Abstract
Over the past few decades, MRI of the prostate has made great strides in improving cancer detection and is being embraced by more clinicians each day. This article aims to review the imaging characteristics of common and uncommon, but consequential lesions involving the seminal vesicles (SV), as seen predominantly on MRI. Many of these findings are seen incidentally during imaging of the prostate. Anatomy and embryology of the SV will be described which will help illustrate the associations of abnormalities seen. Congenital, infectious, neoplastic, and tumor mimics will be explored in detail, with discussion on clinical presentation and treatment strategies.
Keywords: MRI seminal vesicles, prostate cancer, prostate MRI, seminal vesicles
INTRODUCTION

Mahati N Reddy
Recent advances in cross-sectional imaging has expanded our knowledge of other male genitourinary (GU) tract abnormalities. This article aims to review the imaging characteristics of common and uncommon, but significant lesions involving the seminal vesicles (SVs), as seen predominantly on MRI. Many of these findings are incidental during imaging of the prostate or pelvis on CT. Nevertheless, associated clinical findings are discussed, since the SVs are rarely suspected to be the cause of a clinically presenting abnormality and the radiologist has an opportunity to be the first to suggest such a diagnosis. In certain conditions, treatment strategies will be briefly discussed.
ANATOMY
Seminal vesicles (SV) are a pair of accessory glandular structures of the male reproductive system, which are extra-peritoneal in location, interposed between the bladder and the rectum. The vas deferens (VD), which are contiguous with the epididymal tail, terminate and form bilateral outpouchings. These lateral outpouchings are termed the SVs. Each SV itself is approximately 5 cm long and composed of a single coiled tube with irregular diverticula which are connected by fibrous tissue.[1] The main function of the SV is to secrete fluid which forms majority of the ejaculate; however, they are not a reservoir of the semen. The secreted fluid contains fructose, proteins, and other enzymes that provide nutrition for the spermatozoa.[2] The ampulla of the VD and duct of the SV combine at the base of the prostate to form the ejaculatory ducts (EDs). The EDs extend further inferiorly to drain into the prostatic urethra through the verumontanum [Figure 1].
Figure 1.
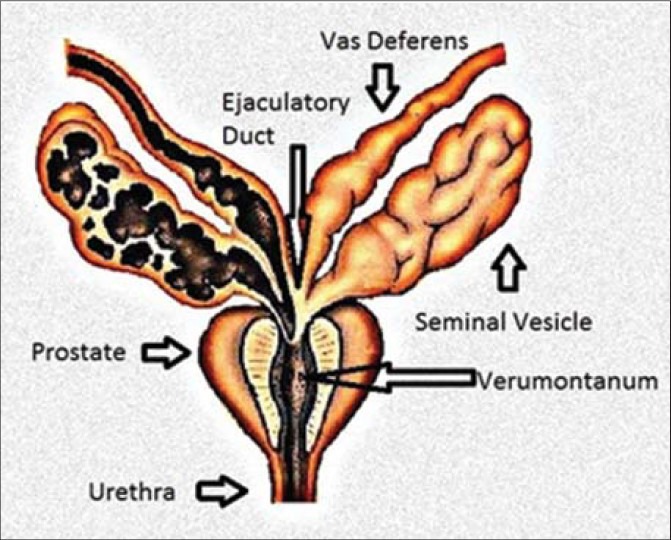
Diagram shows basic anatomy of the seminal vesicle.
On imaging, normal SVs appear as elongated fluid containing structures when commonly evaluated on transrectal US, CT, or MRI [Figure 2]. The SV may be distended with seminal fluid, or relatively collapsed. Although asymmetry of size is common and a normal finding, the signal intensity on MRI should be symmetric. Post-contrast images demonstrate normal enhancement of the septa/wall. Variations in appearance can be seen with hormonal changes and patient's age. The SVs usually shrink after the age of 70 years.[3] Vesiculography (through cannulation of the VD) can provide detailed anatomy of the SV; however, it is not commonly performed today due to the invasive nature of the study and improvement in alternative imaging modalities.[3]
Figure 2.
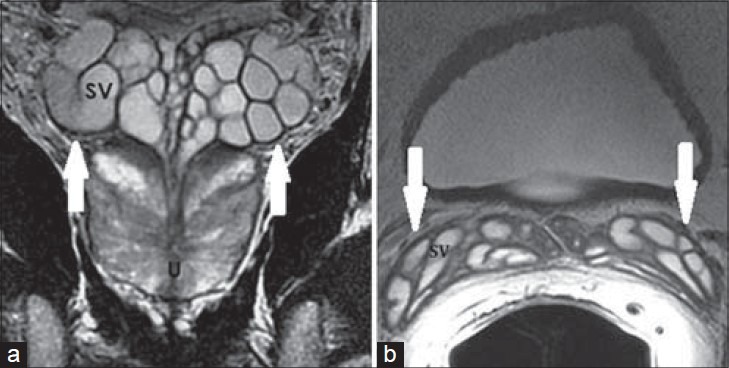
41-year-old asymptomatic male. a) Coronal and b) axial T2-weighted MR images show clustered grape-like appearance of the SVs (white arrows) with high T2 signal intensity of internal content and low T2 signal intensity of the wall of normal SVs.
