Abstract
OBJECTIVE:
To evaluate the role of dehydroepiandrosterone (DHEA) supplementation in women with poor ovarian response (POR) undergoing in vitro fertilization (IVF).
DESIGN:
Prospective case-control study.
SETTING:
Private tertiary fertility clinic.
MATERIALS AND METHODS:
31 infertile women with POR diagnosed as per the Bologna criteria.
INTERVENTIONS:
DHEA supplementation for 2 months and a subsequent IVF cycle, after two previous IVF cycles with POR.
MAIN OUTCOME MEASURE(S):
Dose and duration of gonadotropin therapy, oocyte yield, embryo number and quality, pregnancy and live birth rate.
RESULTS:
No difference was seen in gonadotropin requirement before and after DHEA supplementation. There was a significant increase in total and metaphase II oocytes (5.9 ± 0.68 vs. 2.73 ± 0.24; 4.45 ± 0.47 vs. 2.09 ± 0.26), fertilization (3.65 ± 0.49 vs. 2.00 ± 0.27), Grade I embryos (1.52 ± 0.25 vs. 0.55 ± 0.18), pregnancy rate (30% vs. 9.1%) and live birth rate (25% vs 0%) in those who completed the cycle, following DHEA supplementation.
CONCLUSIONS:
Dehydroepiandrosterone supplementation results in an improvement in oocyte yield, embryo quality, and live birth rate in a group of women with POR having undergone at least two previous failures due to POR.
KEY WORDS: Bologna criteria, dehydroepiandrosterone supplementation, embryo quality, live birth rate, Poor ovarian response
INTRODUCTION
Poor ovarian response (POR) is an important limiting factor to success in in vitro fertilization (IVF). It is encountered in approximately 10-15% of women undergoing conventional IVF.[1,2] Even though the true incidence of POR in various ethnicities is unknown, it is believed that the magnitude varies between women of different ethnicities.[3] The cause of this remains unexplained in the large majority, even though genital tuberculosis may be an important aetiological factor.[4] In addition to the reduced success rate, emotional and financial burden associated with this diagnosis are well-understood.
Lack of consensus for diagnosis of POR makes comparison of efficacy of various stimulation protocols or any beneficial role of adjuvant therapy used in this group of women difficult.[5] Introduction of the Bologna criteria for the definition of POR is a very important step toward applying the diagnosis to a more homogenous group and compare the results of different interventions to draw reliable conclusions.[6] Various interventions before or during ovarian stimulation have been in use in order to improve the ovarian response. These include administration of aromatase inhibitors, androgens or androgen modulating agents, growth hormone, human chorionic gonadotropin (HCG), and luteinizing hormone.[7] The beneficial role if any, of these agents in Indian women with poor response in IVF, is hitherto not evaluated.
Dehydroepiandrosterone (DHEA) has been demonstrated to improve ovarian response in women with POR in a small number of studies including one randomised controlled trial (RCT) study.[8,9,10,11,12,13] DHEA is secreted by adrenal cortex, central nervous system, and ovarian theca cells.[14] It serves as a prohormone for androgens and estrogens, but its conversion may not be symmetrical favoring testosterone over the estradiol and may depend on the steroidogenic enzymes present in peripheral target tissues.[15,16] A most recent in vivo study in the sheep model has shown a high proportion of follicles remaining in antral stage after DHEA supplementation.[17] It is known that androgen receptor mRNA and androgen levels in human granulosa cells from small antral follicular fluid correlate with follicular stimulating hormone (FSH) receptor mRNA expression, signifying the positive role of androgens in the early follicular development.[18] Another mechanism of action attributed to DHEA is an increase in ovarian Insulin-like growth factor-1.[8,15,19] Recently, DHEA is shown to improve the follicular microenvironment by lowering the levels of hypoxic inducible factor 1.[20] Its beneficial effect may be due to rescue of small antral follicles from atresia measured as an increase in the antral follicle count (AFC) and ovarian volume.[21,22] However, it may also act by increasing the recruitment of preantral or small antral follicles seen as an increase in anti-Mullerian hormone (AMH).[11] It offers a relatively inexpensive and simple adjuvant therapy in PORs, and the cost of therapy in India is approximately $28/month. Possible virilising effects such as acne, deepening of voice, and facial hair growth appear to be minimal at the therapeutic dose used.[23,24] However, particular attention may need to be given in women prone to convulsive activity while considering DHEA supplementation.[25] The aim of this study was to prospectively evaluate the impact of DHEA supplementation in PORs of a single ethnicity undergoing IVF on the gonadotropin requirement, endocrine parameters, number of total and mature oocytes, number and quality of embryos; implantation, pregnancy, and live birth rate.
