Abstract
AIM:
The aim of this study was to analyze human seminal plasma proteins in association with male fertility status using the proteomic mass spectrometry technology Surface-Enhanced Laser Desorption Ionization Time-of-Flight (SELDI-TOF-MS).
MATERIALS AND METHODS:
Semen analysis was performed using conventional methods. Protein profiles of the seminal plasma were obtained by SELDI-TOF mass spectrometry over a strong anion exchanger, ProteinChip® Q10 array.
RESULTS AND CONCLUSION:
We found statistically significant differences in motility and sperm count between fertile and infertile men. In addition, we observed ten seminal proteins that are significantly up-regulated in the infertile group. In conclusion, comparison of seminal plasma proteome in fertile and infertile men provides new aspects in the physiology of male fertility and might help in identifying novel markers of male infertility.
KEY WORDS: Fertility, proteomics, seminal plasma, surface-enhanced laser desorption ionization time-of-flight
INTRODUCTION
Semen parameters[1] and functional tests[2,3,4] of sperm cells are evaluated when assessing male fertility status. Nonetheless, the current low reference values for seminal parameters suggested by the World Health Organization (WHO)[5] – misdiagnose almost the entire infertile male population as having normal seminal characteristics, given that normal parameters decrease year after year.[6] Thus, it is necessary to evaluate new seminal parameters to better distinguish fertile and infertile men.
Human seminal plasma is derived from seminal vesicles, prostate, bulbo urethral, and urethral glands. The semen carries the spermatozoa out of the male urethra, at the time of ejaculation, to the female reproductive tract.[7] Human semen contains important proteins for sperm capacitation,[8] regulation of immune responses in the female reproductive tract,[9] and embryo implantation.[10]
Only a few studies have analyzed the proteome in seminal plasma.[11,12,13,14,15,16] I.e. Pilch and Mann reported 923 proteins in human seminal plasma from healthy men by mass spectrometry (MS).[14] Recently, approximately 2300 unique proteins have been identified by MS in semen specimens from fertile control and postvasectomy males.[11] In seminal plasma, 59 putative biomarkers of prostatitis have been recognized that need further validation for diagnosis and monitoring.[12] In addition, Milardi et al.[13] found 919 proteins in the seminal plasma from a group of fertile men and showed that semenogelin I and II are the main proteins in human semen. These proteins were previously reported to play an important role for fertility.[17] However, many seminal plasma proteins are poorly known.[18]
The number of investigations on novel biomarkers for diagnosis, prognosis, or treatment of infertility and urogenital diseases is currently increasing. However, information about the semen proteome is scarce. This study aims to analyze human seminal plasma proteins in association with the male fertility status using the proteomic technology Surface-Enhanced Laser Desorption Ionization Time-of-Flight mass spectrometry (SELDI-TOF-MS).
MATERIALS AND METHODS
Semen samples were obtained from a total of 16 individuals. Nine men had fertility alterations (the female partner did not conceive 2.6 years -1 to 8 years) with no history of urogenital diseases or surgeries and seven healthy men had established fertility. This latter group included men whose partners had at least one pregnancy, whose last child was conceived a maximum of 1 year before the present study, or whose partner was pregnant. Infertility female factor was ruled out. All individuals answered a questionnaire and signed an informed consent prepared according to the Colombian Government Legislation (Resolution 008430-1993). The study was approved by Ethics Review Committee of the Universidad de Antioquia.
After complete semen liquefaction, semen analyses (volume, progressive motility, and viability) were performed according to the WHO guidelines for the examination of human semen.[5,19] The motility was determined using direct observation of an aliquot of the sample at ×40, and graded as follows: I = progressive motility; II = nonprogressive motility and III = immotile. Sperm concentration was determined by using a Makler chamber (Sefi-Medical Instruments, Haifa, Israel)[20] and morphology was evaluated according to the Tygerberg strict criteria.[21] In addition, the teratozoospermia index was calculated as the number of abnormalities present per abnormal spermatozoon. The respective numbers of abnormalities in head, neck/midpiece and tail defects or presence of cytoplasmic droplets are then added together and the resultant sum is divided by the number of abnormal spermatozoa.[5,19]
The semen samples were centrifuged at 2000 g during 15 min to separate seminal plasma. Prior to seminal protein evaluation, the seminal plasma was centrifuged again at 14,000 g for 10 min at 4°C. The semen proteins were measured using NanoDrop® and after diluting semen samples to 0.5 mg/mL in binding buffer (0.1 M Tris-HCl, 0.02% Tween 20%, pH = 9.0); the protein profile of the semen was obtained by SELDI-TOF-MS on-spot over a strong anion exchanger ProteinChip® Q10 array (Ciphergen, Biosystems, CA, USA). All SELDI-TOF analyses have been performed at the Institute for Human Genetics at the University Hospital Jena. The spots were equilibrated three times with 5 μl binding buffer for 5 min and then 5 μl of each sample were added. After incubation for 90 min in a humid chamber, each spot was washed twice with 5 μl binding buffer and twice with 5 μl distilled water. After air drying, the spots were coated with 0.5 μl of energy absorbing matrix (EAM: 20 mg/ml sinapinic acid in 50% acetonitrile and 0.5% trifluoroacetic acid), air dried for 5 min in the dark, and then another 0.5 μl of EAM was applied using the same procedure.