EMBRYOLOGY
During early gestation, both sexes have the mesonephric (Wolffian) ducts and the paramesonephric (Müllerian) ducts, which form the male and female excurrent ducts, respectively. The mesoderm at the nephrogenic ridge gives rise to a pronephros at week 3 of life, which further differentiates into a mesonephros. The pronephros involutes, but a mesonephric duct persists until the end of the 4th week. By the 5th week of life, the ureteric bud arises from the mesonpehric duct and drains through a common duct into the urogenital sinus.[4] In the 6th week of fetal life, the ureteric bud fuses with the metanephric blastema and becomes the primitive kidney. From the 6th to the 8th week, the orifice of the lower mesonephric duct and ureteric bud separate and the ureteric orifice migrates medially and cranially.[5]
In a 9–10-week-old fetus, the mesonephric mesenchymal cells differentiate into Leydig cells, which help produce testosterone. The development of male accessory glandular tissue, SVs, prostate gland, and bulbourethral glands is stimulated by the androgens produced in the 10th-12th weeks of fetal life. The bulk of the mesonephric duct forms the VD and epididymis.[6] At 15 weeks, the rudimentary SV contains a medial horizontal portion and lateral vertical portion. In the mid-second trimester, occasional diverticula or outpouchings form with progressive coiling of the lumen through the third trimester of gestation.[2] In the male fetus, the paramesonephric duct (Müllerian duct) involutes.
CONGENITAL LESIONS
Agenesis of the SV is one of the most commonly encountered congenital abnormalities [Figure 3]. Agenesis of the SV may be unilateral or bilateral, and it is associated with agenesis/ectopia of the VD and ipsilateral kidney, since development of these structures is closely related. In utero insult to the mesonephric duct before ureteral budding will result in ipsilateral renal agenesis, and this is likely to be associated with VD agenesis or ectopia. If the insult occurs after 7 weeks of gestation, the ipsilateral kidney will be present.[7] Bilateral SV agenesis and VD anomalies without associated urinary abnormalities are often seen in patients with cystic fibrosis. In patients with primary genital form of cystic fibrosis, the mechanism of agenesis is postulated to be luminal blockage of the SV and VD precursors from abnormal secretions after 7 weeks of gestation.[8] Although there is no specific symptomatology suggestive of SV agenesis, diagnosis can be readily made on cross-sectional imaging. In patients with symptoms such as infertility, hematospermia, or recurrent epididymitis, an ectopic VD or functional ductal system can be sought. Corrective surgeries can be performed because testicular spermatogenesis is usually intact and fertility may be salvaged.[9]
Figure 3.
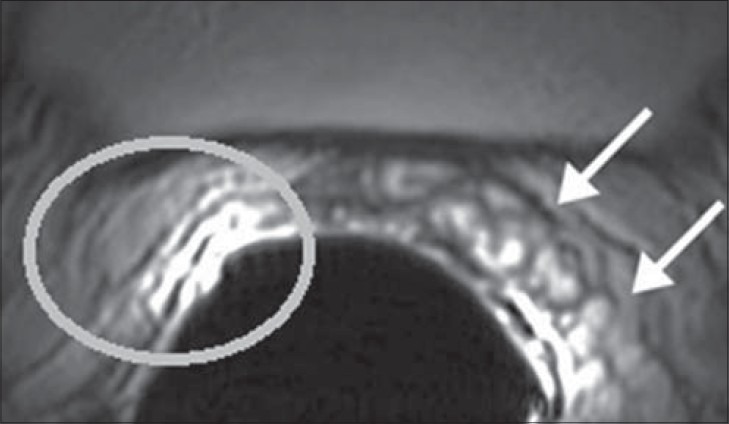
34-year-old male with infertility diagnosed to have hypospermia. Axial T2-weighted MR image using an endorectal coil shows a normal left SV (white arrows) and none on the right (white circle).
Another commonly seen entity in clinical practice is the cysts of the SV, which may be congenital or acquired [Figure 4]. A majority of the congenital cystic lesions are associated with other congenital GU malformations and the etiology is likely to be obstruction of the ED from maldevelopment of the lower mesonephric duct.[10] There may also be associated abnormal migration of the ureteric bud causing improper differentiation of the metanephric blastema resulting in unilateral renal agenesis or dysgenesis. Congenital SV cyst associated with ipsilateral renal agenesis is known as Zinner's syndrome [Figure 5].[11] SV cysts tend to become symptomatic when patients become sexually active, as they enlarge and exert a mass effect.[12] Patients with autosomal dominant polycystic kidney disease (ADPCK) commonly present with associated SV cysts (up to 43%), and the diagnosis must be considered when bilateral SV cysts are present. The cysts themselves do no confer a higher risk of sperm abnormalities.[13]
Figure 4.
58-year-old male with rising PSA levels presented for a prostate MRI. a) Coronal and b) axial T2-weighted MR images show a cyst within the left SV (white arrow), which is non-specific and can be congenital or secondary to other inflammatory etiologies.
Figure 5.