MATERIALS AND METHODS
A prospective case–control study was performed at a private tertiary referral center, between August 2011 and September 2012. The study was approved by the Institutional Review Board of the hospital. Baseline assessment of all study participants included estimation of day 3 FSH, AMH; and a transvaginal ultrasound scan (TVS) to assess the AFC performed by a single radiologist, not involved in the study. Diagnosis of PORs was based on per the Bologna Criteria: All these women had undergone at least one previous cycle of IVF elsewhere with maximal stimulation and documented poor response (<4 oocytes) in the most recent cycle and; an abnormal ORT (AMH < 1.1 ng/ml or AFC < 7) or previous surgery for ovarian endometrioma or cyst. They were counseled to undergo one cycle of IVF with maximal stimulation (Group I) and if unsuccessful, a further IVF cycle following DHEA supplementation for a minimum period of 60-day (Group II). Verbal and written information regarding the presence of only a small body of evidence supporting use of DHEA in PORs was provided to them, and an informed written consent was obtained.
Group I consisted of a total of 31 women less than 40 years of age who fulfilled the selection criteria and agreed to participate in the study: 8 had undergone more than one cycle of IVF elsewhere with the most recent cycle being of POR; 23 had undergone one previous cycle of IVF elsewhere with documented POR. All of them underwent IVF/intracytoplasmic sperm injection (ICSI) with human menopausal gonadotrophin 375 IU (Menogon, Ferring GmbH, Germany) from day 2 of the cycle and received GnRH antagonist in a fixed protocol, 0.25 mg s.c. daily from the fifth day of stimulation onward (Cetrotide, Merck Serono Europe Limited, United Kingdom). Response to ovarian stimulation was monitored by both TVS and hormonal assessment. Ovulation trigger was given with rHCG 250 μg s.c. (Ovitrelle, Merck Serono S.p.A. Modugno (BA), Italy) when the leading follicle reached 18 mm diameter. Cycles were cancelled for lack of response if no dominant follicle was present following 7 days of stimulation and serum estradiol (E2) concentration was <100 pg/ml. Transvaginal sonography was performed using ALOKA scanner (model SSD – 4000 Plus; Hitachi Aloka Medical, Japan). The hormonal assays for FSH, E2 and Progesterone (P) were performed with the automated cobas e 411analyser (Roche Diagnostics, Manneheim Germany). AMH assay was performed with GenII enzyme-linked immunosorbent assay (Beckman Coulter, Inc., USA).
Oocyte retrieval was performed 36 h after HCG administration. ICSI was performed for all, as per the routine practice in PORs in our clinic. Number of oocytes, metaphase II (MII) oocytes, fertilization, cleavage, and number of embryos transferred were assessed and documented by a single embryologist, throughout the study period. Fertilization was assessed 18 h after ICSI and embryo grades were documented on day 2 and day 3.[26] Embryos with eight or more equal blastomeres and without any fragmentation were considered as Grade I on day 3. Embryo transfer was performed on day 3 with K-JETS-7019-SIVF embryo transfer set (Cook Ireland Ltd., Ireland) under transabdominal ultrasound guidance, and a maximum of three embryos were transferred.
Luteal phase was supported by vaginal progesterone (Susten suppositories, Sun Pharmaceutical Ind Ltd., India) 400 mg twice daily from the day of oocyte retrieval. Pregnancy outcome was confirmed by serum beta HCG 12 days after embryo transfer. If positive, a TVS was performed approximately 28 days after embryo transfer and clinical pregnancy was confirmed if fetal cardiac activity was noted on TVS.
