Abstract
CONTEXT:
Various components of follicular fluid are suggested as biochemical predictors of oocyte quality. Previous studies of follicular steroid hormone levels have shown disparate results when related with fertilization outcomes.
AIM:
The objective of the study was to relate the levels of steroid hormones of each individual follicle with oocyte maturation, fertilization results, embryo quality, and pregnancy rates.
SETTINGS AND DESIGN:
Prospective cohort study in a university hospital.
METHODS:
In 31 patients, who underwent intracytoplasmic sperm injection, it was performed an ultrasound guided aspiration of follicular fluid of the first two mature follicles from each ovary. Follicular levels of estradiol, progesterone, testosterone, and dehydroepiandrosterone sulfate were measured by chemiluminescent immunoassay.
STATISTICAL ANALYSIS:
Generalized estimating equation model.
RESULTS:
In follicular fluids with mature oocyte presence, in normal as well as in failed fertilization, there was a positive correlation between follicular testosterone and progesterone (r = 0.794, P = 0.0001 and r = 0.829, P = 0.0001). Progesterone levels were higher in cases of normal fertilization compared to failed fertilization (P = 0.003). B quality embryos came from oocytes immersed in follicular fluids with higher estradiol values and higher estradiol/progesterone and estradiol/testosterone ratios than those of C quality (P = 0.01; P = 0.0009; P = 0.001). Estradiol levels were higher in patients who achieved pregnancy (P = 0.02).
CONCLUSION:
The analysis of follicular hormone composition could be considered as an additional tool in oocyte selection.
KEY WORDS: Fertilisation, follicular fluid, oocyte maturation, pregnancy, steroid
INTRODUCTION
Currently, assisted reproductive techniques seek to achieve a cohort of oocytes of good quality, relegating to a secondary position the objective of achieving the largest possible number of oocytes.[1]
The levels of serum estradiol and follicle size are routine parameters used to monitor follicular development and oocyte maturation.[2] However, each cycle of ovarian stimulation becomes a sequence of obstacles that must be overcome to achieve the ultimate goal, gestation. Not all oocytes from follicles considered optimal based on the indirect parameters mentioned will be mature; of oocytes with nuclear maturation only some experience normal fertilization and of these, not all will generate embryos able to evolve; moreover, embryos from the same patient may be of different quality and finally, not all embryos will result in a pregnancy.
In this situation, it is a priority to find new factors that help optimize the entire process. Various components of follicular fluid, medium in which the oocyte is immersed, are suggested as biochemical predictors of oocyte quality, with subsequent potential for proper fertilization and embryo development.[3] Among these components, steroid hormones have emerged as important elements in reproductive outcomes.
To track each oocyte, it is essential to use intracytoplasmic sperm injection (ICSI) that allows, starting immediately after collection, assessment of nuclear maturation, and the specific results of fertilization and embryo development. Most studies relating steroid hormones with reproductive outcomes use in vitro fertilization as a laboratory technique and thus are limited in their results.[4,5,6,7] Furthermore, in case of sperm injection, fertilization results do not clearly depend on seminal parameters, probably due to the strict selection of a reduced number of sperms, oocyte quality being the critical factor.[8,9]
Higher levels of estradiol and progesterone were found in follicles from which oocytes with higher fertilization rates were obtained.[10,11,12] These observations, however, have not been confirmed by other studies.[13,14] The embryo quality was not related to levels of follicular estradiol and progesterone.[13] Regarding pregnancy rates, both elevated estradiol and progesterone levels in follicular fluid were associated with an increased chance of pregnancy.[3,12,15]
Regarding follicular androgens, some authors find no association between testosterone levels and cases of normal fertilization.[14] However, lower testosterone levels have been found in follicles which produce an oocyte that degenerates as opposed to cases in which the oocyte is fertilized.[10]
The objective of the study is to relate the steroid hormone levels (estradiol, progesterone, testosterone, dehydroepiandrosterone sulfate (DHEAS) and estradiol/progesterone and estradiol/testosterone ratios) of each individual follicle with oocyte maturation, fertilization result, embryo quality, and pregnancy rates.