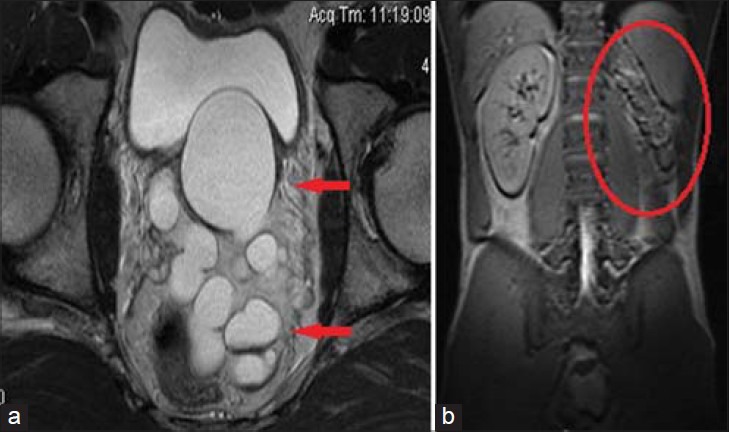
32-year-old male with infertility diagnosed with Zinner's syndrome. a) Axial T2-weighted MRI shows cystic enlargement of the left SV, likely due to an atretic/obstructed ejaculatory duct. b) Coronal localizer image shows associated left renal agenesis. This triad is known as Zinner's syndrome.
On MRI, SV cysts demonstrate high T2 and low T1 signal, much like the surrounding SV. Hemorrhage and proteinaceous content within the SV may cause higher signal on T1 weighted images. The key to diagnosis on imaging is being able to differentiate SV cysts from other mimics such as prostatic cysts, utricular cysts, ectopic ureterocele, etc. MRI is a helpful tool in assessment of potential ectopic insertion of the VD. Affected patients may present with myriad of non-specific GU symptoms such as voiding issues, urinary tract infections, pain, infertility, etc. Currently, laparoscopic partial vesiculectomy is the treatment of choice for symptomatic patients, especially young patients.[11]
INFECTIOUS AND INFLAMMATORY LESIONS
Acute and/or chronic seminal vesiculitis are usually secondary to an infection, which also affect the prostate gland. Urethritis and prostatitis are common clinical entities encountered by urologists. Studies suggest that seminal vesiculitis can be seen in up to 24% of patients with other GU infections, such as gonococcal urethritis.[14] However, clinical assessment of acute seminal vesiculitis is difficult in the setting of concomitant prostatitis, where the prostate gland is enlarged and tender. Chronic and recurrent seminal vesiculitis can also be challenging to diagnose since symptomatology is usually non-specific, when present. However, diagnosis is important due to implications on reproductive health. Symptoms may include pain, hematospermia, spermatocele, infertility, etc.[15]
Classic imaging findings of vesiculitis on US, CT, or MRI include diffuse SV wall thickening with enhancement of the septa on post-contrast CT or MRI [Figure 6]. In endemic countries, TB and schistosomiasis should be considered as possible etiologies.[5] In the acute or subacute phase, cystic dilation of the SVs is present and in the chronic phase, they may shrink with decreased signal on T1- and T2-weighted MR images.[3] When active inflammation is present, the T2 signal is higher than fat and T1 signal may be elevated if a hemorrhagic component is present.[16]
Figure 6.
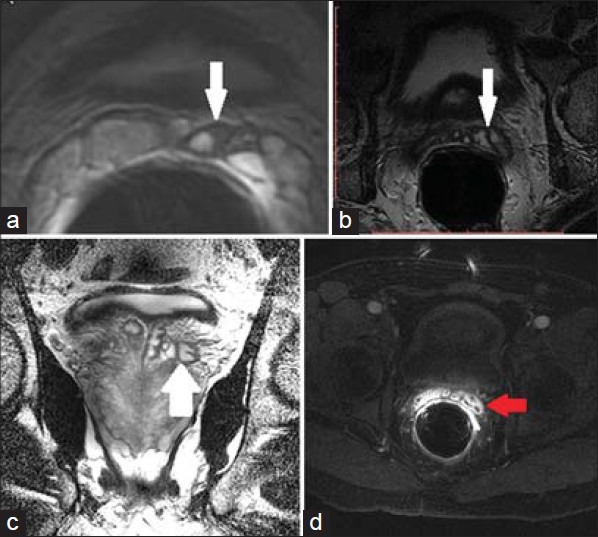
24 year old male with 3 week history of residual pain after treatment for gonococcal infection. a) Axial T2 weighted MRI through the SV shows mild asymmetric dilation of the left SV with focal areas of wall thickening (white arrow). These findings are commonly seen with seminal vesiculitis, although the etiology is non-specific. 53-year-old male presented with acute prostatitis. b) Axial and c) coronal T2-weighted MR images show more diffuse thickening of the SV wall (white arrow). d) Post contrast image shows enhancement (red arrow). These findings are characteristic of seminal vesiculitis.
In an acute presentation, patients are often treated empirically for prostatitis syndrome (which may include concomitant vesiculitis) with antibiotics and no confirmatory diagnostic studies for vesiculitis are obtained. In some patients, a residual chronic form remains as an aftermath of the acute process, but a majority of cases do not have a preceding acute presentation. Chronic and recurrent seminal vesiculitis is initially managed by antimicrobials. However, recurrent inflammation causes obstruction of the highly convoluted tubular glands of the SV and VD and poor drainage of inflammatory exudate. There is also low drug concentration within the SV due to low vascularity.[15] This may lead to abscess formation in the SV. Abscesses are seen in higher frequency in the setting of diabetes mellitus, prior instrumentation, or recent surgical procedures.[5] When an abscess is identified, clinical symptomatology directs treatment and surgical drainage is considered.