Following the first cycle, 27 women had an unsuccessful cycle with POR and agreed for DHEA supplementation. Of these, 2 discontinued DHEA after a few weeks of therapy and two declined any further IVF. Those who agreed for DHEA supplementation received oral micronized DHEA 25 mg 3 times a day (DHEA PREG, Aarkios Health Private Limited, India) for a minimum period of 2 months prior to further IVF. An additional two women from Group I whose pregnancies ended in miscarriage subsequently went on DHEA supplementation prior to further IVF. The IVF/ICSI protocol was identical to that of Group I and the same outcome parameters were recorded.
Statistical analysis
Descriptive statistical analysis was carried out to analyze various parameters. Results on continuous measurements are presented on mean ± standard error and results on categorical measurements are presented in number (%). Results are considered significant if P ≤ 0.05.
Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on a continuous scale between two groups (inter-group analysis) on metric parameters. Levene's test has been performed to assess the homogeneity of variance. Chi-square/Fisher exact test has been used to find the significance of study parameters on the categorical scale between two groups. Power analysis estimated a sample size of 25 needed for this evaluation study, for 80% of statistical power and 5% type I error.
The statistical software SAS 9.2 (SAS Institute Inc, Cary, NC, USA) SPSS 15.0 (SPSS Inc., Chicago, IL), Stata 10.1 (Stat Corp LP, College Station, TX), MedCalc 9.0.1 (MedCalc, Mariakerke, Belgium), Systat 12.0 (Cranes Software, Bangalore, India), and R environment ver. 2.11.1 (GNU Project, Free Software Foundation, Boston, MA) were used for the analysis of the data.
RESULTS
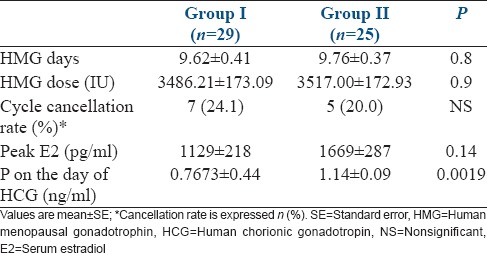
Of the 31 women, who underwent IVF in Group I, two were excluded from all analysis as the oocyte yield was more than 3 in them. Baseline characteristics of the remaining 29 women are shown in Table 1. 18 women were 25-35 years of age and 11 were 36-39 years. They had undergone an average of 1.13 cycles of IVF prior to the commencement of the study. AMH and AFC predicted poor response correctly in 26 and 27 of them with 90% and 95% sensitivity, respectively. Both parameters were within normal range in the two women excluded from the analysis. There was a nonsignificant increase in the AMH concentration in Group II compared to Group I (0.91 ± 0.09 vs. 1.78 ± 2.32; P 0.058). No increment in AFC was documented following DHEA supplementation (4.34 ± 0.33 vs. 4.52 ± 1.98; P 0.753). Table 2 shows the stimulation characteristics of the two groups. There was no difference in the duration of stimulation, or total gonadotropin requirement between the two groups. A nonsignificant reduction in the cycle cancellation rate was seen in Group II. A nonsignificant increase in peak E2 was noted in Group II and the serum P concentration on the day of HCG was significantly higher in Group II compared to Group I. The mean duration of DHEA supplementation was 11.0 weeks (range: 8.5-19.4 weeks).
Table 1.
Baseline characteristics of women in Group I

Table 2.
Stimulation cycle characteristics
On further analysis of the data in those who reached oocyte retrieval, women in Group II showed a significant increase in the oocyte yield, MII oocytes, 2 pronuclei fertilization and Grade I embryos [Table 3]. Despite no difference between the two groups in the number of embryos transferred, a trend toward better implantation rate was noted in Group II [Table 3]. There were two singleton pregnancies in Group I, both of which ended as first-trimester miscarriages. There were six singleton pregnancies in Group II, one of them ending in the first-trimester miscarriage.
Table 3.