METHODS
Patient selection, ovarian stimulation cycle, and analysis of hormones in follicular fluid
Included in the study were 34 patients aged ≤38, with a body mass index <30, and tubal infertility, unknown origin infertility or male infertility.
Causes of infertility with ovarian component of any kind (polycystic ovarian syndrome, endometriosis or diminished ovarian reserve) were excluded.
Eligible subjects, following prior informed consent and Ethics Committee approval, were recruited consecutively from February 2011 to February 2013. All procedures were conducted under the ethical standards of the Helsinki Declaration of 1975 as revised in 2000.
The stimulation protocol used was a long protocol with hypothalamic gonadotropin-releasing hormone agonists and recombinant follicle stimulating hormone (rFSH) exclusively.
Three patients were excluded due to the need to add human menopausal gonadotropin (HMG). The criterion for human chorionic gonadotropin (HCG) administration (250 mcg, subcutaneous) was the presence of two or more follicles >18 mm in diameter associated with consistent serum estradiol levels. The luteal phase was supported with micronized vaginal progesterone (200 mg/12 h).
Follicular growth was monitored by transvaginal ultrasound (HD3 Philips, Eindhoven, Holland). At 36 h after administration of HCG, we performed US-guided follicular aspiration of the oocyte-corona-cumulus complexes and follicular fluid.
Follicular puncture required a puncture needle attached to the ultrasound transducer by a guide, a continuously adjustable vacuum pump (Aspirator Labotect 4014, Gottingen, Germany) and a heat block set at 37°C. The first and second follicle of each ovary, selected by size (18-21 mm) and regular ultrasound contour, was individually aspirated slowly until completely collapsing. Intensely hematic follicular fluids were excluded. Finally, we had a total of 73 follicular fluid samples from 31 patients; one to four follicles per patient.
The follicular fluid sample was cleared by centrifugation and stored at −70°C for later analysis.
The volume of fluid aspirated per individual follicle was correlated with follicular size as described by Wittmaack et al.[16]
Chemiluminescent microparticle immunoassay was used as a method for measuring and hormone quantification of estradiol (ng/mL), progesterone (μg/mL), testosterone (ng/dL), and DHEA-S (μg/dL). Estradiol/progesterone and estradiol/testosterone ratios were also measured.
Estradiol, testosterone, and progesterone were measured on an Architect i2000 (Abbott Diagnostics, Mandaluyong City, Philippines). DHEA-S was measured on an IMMULITE® 2000 immunoassay system (Siemens Healthcare, Erlangen, Germany). The intra-assay variation coefficient was <15%.
The samples were diluted for determination of estradiol (1:1000) and progesterone (1:1000). The measurement of testosterone and DHEAS did not require dilution due to its similarity to serum levels.
Fertilization assessment and embryo selection
The preestablished fertilization method was ICSI. The oocytes were separated from cumulus cells that surround them, and meiotic maturation was assessed by the same biologist. Mature oocytes (metaphase II) were selected by the presence of the first polar body. The immature (metaphase I) or those in germinal vesicle stage and degenerates were rejected for ICSI.
Fertilization results were assessed at 19-21 h after ICSI. Fertilization was considered normal by the presence of two pronuclei and two polar bodies. Fertilization failure (no pronuclei) and abnormal fertilization (one or three pronuclei) were grouped as fertilization failure.
The quality of the embryos coming from oocytes that had been immersed in the studied follicular fluids was established as rated by Association for Studying the Biology of Reproduction.[17] Embryos were divided into four decreasing grades: A, B, C, D, which were assigned at day 3 based on the number of cells. We also evaluated the percentage of fragmentation, the presence of vacuoles, multi-nucleation, and similarity between the cells, the pronuclei, and the presence of acytoplasmic ring at day 3. According to these characteristics, allocation was modified downward.
One to two embryos with the highest number of blastomeres and the best morphological grade were selected for fresh embryo transfer per patient. Remaining embryos were cryopreservated for future transfers. In each patient, at least one embryo proceeding from an oocyte whose follicular liquid had been analyzed was transferred either fresh or frozen.