In endemic areas, tuberculosis (TB) must be considered as a possible etiology of a GU abscess. The most common extra-pulmonary manifestation of TB is in the GU tract. As with other infectious processes, tuberculous involvement of the SV is almost always accompanied by infection of the prostate and usually the upper urinary tract (kidneys and ureters) leading to theories that bacteria seed within the lower GU tract as they travel through the urine.[17] However, subsequent case reports of primary genital TB have been published. In the initial stages, enlarged SV with destruction of convolutions, hypoechoic areas on US, and hypodense areas on CT are present with surrounding hypervascularity, perivesicle inflammation, and bladder wall thickening.[18,19] Abscess formation follows with caseation, cavitation, and fibrosis.[20] Eventually, a calcified mass may be seen.
Schistosomiasis is a disease caused by several species of a parasite whose first hosts are snails in freshwater; therefore, humans contract the disease in contaminated water. Schistosoma haematobium, endemic in Africa and the Middle East, is the most common species affecting the GU system.[21,22] In endemic regions such as Zambia, postmortem studies have found parasite eggs in the SV of approximately 58% of the male cadavers.[23] Seminal vesiculitis is usually seen in the subacute or chronic phase of genital schistosomiasis.[22] The schistosome eggs are deposited into the wall of the SV tissue rather than the lumen, which may lead to wall calcifications that do not resolve with treatment, usually best seen on CT or US. As with other forms of seminal vesiculitis, there may be dilation of the SV and ED due to distal fibrosis and obstruction, which can resolve after specific treatment.[24] This diagnosis should be considered when SV and VD calcifications are seen in a symptomatic patient traveling to an endemic area, and confirmation can be obtained with parasitological study of the semen and urine.
NEOPLASMS
Primary neoplasms of the SV, which arise from the epithelial or mesenchymal elements, are very rare.[25] A variety of tumors have been reported in isolated case reports, with benign etiologies even less common than malignant. There are less than 20 reported cases of cystadenomas (epithelial stromal tumor) of the SV, which usually present as a well-demarcated, retrovesicular, multi-loculated cystic mass in an asymptomatic patient or a patient complaining of hematuria.[26,27] Imaging is non-specific and high-grade cytologic features may be present on microscopic evaluation. Therefore, the mass should be completely resected and radical cysto-prostatectomy should be considered if high-grade elements are identified.[28] Another benign lesion of the SV reported in the literature is a leiomyoma, which arises from the smooth muscle of the SV. MRI demonstrates low T2 and iso to low T1 signal of the lesion with post-contrast enhancement, and CT may demonstrate coarse, dense calcifications as seen with leiomyomas of the uterus.[29,30] Rare reports of other benign tumors such as fibromas, schwannomas, and paragangliomas are also found in the literature.
A mass in the SV with an infiltrative growth pattern is suggestive of a malignancy. Of the primary malignant SV tumors, adenocarcinoma arising from the glandular epithelium is the most common.[25] There are established, stringent criteria for diagnosis of carcinoma of the SV. The criteria include no concomitant primary tumor of the prostate, presence of mucus production in anaplastic variant (to distinguish from anaplastic prostate carcinoma), and negative immunohistochemistry staining for prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP).[31] Adenocarcinoma of the SV is also usually negative for carcinoembryonic antigen (CEA) and positive for CA-125.[31] Due to rarity of the disease, diagnosis is usually delayed and treatment strategies are poorly defined, which further contribute to a poor prognosis. Unfortunately, there are no reliable imaging features that distinguish a primary from a secondary form of malignancy. Several types of sarcomas and seminomas originating in the SV have been reported in the literature. Also without any specific imaging or clinical manifestations it is difficult to identify an accurate diagnosis without tissue sampling [Figure 7].
Figure 7.
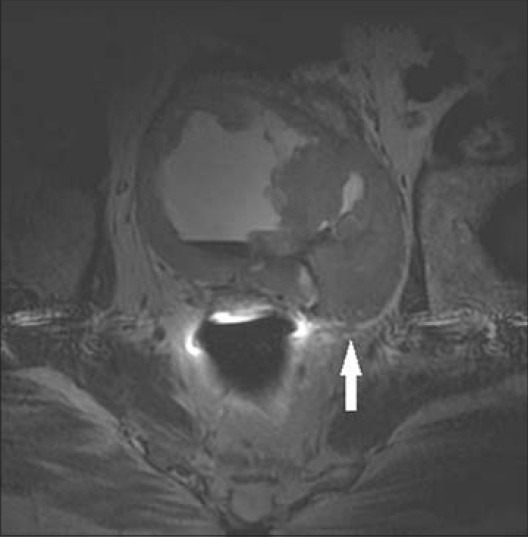
66-year-old male presented with hematuria and was diagnosed with sarcoma of the left SV. Axial T2-weighted MRI of the left SV demonstrates a large low T2 signal infiltrative mass with invasion of the bladder base. On histologic evaluation, the mass was found to be a sarcoma originating from the left SV.