Oocyte, embryo and pregnancy parameters in women undergoing oocyte retreival
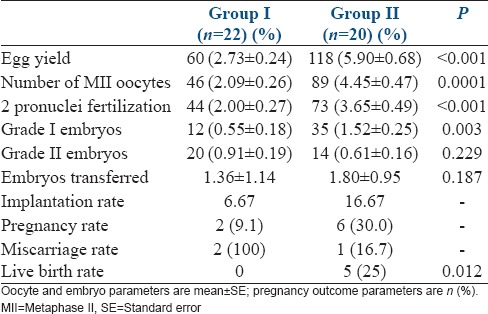
A subgroup analysis in Group II of oocyte yield and total number of embryos in younger women (≤35 years of age) and older women (36-40 years of age), did not show any significant difference between the two groups (oocyte yield 5.7 ± 2.31 vs. 6.1 ± 3.81; total number of embryos 3.6 ± 1.95 vs. 3.7 ± 2.54).
Pregnancy outcome
The outcome of the five pregnancies in Group II is as follows: 4 of them have delivered live singleton babies at term, and one had a preterm delivery at 32 weeks of gestation and the neonate required admission to NICU for a week, in view of prematurity. Live birth rate was significantly higher in Group II compared to Group I [Table 3].
Side effects
Apart from two women, in Group II, reporting a marginal lengthening of their menstrual cycles following DHEA supplementation, no other side effect was encountered.
DISCUSSION
Voluntary delayed childbearing has increased the need for IVF globally.[27] POR due to either advanced age or other underlying causes affect the outcome adversely[4,28,29,30,31] and this appears to occur at a younger age in women of Indian ethnicity compared to Caucasian women.[3] With the increasing burden of POR, various adjuvant therapies are in use in an attempt to improve the outcome even though such a practice has been questioned in the light of very limited evidence available.[32]
There is a paucity of studies diagnosing POR based on the Bologna criteria and evaluating the role of adjuvant therapy in PORs. To our knowledge, this is the first study adopting the Bologna criteria for diagnosis of POR, further, including a very homogenous group of PORs, all having undergone two cycles of IVF with maximal stimulation, and documented POR prior to DHEA supplementation and in a population considered to have a high incidence of POR. Such a study design would rule out the possibility of an improvement seen in the subsequent cycle solely due to “regression to the mean” with recruitment of a larger cohort.[33]
The average age of women included in this study is younger compared to previous reports.[10,11,13,20,34] We believe that it reflects the occurrence of POR earlier in women studied, as has been documented previously in certain ethnicities.[3] This study did not show any improvement in cycle parameters such as duration of treatment or dose of gonadotropins, as has been documented previously and only a nonsignificant reduction in cancellation rate.[10,20] Unlike previous reports, the changes noted in peak E2 levels were only modest.[20,22] Shorter duration of DHEA supplementation compared to previous studies may explain these findings.[10,20,22] However, it is known that parameters like improvement in oocyte quality leading to a reduction in aneuploidy may be seen with a shorter duration of supplementation.[8,12,35] The decision to limit DHEA supplementation to 2 months was based on the above evidence and in view of the concerns of prolonged delay in active treatment in a group of women with severely diminished ovarian reserve.[36] As documented previously, we also noted a significant increase in the P level on the day of HCG without any adverse effect on implantation.[37]
We noted an improvement in a number of total oocytes and mature oocytes as has been shown previously.[9,10,20,22] Our findings also support the previous evidence of improvement in number and quality of embryos.[10,13,22,38] As noted previously, there was a trend toward improvement in implantation and pregnancy rate despite a lack of significant difference between the two groups in the number of embryos transferred.[11] Similar to previous reports, we noted a significant increase in the live birth rate.[13] Previous studies have documented a reduction in aneuploidy following DHEA supplementation resulting in a reduction in miscarriage.[34,35] However, the small number included in the study precludes any conclusion to be drawn in this regard. Similar to previous studies, DHEA was well-tolerated, and there was a high compliance among the women in Group II.[10,20]
It has been suggested that in addition to the direct benefits of DHEA supplementation, reduction in duration or dose of treatment will help reducing the financial burden inherent with treatment.[36] It is believed that the beneficial effects despite an apparent lack of improvement in ovarian reserve markers such as AMH and AFC as was noted in our study could be due to an improvement in intra-ovarian environment secondary to increased intra-ovarian androgens.[39,40] The overall benefits of reducing the number of repeated IVF cycles and avoiding egg donation cycles in up to 20% of PORs is multidimensional and may be difficult to evaluate.