The protein chip arrays were analyzed using the ProteinChip SELDI System Series 4000 (Ciphergen). Arrays were exposed to 2200 nJ laser energy to detect proteins in the range of 2000-20000 Da, as well as to 3500 nJ for proteins in the range of 20,000-200.000 Da below a pressure of 150 μPa.
For the purposes of protein identification, searchs in the semen proteins databases[14,22,23] UniProt, IPI and NCBI were performed by using the m/z value obtained for each peak with differential expression between the groups.
Statistical analysis
The seminal parameters in fertile and infertile men were compared using the Mann-Whitney test (a nonparametric, independent sample median test). Statistical significance was defined as P < 0.05, and the statistical analysis was processed with Graphpad Prism 5.0.
Spectra were analyzed using the Ciphergen Express Client 3.1 software (Ciphergen) to differentiate seminal protein expression from fertile and infertile men. Receiver operating characteristic (ROC) curves were performed to determine the area under the curve, and a cut-off value was selected for optimal sensitivity and specificity.
RESULTS
Analysis of seminal parameters
Statistically significant decreased in sperm progressive [Figure 1D], and sperm count in the infertile group [Figure 1f] was observed. The other semen parameters were not significantly different between fertile and infertile groups (P > 0.05; Figure 1).
Figure 1.
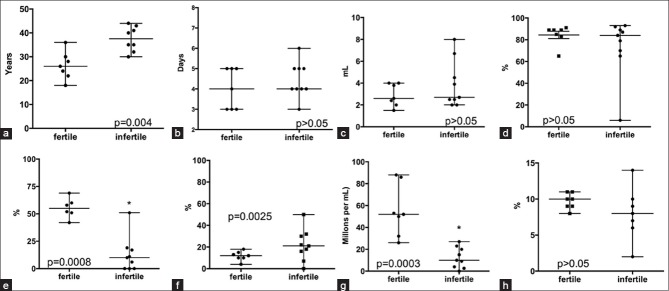
Seminal parameters in samples from fertile (n = 7) and infertile (n = 9) males. a: Years; b: Sexual abstinence; c: Volume of ejaculate; d: Sperm vitality; e: Sperm motility I, progressive motility; f: Sperm motility II, non-progressive; g: Sperm count, and h: Normal morphology. Mann-Whitney test *P < 0.05
Expression profile of seminal proteins
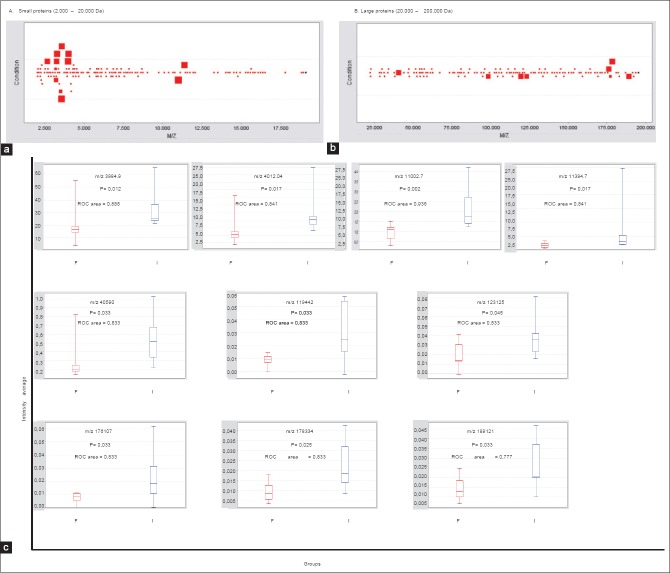
Ten seminal proteins were detected with a statistical difference and a ROC value > 0.77 between the fertile and infertile groups: There were four peaks in the low mass range corresponding to small proteins (2000–20,000 Da) and six peaks in the high mass range corresponding to large proteins (20,000–2,00,000 Da) which were statistically (P < 0.05) differentially expressed in seminal samples from fertile and infertile individuals [Figure 2a-c]. In some cases, due to the similar molecular weight we tentatively grouped these as being likely the same protein.