We analyzed the level of serum beta-HCG after 14 days of embryo transfer, making the diagnosis of pregnancy after transvaginal ultrasound and following its evolution by reviewing medical records and subsequent telephone calls to the patients at the end of the cycle.
Statistical analysis
The correlation between continuous quantitative variables was studied by the Pearson correlation coefficient.
Performing more than one follicular puncture per patient required that a statistical analysis of repeated measures be carried out. Repeated measures were adjusted with a generalized estimating equation model with normal marginal distribution, with identity link and interchangeable correlation structure.
Statistical significance was established for values of P < 0.05, considering all bilateral statistical tests. Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The mean volume aspirated was 5.02 ± 1.03 cc with an average follicle size of 19.05 ± 1.03 mm. Regarding the final result of the cycle in each patient, the mean number of punctured complexes was 9.03 ± 4.10, with a total of 8.90 ± 4.18 oocytes. 7.81 ± 3.66 of these were mature ones and 5.29 ± 3.43 fertilized normally, being obtained a total average of 4.39 ± 2.90 embryos per patient.
In the samples analyzed of follicular fluid, 59 (81%: 59/73) contained an oocyte in metaphase II while the remaining 19% (14/73) did not. No oocytes were obtained in metaphase I.
Thirty-eight of 59 oocytes underwent normal fertilization while the remaining 21 suffered fertilization failure. Of the embryos obtained, 21 were transferred, and 10 were cryopreserved.
Eleven of 31 (35.5%) patients achieved a pregnancy. Four of the 11 (36.4%) ended with abortion in the first trimester and the remaining 7 (63.6%) achieved a pregnancy whose outcome was a healthy live birth. The rate of live newborns at home was 22.5% (7/31).