Limitations of this study are the inclusion of small numbers both due to this being a single center study and strict selection criteria; and nonrandomized study design. However, easy availability and safety of DHEA and difficulty in conducting RCT in this challenging subgroup of women may mean obtaining evidence through other study designs.[41]
CONCLUSIONS
This is the first study including a very homogenous group of PORs, recruited using the Bologna criteria, with POR in two successive IVF cycles with maximal stimulation. Even though the sample size is the limiting factor, a significant improvement in many of the outcome parameters including embryo quality, resulting in a better live birth rate is of importance to note. The results of this study add to the growing body of evidence in support of DHEA supplementation in PORs. Indirect benefits of reducing the number of attempts to achieve pregnancy and avoidance of egg donation in up to 20% of this challenging subgroup of women is encouraging. Further studies including a larger number of poor responders are warranted to define the place of DHEA supplementation in this challenging clinical situation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pellicer A, Lightman A, Diamond MP, Russell JB, DeCherney AH. Outcome of in vitro fertilization in women with low response to ovarian stimulation. Fertil Steril. 1987;47:812–5. doi: 10.1016/s0015-0282(16)59170-5. [DOI] [PubMed] [Google Scholar]
- 2.Tanbo T, Dale PO, Abyholm T, Stokke KT. Follicle-stimulating hormone as a prognostic indicator in clomiphene citrate/human menopausal gonadotrophin-stimulated cycles for in-vitro fertilization. Hum Reprod. 1989;4:647–50. doi: 10.1093/oxfordjournals.humrep.a136959. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud G, López Bernal A, Yudkin P, Ledger W, Barlow DH. A controlled assessment of the in vitro fertilization performance of British women of Indian origin compared with white women. Fertil Steril. 1995;64:103–6. [PubMed] [Google Scholar]
- 4.Malhotra N, Sharma V, Bahadur A, Sharma JB, Roy KK, Kumar S. The effect of tuberculosis on ovarian reserve among women undergoing IVF in India. Int J Gynaecol Obstet. 2012;117:40–4. doi: 10.1016/j.ijgo.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil Steril. 2009;91:749–66. doi: 10.1016/j.fertnstert.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 6.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum Reprod. 2011;26:1616–24. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 7.Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis L, et al. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:127–45. doi: 10.1093/humupd/dmr051. [DOI] [PubMed] [Google Scholar]
- 8.Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: A case series. Hum Reprod. 2000;15:2129–32. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 9.Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84:756. doi: 10.1016/j.fertnstert.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21:2845–9. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- 11.Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010;21:360–5. doi: 10.1016/j.rbmo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR) Reprod Biol Endocrinol. 2011;9:67. doi: 10.1186/1477-7827-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: A randomized prospective study. Hum Reprod. 2010;25:2496–500. doi: 10.1093/humrep/deq220. [DOI] [PubMed] [Google Scholar]
- 14.Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3–5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 15.Buster JE, Casson PR, Straughn AB, Dale D, Umstot ES, Chiamori N, et al. Postmenopausal steroid replacement with micronized dehydroepiandrosterone: Preliminary oral bioavailability and dose proportionality studies. Am J Obstet Gynecol. 1992;166:1163–8. [PubMed] [Google Scholar]
- 16.Haning RV, Jr, Hackett RJ, Flood CA, Loughlin JS, Zhao QY, Longcope C. Plasma dehydroepiandrosterone sulfate serves as a prehormone for 48% of follicular fluid testosterone during treatment with menotropins. J Clin Endocrinol Metab. 1993;76:1301–7. doi: 10.1210/jcem.76.5.8496321. [DOI] [PubMed] [Google Scholar]
- 17.Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2014;29:146–54. doi: 10.1093/humrep/det408. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Møllgård K, Wreford Andersen E, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17:63–70. doi: 10.1093/molehr/gaq073. [DOI] [PubMed] [Google Scholar]