Figure 2.
P value plot of proteins in seminal plasma. Each square stands for a differentially expressed protein (a and b). Large squares show the proteins with a differential expression (P < 0.05) between semen samples from fertile and infertile men. Squares around similar molecular weight were grouped (ten proteins). The behavior of these proteins in the two groups of patients is shown in the box and whisker graph. F: Fertile men; I: infertile men (c). m/z: Mass to charge ratio
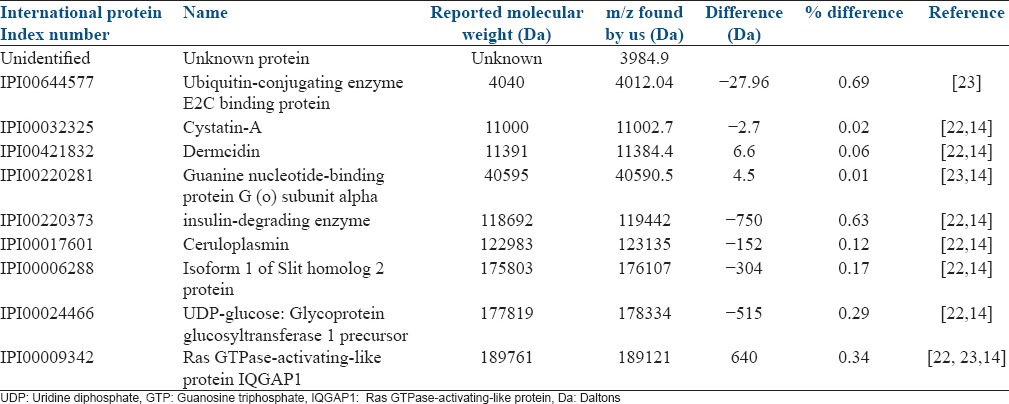
The differential expression of proteins was visualized by using a heatmap based on sample group and mass to charge (m/z) ratio. This shows the relative up- and down-regulated expression of proteins in each individual seminal plasma sample. All differentially expressed proteins were up-regulated in the seminal plasma from the infertile group [Figure 3a, b]. Using the previously published database for seminal proteins,[14,23] we were able to propose the potential identity of some proteins [Table 1].
Figure 3.
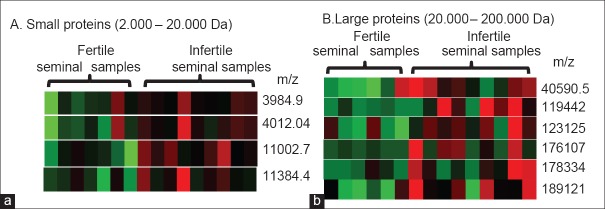
The heat map represents relative expression of proteins with significant differences in fertile and infertile seminal samples. Red represents over-expression, green represents under expression
Table 1.
Approach to protein identification
DISCUSSION
Male infertility is generally diagnosed in terms of concentration, motility, and morphology of spermatozoa. Often, these parameters do not accurately reflect the real capacity of the semen for fecundation.[2] We found decreased progressive motility and sperm count in semen samples from infertile males. However, all further semen parameters were classified as normal following the reference values of the WHO 2010.[5,6] The application of proteomic techniques in seminal evaluation might be useful to classify such individuals, whose spermatozoa fulfill the current parameters of normality but have fertility problems, and eventually further define the etiology of infertility. The advantage of SELDI-TOF-MS is its ability to provide rapid protein expression profiles from complex biological samples like seminal plasma, with a minimal requirement for purification and separation of protein prior to mass spectrometry. The method is not applicable for routine diagnostics because of low accessibility to SELDI-TOF spectrometers for IVF centers.
In our analysis several proteins were differentially expressed in the semen of fertile and infertile males. Although we did not perform the final identification of these proteins, the databank search provided several candidates which will be discussed in the following section, provided our suggestion corresponds to the reality, which we believe is highly likely to be the case. Smaller molecules in the peptide range may also be important for activation of maternal immunoprotective responses[24] but were not investigated in this study.