In case of follicular fluid without oocyte, there was a positive correlation between testosterone and estradiol (r = 0.552, P = 0.041) and between testosterone and DHEAS (r = 0.644, P = 0.013) [Table 1].
Table 1.
Correlation among follicular fluid hormone levels according to the presence of oocyte and type of fertilization
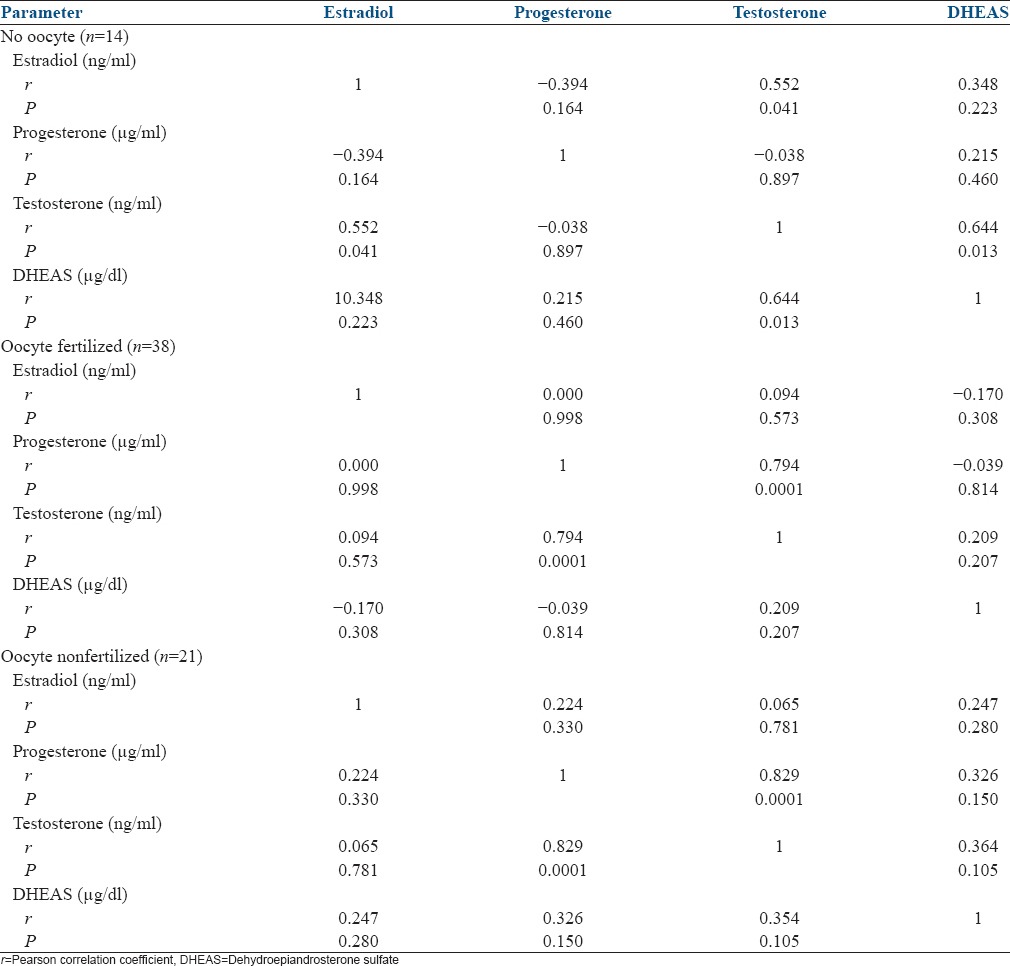
In the follicular fluid with mature oocyte presence, in normal as well as failed fertilization, there was a positive correlation between follicular testosterone and progesterone (r = 0.794, P = 0.0001 and r = 0.829, P = 0.0001) [Table 1].
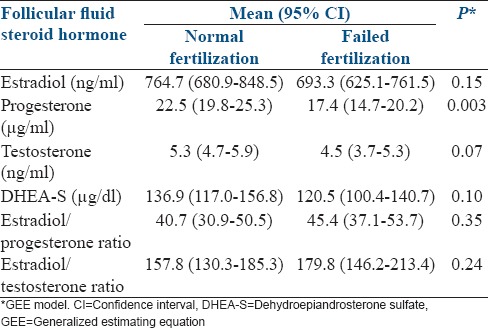
The levels of estradiol, progesterone, testosterone, and DHEAS were higher in cases of normal fertilization than in cases of failed fertilization. The association reached statistical significance in the case of progesterone (P = 0.003) [Table 2].
Table 2.
Relationship between follicular fluid hormone levels and type of fertilization
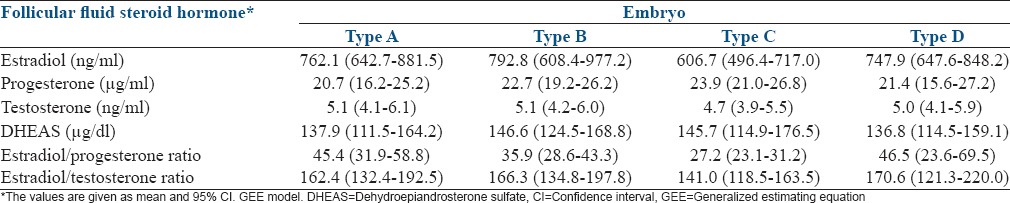
The relationship between hormone levels in follicular fluid and embryo quality is described in Table 3. In estradiol figures, there were statistically significant differences between embryo grades B and C and between grades C and D (P = 0.01; P = 0.03). In the case of the ratios, there were statistically significant differences between embryo qualities A and C, and B and C, in the estradiol/progesterone ratio (P = 0.003; P = 0.0009) and between qualities B and C in the case of estradiol/testosterone ratio (P = 0.001).
Table 3.
Relationship between follicular fluid hormone levels and embryo quality
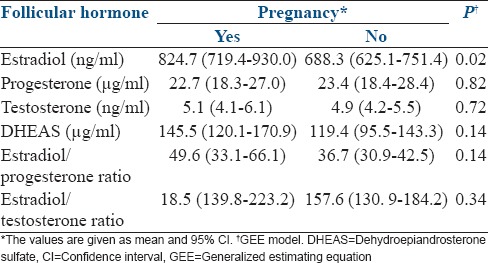
Estradiol levels were higher in patients who achieved a pregnancy (P = 0.02) [Table 4].