- 19.Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: A six-month trial. Fertil Steril. 1998;70:107–10. doi: 10.1016/s0015-0282(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 20.Artini PG, Simi G, Ruggiero M, Pinelli S, Di Berardino OM, Papini F, et al. DHEA supplementation improves follicular microenviroment in poor responder patients. Gynecol Endocrinol. 2012;28:669–73. doi: 10.3109/09513590.2012.705386. [DOI] [PubMed] [Google Scholar]
- 21.Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98:380–8. doi: 10.1210/jc.2012-3071. [DOI] [PubMed] [Google Scholar]
- 22.Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Gal M, Alerhand S, et al. DHEA supplementation may improve IVF outcome in poor responders: A proposed mechanism. Eur J Obstet Gynecol Reprod Biol. 2013;168:49–53. doi: 10.1016/j.ejogrb.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: A review. J Clin Pharmacol. 1999;39:327–48. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 24.Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–5. doi: 10.1016/j.maturitas.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Karp G, Bentov Y, Masalha R, Ifergane G. Onset of late posttraumatic seizure after dehydroepiandrosterone treatment. Fertil Steril. 2009;91:931.e1–2. doi: 10.1016/j.fertnstert.2008.08.115. [DOI] [PubMed] [Google Scholar]
- 26.Veeck LL. The morphological assessment of human oocytes and early concepti. In: Keel BA, Webster BW, editors. Handbook of the Laboratory Diagnosis and Treatment of Infertility. Raton, FL: CRC Press; 1990. pp. 353–69. [Google Scholar]
- 27.te Velde ER. Ovarian ageing and postponement of childbearing. Maturitas. 1998;30:103–4. [PubMed] [Google Scholar]
- 28.Sutherland AM. The changing pattern of tuberculosis of the female genital tract. A thirty year survey. Arch Gynecol. 1983;234:95–101. doi: 10.1007/BF00207681. [DOI] [PubMed] [Google Scholar]
- 29.Dam P, Shirazee HH, Goswami SK, Ghosh S, Ganesh A, Chaudhury K, et al. Role of latent genital tuberculosis in repeated IVF failure in the Indian clinical setting. Gynecol Obstet Invest. 2006;61:223–7. doi: 10.1159/000091498. [DOI] [PubMed] [Google Scholar]
- 30.Sangi-Haghpeykar H, Poindexter AN., 3rd Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85:983–92. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- 31.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. doi: 10.1016/s0889-8545(02)00050-5. vii. [DOI] [PubMed] [Google Scholar]
- 32.Urman B, Yakin K. DHEA for poor responders: Can treatment be justified in the absence of evidence? Reprod Biomed Online. 2012;25:103–7. doi: 10.1016/j.rbmo.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Klinkert ER, Broekmans FJ, Looman CW, Te Velde ER. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil Steril. 2004;81:1247–53. doi: 10.1016/j.fertnstert.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24:629–34. doi: 10.1007/s10815-007-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: Direct evidence from preimplantation genetic screening (PGS) Reprod Biol Endocrinol. 2010;8:140. doi: 10.1186/1477-7827-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–40. doi: 10.1093/humrep/der464. [DOI] [PubMed] [Google Scholar]
- 37.Weissman A, Horowitz E, Ravhon A, Golan A, Levran D. Dehydroepiandrosterone supplementation increases baseline follicular phase progesterone levels. Gynecol Endocrinol. 2011;27:1014–7. doi: 10.3109/09513590.2011.569611. [DOI] [PubMed] [Google Scholar]
- 38.Sönmezer M, Cil AP, Oktay K. Ongoing pregnancies from early retrieval of prematurely developing antral follicles after DHEA supplementation. Reprod Biomed Online. 2009;19:816–9. doi: 10.1016/j.rbmo.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Yeung TW, Chai J, Li RH, Lee VC, Ho PC, Ng EH. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil Steril. 2014;102:108–115.e1. doi: 10.1016/j.fertnstert.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 40.Gleicher N, Kim A, Weghofer A, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR) J Assist Reprod Genet. 2013;30:49–62. doi: 10.1007/s10815-012-9890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gleicher N, Barad DH. Misplaced obsession with prospectively randomized studies. Reprod Biomed Online. 2010;21:440–3. doi: 10.1016/j.rbmo.2010.06.042. [DOI] [PubMed] [Google Scholar]