We found the ubiquitin-activating enzymes (E2) increased in semen from infertile males. This protein is involved in the ubiquitin transfer to targets in the proteasome system. This increased enzyme in infertile patients might be involved in the ubiquitin transfer to the surface of defective sperm during epididymal passage[25] or ejaculation. Ubiquitin was detected in human epididymal cells[25] and seminal plasma[26] and a relationship with sperm quality was proposed.[27,28] In humans and other mammalian species, male infertility has been associated with a higher content of sperm surface proteins that cross-react with antiubiquitin antibodies[28] and bind predominantly to the surface of defective spermatozoa.[27]
We have demonstrated in the infertile group an increase in the concentration of a protein with a molecular weight of 11002.7 Da which may be identical with the cysteine protease inhibitor cystatin A. Cystatins are endogenous inhibitors of cysteine proteinases. They inhibit cysteine proteinases such as the mammalian cathepsins B, H, L, and S. Previously, it has been shown that cystatin A is expressed in the basal epithelial cells of normal prostate and that this expression disappears in prostatic carcinoma.[29] However, the function of this protein remains unclear.
In the group of infertile patients, we found an increase of a protein with the m/z value identical with that of ceruloplasmin. This protein is a serum ferroxidase that contains over 95% of the copper found in plasma. In vitro ceruloplasmin is capable of catalyzing the oxidation of a number of different substrates. Ceruloplasmin is a member of the multicopper oxidase family, an evolutionarily conserved group of proteins that utilize copper to couple substrate oxidation with the four-electron reduction of oxygen to water.[30,31] Previously, ceruloplasmin has been measured in seminal fluid and circulating blood of normal and vasectomized subjects,[32] but not in fertile versus infertile individuals.
All the other proteins such as the alpha-subunit of the guanine nucleotide-binding protein G, the isoform 1 of slit homolog 2 protein and UDP-glucose glycoprotein glucosyltransferase 1 precursor were predominantly up-regulated in the semen of infertile men. Dermcidin is an antimicrobial agent secreted and transported via sweat to the epidermal surface.[33] The insulin degrading enzyme (IDE), an ubiquitously expressed zinc-metalloprotease that degrades several pathophysiologically significant extracellular substrates, is the major enzyme responsible for insulin degradation in vitro.[34] The Ras GTPase-activating-like protein IQGAP1 is an ubiquitously expressed protein involved in regulation of various cellular processes ranging from the organization of the actin cytoskeleton, transcription, and cellular adhesion to regulate the cell cycle.[35] The possible role of these proteins in human fertility is unknown.
Summarizing, the Seldi TOF proteomic analysis of seminal plasma proteins has yielded identification of differential expression in fertile and infertile individuals. These might indicate pathways of research to better understand the patophysiology of male fertility, and might help to identify novel markers of male infertility. Already, we believe that the presented candidates may serve as novel markers for infertility in men and may be recommended for analyses by using specific antibody-based methods in future double blind larger prospective studies.
ACKNOWLEDGEMENTS
AA and WCM were granted a research scholarship from Colciencias, Colombia. We also thank Prof. F.v.Eggeling, Prof. G. Chaouat and Prof. David A. Clark for helpful suggestions regarding the manuscript.
Footnotes
Source of Support: University of Antioquia (Estrategia de Sostenibilidad Universidad de Antioquia 2013-2014) and Boehringer Ingelheim
Conflict of Interest: None declared.