Table 4.
Relationship between follicular fluid hormone levels and pregnancy
DISCUSSION
The purpose of the study is to assess the relationship of hormone levels in follicular fluid with different reproductive outcomes. That is why it has been necessary to conduct it under the most basal conditions possible, trying to avoid possible interferences in hormone levels. We included patients with good quantitative ovarian reserve, conducted ovarian stimulation with only rFSH and causes of infertility with ovarian component of any kind were excluded. Several authors employ stimulation protocols with HMG, thus shifting away from the ideal baseline.[3,7]
The first and second follicles of each ovary were included as samples. This was intended to avoid loss of a potential oocyte that could have remained in the system with recovery of liquid from the first punctured follicle, assuming the possibility of an oocyte corresponding to the first follicle being classified as the second. An ideal study would aspirate each follicle individually with multiple vaginal punctures, thus being limited in clinical practice. Individual puncture of every patient follicle is limited in clinical practice by the need of multiple punctures to assure an oocyte and follicular content correct and complete from each individual follicle. Follicular selection was carried out using follicular size, assuming that the optimum size for oocyte maturation is between 14 and 21 mm, being those with ≥ 18 mm the ones with better oocyte maturation rates and fecundation capacity.[6,18] Thus, these 2 follicles of each ovary considered optimums were the ones included as patient's samples.
The potential increase in the variability of the variation coefficients of the hormones measured could pose a limitation. The need for serial dilutions in the case of follicular estradiol and progesterone could help elevate these coefficients, which could attenuate the results.[10] A linearity survey was conducted to verify the necessary dilution and a repeatability study was performed to validate tests used in follicular fluid use.
There are multiple studies that address the hormone issue and its relationship with the various outcomes of assisted reproduction, but none covers the various stages of the response to the stimulation cycle, from its normal or failed fertilization until pregnancy through oocyte and embryo quality.
By limiting the study to ICSI, the evaluation of nuclear maturation in a short period of time from its recovery is allowed. This usually can only be made in ICSI cases because in conventional IVF meiotic evaluation is usually carried out in the following day and oocytes may have completed its maturation when cultivated.[3]
The nuclear component of oocyte maturation is easily identifiable; however, the cytoplasmic component is difficult to assess. Failures in fertilization and subsequent embryo development have arisen as a result of defects related to cytoplasmic maturation.[19] In cases with the presence of metaphase-II oocytes, a positive correlation between follicular testosterone and progesterone was found.
In the study, fertilization was classified as normal and failed fertilization, as the two valid options from a practical standpoint. The cases of normal fertilization came from oocytes immersed in follicular fluid with higher levels of progesterone than the cases of failed fertilization. These results agree with those obtained by some authors.[10,11] However, other authors found no difference in follicular levels of estradiol, progesterone, and testosterone between fertilized or unfertilized oocytes, but described a tendency toward lower levels of these hormones in fertilized oocytes, data completely opposite from ours.[14] In our results, there was a trend to higher testosterone levels in favor of normal fertilization, without reaching statistical significance (P = 0.07), but in line with the positive correlation obtained between follicular progesterone and testosterone in cases of oocytes with normal fertilization. Lamb et al. did not report differences in testosterone levels among their cases with normal fertilization compared with cases of unsuccessful fertilization.[10] Wen et al. describe a positive correlation between progesterone and testosterone only in cases of failed fertilization.[14]