Malignancy within the SV is most commonly secondary to carcinoma of the prostate, rectum, or bladder. Recent improvements in imaging of prostate carcinoma with MRI using an endorectal coil have significantly improved the sensitivity of determining SV invasion, which affects the prognosis and may alter the course of treatment. Route of SV invasion in prostate cancer is unknown and the possibilities include retrograde tumor extension through the ED, spread from fat tissue, tumor deposits, or direct spread across the prostatic base.[5] The most sensitive and specific features on MRI to determine SV invasion are low signal intensity within the SV and lack of preservation of the normal SV architecture, respectively [Figure 8]. When these features are combined with a visible malignancy at the prostate base extending beyond the capsule, SV invasion is highly predictive.[32] Tumor extension from the bladder or rectum can be identified as a large contiguous soft tissue mass. Other soft tissue masses can represent metastatic deposits on the SV.
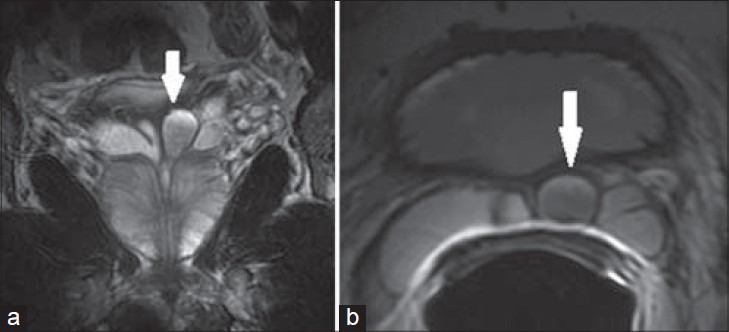
Figure 8.
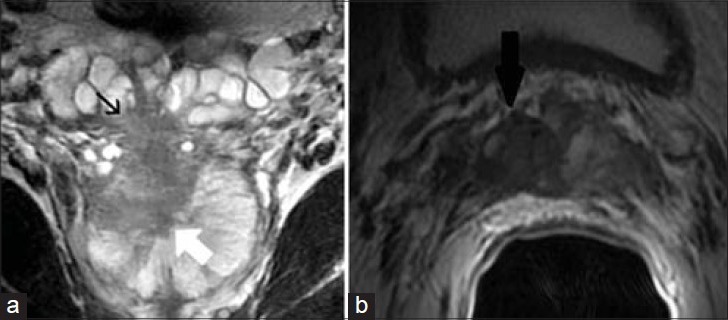
56-year-old male with an elevated PSA diagnosed with prostatic adenocarcinoma. a) Coronal and b) axial T2-weighted MR images demonstrate a prostatic mass at the right base (white arrow) extending through the capsule into the right SV (black arrow). Findings are consistent with prostatic adenocarcinoma with invasion of the right SV.
MIMICS OF NEOPLASM
Asymptomatic, localized amyloid deposits within the SV have been a well-known entity for several years. However, with recent increase in prostate carcinoma imaging, accurate characterization of this pathology has become critical since it can be misinterpreted as tumor. Amyloidosis describes a heterogeneous group of disorders characterized by localized or systemic deposition of amyloid fibrils, which are extracellular substances that stain positive for Congo red and demonstrate apple-green birefringence under polarized light.[33] Amyloid found in the SV may be localized, part of systemic amyloid, or a mixture of both.[34] The localized form has different histochemical and immunohistochemical properties from the systemic form and it is unlikely to be symptomatic.[34,35] Isolated cases of SV amyloidosis causing hematospermia and perineal pain are present, but they are rare when compared to the high prevalence of the disease in males older than 75 years of age. MRI findings of the SV demonstrate luminal narrowing, thickening of the wall with low T2 signal, and usually lack of normal post-contrast enhancement of the wall on T1-weighted images.[36] On diffusion-weighted imaging (DWI), there is lack of restricted diffusion within the SVs [Figure 9].
Figure 9.
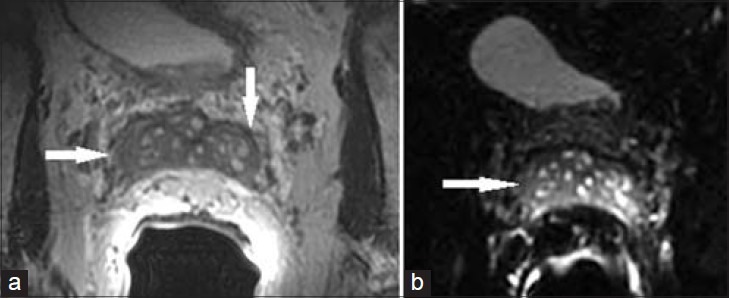
76-year-old with elevated PSA presented for initial prostate MRI. (a) Axial T2 MR image and b) axial diffusion-weighted image of the SV demonstrate diffusely thickened SV wall and luminal narrowing. Lack of diffusion restriction helped exclude tumor and lack of contrast enhancement helped exclude seminal vesiculitis. These changes were found to be secondary to amyloidosis.