REFERENCES
- 1.de los Rios J, Cardona WD, Berdugo JA, Correa C, Arenas A, Olivera-Angel M, et al. Sperm parameters in 113 subjects after recent fatherhood did not correlate with WHO standards. Arch Esp Urol. 2004;57:147–52. [PubMed] [Google Scholar]
- 2.Cardona Maya WD, Berdugo Gutierrez JA, de los Rios J, Cadavid Jaramillo AP. Functional evaluation of sperm in Colombian fertile men. Arch Esp Urol. 2007;60:827–31. doi: 10.4321/s0004-06142007000700019. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Role of male factor in early recurrent embryo loss: Do antioxidants have any effect? Fertil Steril. 2009;92:565–71. doi: 10.1016/j.fertnstert.2008.07.1715. [DOI] [PubMed] [Google Scholar]
- 4.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril. 2010;94:1465–72. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 5.WHO. 5th ed. 2010. Laboratory manual for the examination and processing of human semen. [PubMed] [Google Scholar]
- 6.Cardona Maya W. World Health Organization manual for the processing of human semen-2010. Actas Urol Esp. 2010;34:577–8. [PubMed] [Google Scholar]
- 7.Risbridger GP, Taylor RA. Ch. 23 - Physiology of the male accessory sex structures: The prostate gland, seminal vesicles, and bulbourethral glands. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, et al., editors. Knobil and Neill's Physiology of Reproduction. 3rd ed. St Louis: Academic Press; 2006. pp. 1149–72. [Google Scholar]
- 8.De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11:205–14. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- 9.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology. 2006;66:2091–103. doi: 10.1016/j.theriogenology.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Batruch I, Lecker I, Kagedan D, Smith CR, Mullen BJ, Grober E, et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–53. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 12.Kagedan D, Lecker I, Batruch I, Smith C, Kaploun I, Lo K, et al. Characterization of the seminal plasma proteome in men with prostatitis by mass spectrometry. Clin Proteomics. 2012;9:2. doi: 10.1186/1559-0275-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milardi D, Grande G, Vincenzoni F, Messana I, Pontecorvi A, De Marinis L, et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil Steril. 2012;97:67–73. doi: 10.1016/j.fertnstert.2011.10.013. e61. [DOI] [PubMed] [Google Scholar]
- 14.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolland AD, Lavigne R, Dauly C, Calvel P, Kervarrec C, Freour T, et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod. 2013;28:199–209. doi: 10.1093/humrep/des360. [DOI] [PubMed] [Google Scholar]
- 16.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, Souza GH, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–8. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson M, Frohm B, Malm J. Binding of semenogelin I to intact human spermatozoa studied by flow cytometry and surface plasmon resonance. J Androl. 2010;31:560–5. doi: 10.2164/jandrol.109.008672. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: What role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Cambridge: Cambridge University Press; 1999. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. [Google Scholar]
- 20.Cardona-Maya W, Berdugo J, Cadavid A. Comparing the sperm concentration determined by the Makler and the Neubauer chambers. Actas Urol Esp. 2008;32:443–5. doi: 10.1016/s0210-4806(08)73860-9. [DOI] [PubMed] [Google Scholar]
- 21.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 22. [Last accessed on 2014 Mar 03]. Available from: http://proteome.biochem.mpg.de/seminal/
- 23.Wang J, Wang J, Zhang HR, Shi HJ, Ma D, Zhao HX, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11:484–91. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark DA, Rahmati M, Gohner C, Bensussan A, Markert UR, Chaouat G. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion-prone CBAxDBA/2 model. J Reprod Immunol. 2013;99:46–53. doi: 10.1016/j.jri.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Fraile B, Martin R, De Miguel MP, Arenas MI, Bethencourt FR, Peinado F, et al. Light and electron microscopic immunohistochemical localization of protein gene product 9.5 and ubiquitin immunoreactivities in the human epididymis and vas deferens. Biol Reprod. 1996;55:291–7. doi: 10.1095/biolreprod55.2.291. [DOI] [PubMed] [Google Scholar]
- 26.Lippert TH, Seeger H, Schieferstein G, Voelter W. Immunoreactive ubiquitin in human seminal plasma. J Androl. 1993;14:130–1. [PubMed] [Google Scholar]
- 27.Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci. 2001;114:1665–75. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- 28.Sutovsky P, Terada Y, Schatten G. Ubiquitin-based sperm assay for the diagnosis of male factor infertility. Hum Reprod. 2001;16:250–8. doi: 10.1093/humrep/16.2.250. [DOI] [PubMed] [Google Scholar]
- 29.Mirtti T, Alanen K, Kallajoki M, Rinne A, Soderstrom KO. Expression of cystatins, high molecular weight cytokeratin, and proliferation markers in prostatic adenocarcinoma and hyperplasia. Prostate. 2003;54:290–8. doi: 10.1002/pros.10196. [DOI] [PubMed] [Google Scholar]
- 30.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–58. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 31.Schittek B. The multiple facets of dermcidin in cell survival and host defense. J Innate Immun. 2012;4:349–60. doi: 10.1159/000336844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando C, Caldini AL, Barni T, Wood WG, Strasburger CJ, Natali A, et al. Ceruloplasmin and transferrin in human seminal plasma: Are they an index of seminiferous tubular function? Fertil Steril. 1985;43:290–4. doi: 10.1016/s0015-0282(16)48388-3. [DOI] [PubMed] [Google Scholar]
- 33.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 34.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: Progress and potential. Endocr Rev. 1998;19:608–24. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 35.Girouard J, Frenette G, Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl. 2011;34:e475–86. doi: 10.1111/j.1365-2605.2011.01203.x. [DOI] [PubMed] [Google Scholar]