Serving as a basis for steroidogenesis, progesterone is the precursor of androgens, and these are an essential substrate for the production of estrogen. Androgens could be introduced as an additional element in the theory of two cells-two gonadotropins in follicular estradiol synthesis.[20] The conversion of androgens to estrogens is favored by FSH, so one would think that follicular fluid with higher androgen levels would also have increased levels of estrogen, having more substrate from which to obtain it. The predominant follicular androgenic environment can lead to follicular atresia although a certain amount of follicular androgens are necessary for optimal follicle growth.[21]
Few studies assess variables related to embryos correlated with follicular steroid hormones. Assuming that other factors may influence the embryo quality, it has been tried to evaluate the implication that steroid follicular hormones could have in this quality, insofar these hormones may determine oocyte quality. Asimakopoulos et al. did not find an association between hormone levels of follicular estradiol or progesterone and embryo quality.[13]
Very possibly determined by the highest levels of estradiol in both cases, the estradiol/progesterone ratio was higher in A and B quality than in C quality embryos, and estradiol/testosterone ratio was higher in B quality embryos than in those of C quality. Considering our results, it is not possible to establish a gradual relation between follicular steroid hormones and morphological grades of embryo quality. Estradiol figures did not decrease linearly with the embryo grades. Our results showed that follicular estradiol figures would be discriminatory mainly in intermediate embryo qualities (B and C) but considering gestation as the ultimate goal, in examining overall pregnancy rates, there were higher estradiol levels in follicular fluid in cases of pregnancy, as reported by Mendoza et al.[3]
Although little is known about DHEA and DHEAS levels in follicular fluid and how they relate to the results of fertilization and pregnancy, only a few studies address the issue. DHEA is a precursor used by the granulosa cells in the production of estrogens and androgens.[22] A recent meta-analysis has related androgen adjuvants (testosterone, DHEA) with higher pregnancy rates.[23] The determination of DHEAS could be seen as an indirect measurement of follicular DHEA considering the possibility of cross detection of DHEA and DHEAS in the results presented. DHEAS levels have not appeared to be relevant in various reproductive outcomes assessed.
It could be concluded that a follicular environment rich in estradiol, progesterone, and testosterone is key to good oocyte development. High rates of progesterone and to a lesser extent, testosterone would be crucial for determining good oocyte quality and key for normal fertilization, as well as an essential step for success in assisted reproduction. Among the oocytes immersed in a follicular environment rich in progesterone and testosterone, those with higher levels of estradiol obtained higher pregnancy rates. In the future, analysis of follicular hormone composition could be considered as an additional tool in oocyte selection.
ACKNOWLEDGMENT
To the staff of the Reproduction, Clinical Analyzes and Biostatistics Departments at La Paz University Hospital for their invaluable assistance in conducting this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 2.Mikkelsen AL, Smith S, Lindenberg S. Impact of oestradiol and inhibin A concentrations on pregnancy rate in in-vitro oocyte maturation. Hum Reprod. 2000;15:1685–90. doi: 10.1093/humrep/15.8.1685. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17:1017–22. doi: 10.1093/humrep/17.4.1017. [DOI] [PubMed] [Google Scholar]
- 4.Tarlatzis BC, Pazaitou K, Bili H, Bontis J, Papadimas J, Lagos S, et al. Growth hormone, oestradiol, progesterone and testosterone concentrations in follicular fluid after ovarian stimulation with various regimes for assisted reproduction. Hum Reprod. 1993;8:1612–6. doi: 10.1093/oxfordjournals.humrep.a137900. [DOI] [PubMed] [Google Scholar]
- 5.Andersen CY. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J Clin Endocrinol Metab. 1993;77:1227–34. doi: 10.1210/jcem.77.5.7521343. [DOI] [PubMed] [Google Scholar]