As described previously, tumor involvement of the SV is commonly seen with prostate cancer. After treatment, another challenge faced by clinicians is differentiating tumor recurrence from radiation-related morphologic changes. Histologically, the perivesicle fibroadipose tissue is replaced by dense fibrous tissue after radiation.[37] The lumen of the tubules is progressively decreased; the SVs appear atrophied and can sometimes be obliterated secondary to peritubular fibrosis [Figure 10].[37] The fibrosis can mimic wall thickening of vesiculitis or tumor invasion. Bilateral, symmetric nature of the abnormality can help differentiate fibrosis for tumor recurrence.
Figure 10.
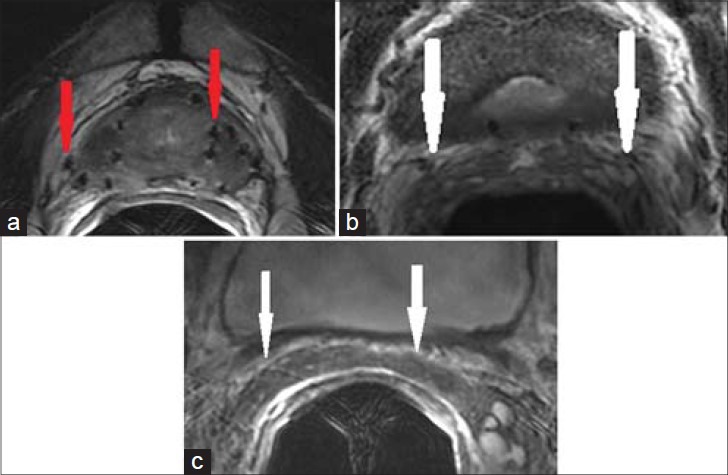
69-year-old male with history of treated prostate carcinoma presented for a follow-up study. a) Axial and b) coronal T2-weighted MR images through the pelvis show scattered foci of susceptibility, consistent with radiation seeds. The SVs are diffusely atrophied with low T2 signal, consistent with radiation fibrosis.
Hemorrhage is a commonly encountered abnormality on prostate MRI since many patients are imaged after biopsy. Hemorrhage within the SV with hypointense T2 signal can simulate tumor and cause confounding findings on DWI. Therefore, prostate MRI is commonly deferred at least 4 weeks post biopsy, but hemorrhage may persist up to 4 months.[38] If methemoglobin is present, corresponding abnormality demonstrates hyperintense T1 signal [Figure 11]. High T1 signal can also be seen with inspissated secretions. However, hemorrhage at different stages of evolution may not demonstrate high T1 signal, making the diagnosis very challenging.[39] SV hemorrhage is not only iatrogenic, but associated with other abnormalities, some of which are described above. Hematospermia is most commonly seen with GU infections, but can also be seen with calculi and tumors. SV calculi are rare and extremely non-specific in etiology, but radiologists need to be aware of the entity so that it is not confused with distal ureteral calculi or tumor on MRI [Figure 12].[40]
Figure 11.
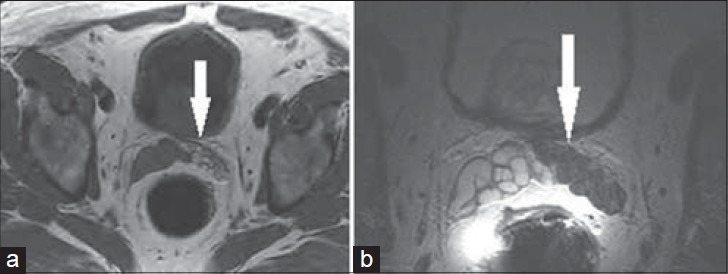
71-year-old male with rising PSA presented for a prostate MRI 2 weeks after prostate biopsy. a) Axial T1-weighted and b) axial T2-weighted MRI of the left SV show low T2 and high T1 signal throughout the left SV (white arrow), consistent with hemorrhage.
Figure 12.
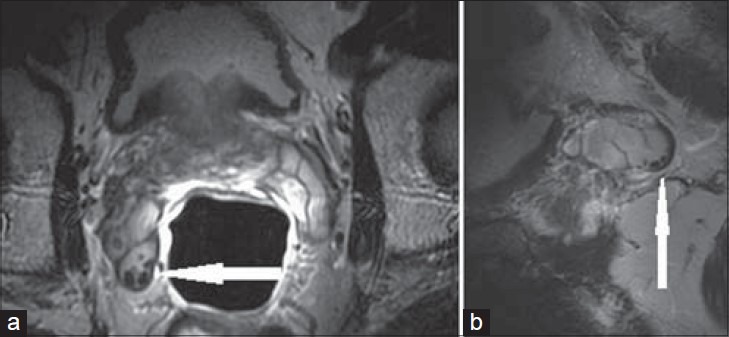
29-year-old male presented with hematospermia. a) Axial T2-weighted and b) sagittal T2-weighted MRI through the right SV show punctate foci of low signal along the periphery of the SV (white arrow), consistent with calculi.
