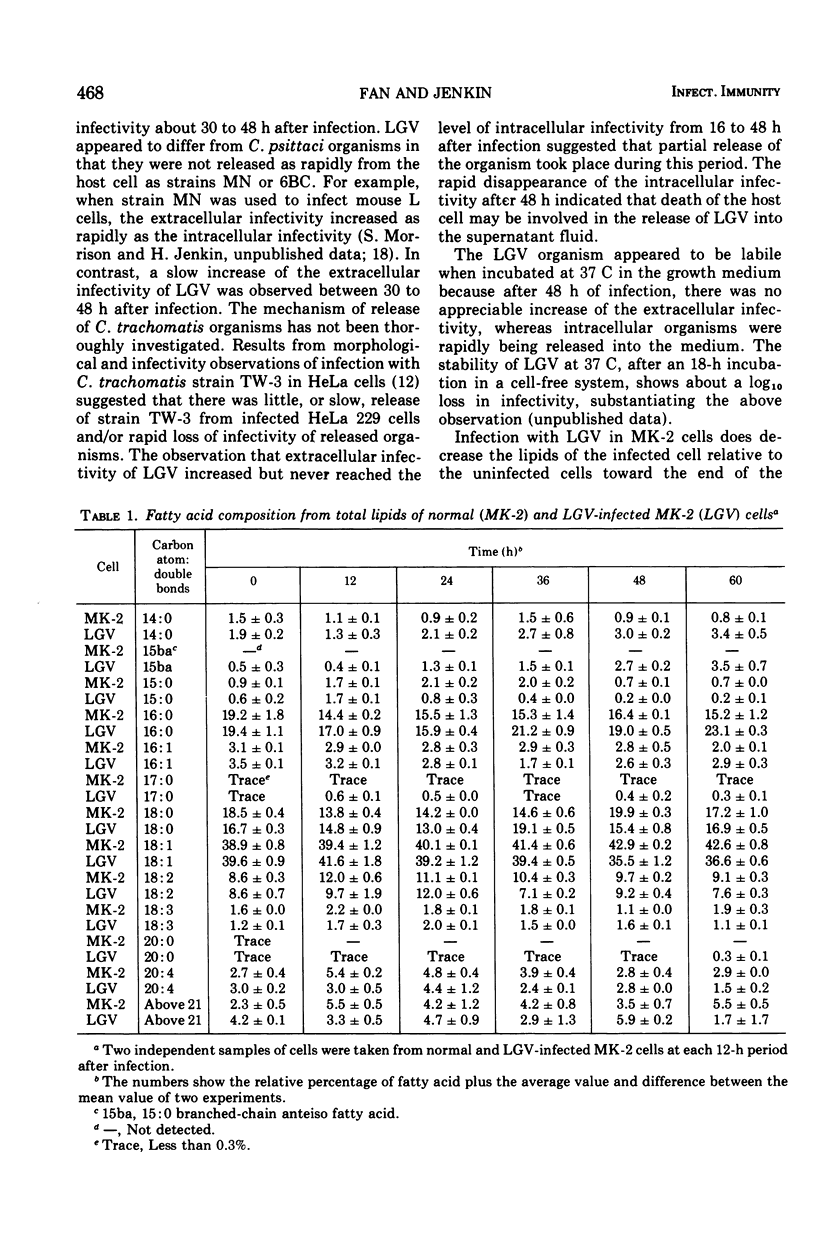
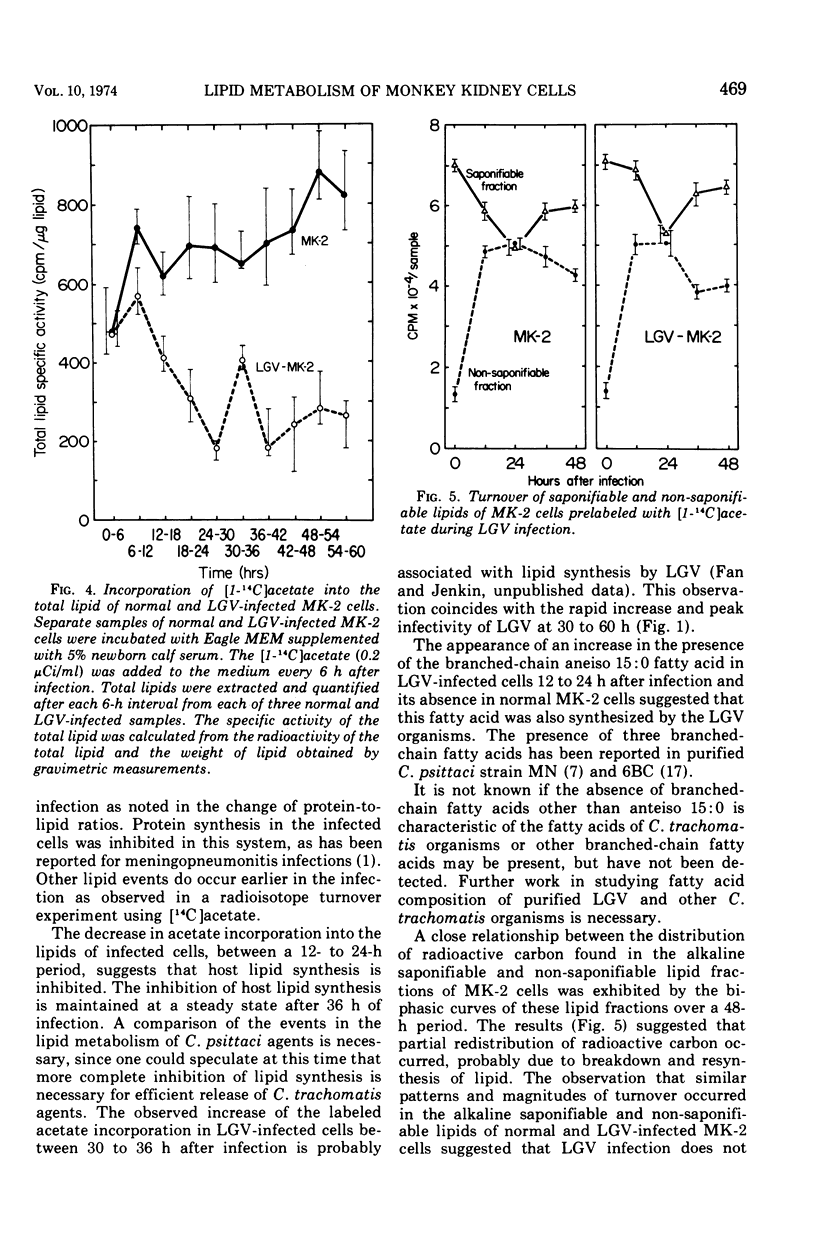
Lipid metabolism of monkey kidney (LLC-MK-2) cells and cells infected with a Chlamydia trachomatis strain lymphogranuloma venereum (LGV) was studied. The protein-to-lipid ratio of normal MK-2 cells was found to increase linearly over a 60-h period of incubation. The protein-to-lipid ratio of the infected cells was similar to that in normal cells until 36 h after infection, when a plateau in the ratio was observed. Lipid synthesis of the infected cells was found to be inhibited after 48 h of infection. Turnover of host lipids did not appear to be markedly altered by infection with LGV over a 48-h period of incubation. An anteiso branched chain of 15:0 fatty acid was found in infected cells but not in normal cells. The appearance of this fatty acid, correlated with a rise in the infectivity of LGV, suggests that synthesis of specific lipids was associated with the infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banks J., Eddie B., Schachter J., Meyer K. F. Plaque formation by Chlamydia in L cells. Infect Immun. 1970 Mar;1(3):259–262. doi: 10.1128/iai.1.3.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gaugler R. W., Neptune E. M., Adams G. M., Sallee T. L., Weiss E., Wilson N. N. Lipid synthesis by isolated Chlamydia psittaci. J Bacteriol. 1969 Nov;100(2):823–826. doi: 10.1128/jb.100.2.823-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E. The effect of oleic acid on the growth of monkey kidney cells (LLC-MK2). Exp Cell Res. 1970 Jan;59(1):6–10. doi: 10.1016/0014-4827(70)90616-6. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. Comparative lipid composition of psittacosis and trachoma agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1087–1098. doi: 10.1016/0002-9394(67)94087-1. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. The continuous passage of agents of trachoma in cell culture. I. Characteristics of TW-3 and Bour strains of trachoma cultivated in serial passage in HeLa 229 cells. J Infect Dis. 1966 Jun;116(3):390–399. doi: 10.1093/infdis/116.3.390. [DOI] [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makino S., Jenkin H. M., Yu H. M., Townsend D. Lipid composition of Chlamydia psittaci grown in monkey kidney cells in defined medium. J Bacteriol. 1970 Jul;103(1):62–70. doi: 10.1128/jb.103.1.62-70.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Jenkin H. M. Growth of Chlamydia psittaci strain meningopneumonitis in mouse L cells cultivated in a defined medium in spinner cultures. In Vitro. 1972 Sep-Oct;8(2):94–100. doi: 10.1007/BF02615966. [DOI] [PubMed] [Google Scholar]
- Schachter J., Meyer K. F. Lymphogranuloma venereum. II. Characterization of some recently isolated strains. J Bacteriol. 1969 Sep;99(3):636–638. doi: 10.1128/jb.99.3.636-638.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- WANG S. P., GRAYSTON J. T. EGG INFECTIVITY ASSAY OF TRACHOMA VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:587–591. doi: 10.3181/00379727-115-28978. [DOI] [PubMed] [Google Scholar]