- 6.Rosen MP, Shen S, Dobson AT, Rinaudo PF, McCulloch CE, Cedars MI. A quantitative assessment of follicle size on oocyte developmental competence. Fertil Steril. 2008;90:684–90. doi: 10.1016/j.fertnstert.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara T, Lambert-Messerlian G, Sidis Y, Leykin L, Isaacson K, Toth T, et al. Analysis of follicular fluid hormone concentrations and granulosa cell mRNA levels for the inhibin-activin-follistatin system: Relation to oocyte and embryo characteristics. Fertil Steril. 2000;74:348–55. doi: 10.1016/s0015-0282(00)00652-x. [DOI] [PubMed] [Google Scholar]
- 8.Göker EN, Sendag F, Levi R, Sendag H, Tavmergen E. Comparison of the ICSI outcome of ejaculated sperm with normal, abnormal parameters and testicular sperm. Eur J Obstet Gynecol Reprod Biol. 2002;104:129–36. doi: 10.1016/s0301-2115(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Hassan D, Koester F, Shoepper B, Schultze-Mosgau A, Asimakopoulos B, Diedrich K, et al. Comet assay of cumulus cells and spermatozoa DNA status, and the relationship to oocyte fertilization and embryo quality following ICSI. Reprod Biomed Online. 2006;12:447–52. doi: 10.1016/s1472-6483(10)61997-9. [DOI] [PubMed] [Google Scholar]
- 10.Lamb JD, Zamah AM, Shen S, McCulloch C, Cedars MI, Rosen MP. Follicular fluid steroid hormone levels are associated with fertilization outcome after intracytoplasmic sperm injection. Fertil Steril. 2010;94:952–7. doi: 10.1016/j.fertnstert.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza C, Cremades N, Ruiz-Requena E, Martinez F, Ortega E, Bernabeu S, et al. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum Reprod. 1999;14:628–35. doi: 10.1093/humrep/14.3.628. [DOI] [PubMed] [Google Scholar]
- 12.Artini PG, Battaglia C, D’Ambrogio G, Barreca A, Droghini F, Volpe A, et al. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Hum Reprod. 1994;9:902–6. doi: 10.1093/oxfordjournals.humrep.a138614. [DOI] [PubMed] [Google Scholar]
- 13.Asimakopoulos B, Abu-Hassan D, Metzen E, Al-Hasani S, Diedrich K, Nikolettos N. The levels of steroid hormones and cytokines in individual follicles are not associated with the fertilization outcome after intracytoplasmic sperm injection. Fertil Steril. 2008;90:60–4. doi: 10.1016/j.fertnstert.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 14.Wen X, Li D, Tozer AJ, Docherty SM, Iles RK. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod Biol Endocrinol. 2010;8:117. doi: 10.1186/1477-7827-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enien WM, el Sahwy S, Harris CP, Seif MW, Elstein M. Human chorionic gonadotrophin and steroid concentrations in follicular fluid: The relationship to oocyte maturity and fertilization rates in stimulated and natural in-vitro fertilization cycles. Hum Reprod. 1995;10:2840–4. doi: 10.1093/oxfordjournals.humrep.a135804. [DOI] [PubMed] [Google Scholar]
- 16.Wittmaack FM, Kreger DO, Blasco L, Tureck RW, Mastroianni L, Jr, Lessey BA. Effect of follicular size on oocyte retrieval, fertilization, cleavage, and embryo quality in in vitro fertilization cycles: A 6-year data collection. Fertil Steril. 1994;62:1205–10. doi: 10.1016/s0015-0282(16)57186-6. [DOI] [PubMed] [Google Scholar]
- 17.Ardoy M, Calderón G. Morphological evaluation criteria of oocytes, early embryos and human blastocists. Ed: ASEBIR. 2007 [Google Scholar]
- 18.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: Relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–7. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- 19.Nasr-Esfahani MH, Razavi S, Mardani M, Shirazi R, Javanmardi S. Effects of failed oocyte activation and sperm protamine deficiency on fertilization post-ICSI. Reprod Biomed Online. 2007;14:422–9. doi: 10.1016/s1472-6483(10)60888-7. [DOI] [PubMed] [Google Scholar]
- 20.Hugues JN, Durnerin IC. Impact of androgens on fertility-physiological, clinical and therapeutic aspects. Reprod Biomed Online. 2005;11:570–80. doi: 10.1016/s1472-6483(10)61165-0. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Møllgård K, Wreford Andersen E, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17:63–70. doi: 10.1093/molehr/gaq073. [DOI] [PubMed] [Google Scholar]
- 22.Arlt W, Justl HG, Callies F, Reincke M, Hübler D, Oettel M, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: Pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928–34. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- 23.Sunkara SK, Coomarasamy A. Androgen pretreatment in poor responders undergoing controlled ovarian stimulation and in vitro fertilization treatment. Fertil Steril. 2011;95:e73–4. doi: 10.1016/j.fertnstert.2011.04.083. [DOI] [PubMed] [Google Scholar]