CONCLUSION
The seminal vesicles (SVs) are one of the three main accessory glands of the male reproductive system. Most pathology and symptomatology affecting the SVs also affects the prostate and, in many instances, arises initially within the prostate gland. This pattern has resulted in pathology being masked or ignored within the SV. Nevertheless, with increase in cross-sectional imaging and improvement in techniques of prostate imaging, discrete lesions of the SV are being critically examined. Imaging characteristics of various congenital, infectious, inflammatory, and neoplastic diseases are described in detail with discussion on clinical implications.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/61/143734
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Standring S. London, UK: Churchill Livingstone; 2008. Gray's Anatomy: The Anatomical Basis of Clinical Practice; pp. 1261–77. [Google Scholar]
- 2.Ernst LM, Ruchelli ED, Huff DS. New York: Springer; 2011. Color Atlas of Fetal and Neonatal Histology; pp. 157–61. [Google Scholar]
- 3.Secaf E, Nuruddin RN, Hricak H, McClure RD, Demas B. MR imaging of the seminal vesicles. AJR Am J Roentgenol. 1991;156:989–94. doi: 10.2214/ajr.156.5.2017966. [DOI] [PubMed] [Google Scholar]
- 4.Kenney PJ, Spirt BA, Leeson MD. Genitourinary anomalies: Radiologic-anatomic correlations. Radiographics. 1984;4:233–60. [Google Scholar]
- 5.Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King BF., Jr Imaging of the seminal vesicle and vas deferens. Radiographics. 2009;29:1105–21. doi: 10.1148/rg.294085235. [DOI] [PubMed] [Google Scholar]
- 6.Schoenwolf GC. Philadelphia: Churchill Livingstone/Elsevier; 2009. Larsen's Human Embryology; pp. 479–541. [Google Scholar]
- 7.Campbell MF, Walsh PC, Retik AB. Philadelphia: Saunders; 2002. Campbell's Urology; pp. 3869–85. [Google Scholar]
- 8.Arora SS, Breiman RS, Webb EM, Westphalen AC, Yeh BM, Coakley FV. CT and MRI of congenital anomalies of the seminal vesicles. AJR Am J Roentgenol. 2007;189:130–5. doi: 10.2214/AJR.06.1345. [DOI] [PubMed] [Google Scholar]
- 9.Wu HF, Qiao D, Qian LX, Song NH, Feng NH, Hua LX, et al. Congenital agenesis of seminal vesicle. Asian J Androl. 2005;7:449–52. doi: 10.1111/j.1745-7262.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 10.Cihan A, Cimen S, Secil M, Kefi A, Aslan G. Congenital seminal vesicle cyst accompanying ipsilateral renal agenesis and rudimentary ureter. Int Urol Nephrol. 2006;38:133–5. doi: 10.1007/s11255-005-3152-2. [DOI] [PubMed] [Google Scholar]
- 11.Pereira BJ, Sousa L, Azinhais P, Conceição P, Borges R, Leão R, et al. Zinner's syndrome: An up-to-date review of the literature based on a clinical case. Andrologia. 2009;41:322–30. doi: 10.1111/j.1439-0272.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 12.Casey RG, Stunell H, Buckley O, Flynn R, Torreggiani WC. A unique radiological pentad of mesonephric duct abnormalities in a young man presenting with testicular swelling. Br J Radiol. 2008;81:e93–6. doi: 10.1259/bjr/31182823. HYPERLINK “http://www.ncbi.nlm.nih.gov/pubmed/?term=A+unique+radiological+pentad+of+mesonephric+duct+abnormalities+in+a+young+man+presenting+with+testicular #x002B;swelling” \o “Th e British journal of radiology.” . [DOI] [PubMed] [Google Scholar]
- 13.Torra R, Sarquella J, Calabia J, Martí J, Ars E, Fernández-Llama P, et al. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2008;3:790–3. doi: 10.2215/CJN.05311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya R, Takahashi S, Furuya S, Saitoh N, Ogura H, Kurimura Y, et al. Is urethritis accompanied by seminal vesiculitis? Int J Urol. 2009;16:628–31. doi: 10.1111/j.1442-2042.2009.02314.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Li P, Niu X, Zhang X, Wang Z, Qin C, et al. A new method of chronic and recurrent seminal vesiculitis treatment. J Endourol. 2011;25:1815–8. doi: 10.1089/end.2010.0456. [DOI] [PubMed] [Google Scholar]
- 16.Sue DE, Chicola C, Brant-Zawadzki MN, Scidmore GF, Hart JB, Hanna JE. MR imaging in seminal vesiculitis. J Comput Assist Tomogr. 1989;13:662–4. doi: 10.1097/00004728-198907000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Henline RB. Prostatitis and seminal vesiculitis: Acute and chronic. J Am Med Assoc. 1943;123:608–15. [Google Scholar]
- 18.Eastham JA, Spires KS, Abreo F, Johnson JB, Venable DD. Seminal vesicle abscess due to tuberculosis: Role of tissue culture in making the diagnosis. South Med J. 1999;92:328–9. doi: 10.1097/00007611-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Premkumar A, Newhouse JH. Seminal vesicle tuberculosis: CT appearance. J Comput Assist Tomogr. 1988;12:676–7. doi: 10.1097/00004728-198807000-00035. [DOI] [PubMed] [Google Scholar]
- 20.Joseph J, Narayanan H, Babu H, Praveen A. Tuberculosis of the prostate and seminal vesicles. J HK Coll Radiol. 2010;13:40–2. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Parsites-Schistosomiasis: Epidemiology and Risk Factors. [Last accessed on 2012 Jul 11]. Available from: http://www.cdc.gov/parasites/schistosomiasis/epi.html .
- 22.Shebel HM, Elsayes KM, Abou El Atta HM, Elguindy YM, El-Diasty TA. Genitourinary schistosomiasis: Life cycle and radiologic-pathologic findings. Radiographics. 2012;32:1031–46. doi: 10.1148/rg.324115162. [DOI] [PubMed] [Google Scholar]
- 23.Patil PS, Elem B. Schistosomiasis of the prostate and the seminal vesicles: Observations in Zambia. J Trop Med Hyg. 1988;91:245–8. [PubMed] [Google Scholar]
- 24.Vilana R, Corachán M, Gascón J, Valls E, Bru C. Schistosomiasis of the male genital tract: Transrectal sonographic findings. J Urol. 1997;158:1491–3. [PubMed] [Google Scholar]
- 25.Ramchandani P, Banner MP, Pollack HM. Imaging of the seminal vesicles. Semin Roentgenol. 1993;28:83. doi: 10.1016/s0037-198x(05)80115-4. HYPERLINK “http://www.ncbi.nlm.nih.gov/pubmed/8465210” \o “Seminars in roentgenology” . [DOI] [PubMed] [Google Scholar]
- 26.Bullock KN. Cystadenoma of the seminal vesicle. J R Soc Med. 1988;81:294–5. doi: 10.1177/014107688808100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorber G, Pizov G, Gofrit ON, Pode D. Seminal vesicle cystadenoma: A rare clinical perspective. Eur Urol. 2011;60:388–91. doi: 10.1016/j.eururo.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Hoshi A, Nakamura E, Higashi S, Segawa T, Ito N, Yamamoto S, et al. Epithelial stromal tumor of the seminal vesicle. Int J Urol. 2006;13:640–2. doi: 10.1111/j.1442-2042.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 29.Shiotani T, Kawai N, Sato M, Minamiguchi H, Takeuchi T, Tanihata H, et al. Leiomyoma of the seminal vesicle. Jpn J Radiol. 2009;27:218–20. doi: 10.1007/s11604-009-0321-8. [DOI] [PubMed] [Google Scholar]
- 30.Lallemand B, Busard P, Leduc F, Vaesen R. Laparoscopic resection of a leiomyoma of the seminal vesicle. Indian J Urol. 2007;23:70–1. doi: 10.4103/0970-1591.30272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiel R, Effert P. Primary adenocarcinoma of the seminal vesicles. J Urol. 2002;168:1891–6. doi: 10.1016/S0022-5347(05)64260-7. [DOI] [PubMed] [Google Scholar]
- 32.Sala E, Akin O, Moskowitz CS, Eisenberg HF, Kuroiwa K, Ishill NM, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: Diagnostic accuracy and multivariate feature analysis. Radiology. 2006;238:929–37. doi: 10.1148/radiol.2383050657. [DOI] [PubMed] [Google Scholar]
- 33.Cotran RS, Kumar V, Collins T. Diseases of immunity. In: Cotran RS, Collins T, editors. Robbins Pathologic Basis of Disease. 6th ed. Vol. 6. Philadelphia: W.B. Saunders; 1999. pp. 189–259. [Google Scholar]
- 34.Maroun L, Jakobsen H, Kromann-Andersen B, Horn T. Amyloidosis of the seminal vesicle: A case report and review of the literature. Scand J Urol Nephrol. 2003;37:519–21. doi: 10.1080/00365590310001764. [DOI] [PubMed] [Google Scholar]
- 35.Furuya S, Masumori N, Furuya R, Tsukamoto T, Isomura H, Tamakawa M. Characterization of localized seminal vesicle amyloidosis causing hemospermia: An analysis using immunohistochemistry and magnetic resonance imaging. J Urol. 2005;173:1273–7. doi: 10.1097/01.ju.0000152291.44802.9f. [DOI] [PubMed] [Google Scholar]
- 36.Kaji Y, Sugimura K, Nagaoka S, Ishida T. Amyloid deposition in seminal vesicles mimicking tumor invasion from bladder cancer: MR findings. J Comput Assist Tomogr. 1992;16:989–91. doi: 10.1097/00004728-199211000-00032. [DOI] [PubMed] [Google Scholar]
- 37.Chan TW, Kressel HY. Prostate and seminal vesicles after irradiation: MR appearance. J Magn Reson Imaging. 1991;1:503–11. doi: 10.1002/jmri.1880010502. [DOI] [PubMed] [Google Scholar]
- 38.White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: Effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195:385–90. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 39.Mirowitz SA. Seminal vesicles: Biopsy-related hemorrhage simulating tumor invasion at endorectal MR imaging. Radiology. 1992;185:373–6. doi: 10.1148/radiology.185.2.1410340. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal S, Ansari MS. Idiopathic bilateral giant seminal vesicle calculi and calcification of the male ejaculatory system: Current review of diagnosis and management. Indian J Surg. 2006;68:38–40. [Google Scholar]


