Abstract
Introduction
Disease relapses are frequent in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV). We evaluated the outcomes of patients re-treated with rituximab (RTX) and prednisone for severe disease relapses.
Methods
The Rituximab in AAV trial was a randomized, double-blind, placebo-controlled trial comparing the rates of remission induction among patients treated with RTX (n = 99) and patients treated with cyclophosphamide (CYC) followed by azathioprine (AZA) (n = 98). Prednisone was tapered to discontinuation after 5.5 months. After achieving remission, patients who experienced a severe disease relapse between months 6 and 18 were eligible to receive RTX and prednisone on an open-label basis according to a pre-specified protocol. Investigators remained blinded to the original treatment assignment.
Results
Twenty-six patients received treatment with RTX for disease relapse after initially achieving remission on their originally assigned treatment. Fifteen patients were initially randomized to RTX and 11 to CYC/AZA. Thirteen (87%) of the patients originally assigned to RTX and 10 (91%) originally assigned to CYC/AZA achieved remission again with open-label RTX, an overall percentage of 88%. Half of the patients treated with open-label RTX were able to discontinue prednisone entirely. Patients in this cohort experienced fewer adverse events compared to the overall study population (4.7 adverse events/patient-year versus 11.8 adverse events/patient-year, respectively).
Conclusion
Re-treatment of AAV relapses with RTX and glucocorticoids appears to be a safe and effective strategy, regardless of previous treatment.
Introduction
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) that affect small- and medium-sized blood vessels. Treatment of systemic AAV with cyclophosphamide (CYC)-based regimens combined with high-dose glucocorticoids dramatically altered the prognosis of this group of diseases but is associated with significant concerns about treatment-related morbidity, particularly infection, infertility and the long-term risk of malignancy.1–3 The Rituximab in ANCA-associated Vasculitis (RAVE) trial demonstrated that a regimen of rituximab (RTX) plus glucocorticoids is non-inferior to CYC plus glucocorticoids followed by azathioprine (AZA) for remission induction in severe AAV.4 This trial also demonstrated superiority of the RTX regimen for remission induction in patients with relapsing disease.
A large majority of patients with AAV now achieve disease remission with regimens based on either RTX or CYC, but relapses remain common. Previous studies have demonstrated relapses in up to 55% of patients within the first three years after achieving remission and a persistent risk of relapse over long-term follow-up.5,6 In addition, a substantial percentage of patients fails treatment induction regimens because of persistent or recurrent active AAV within the first six months of remission induction therapy, regardless of whether RTX- or CYC-based regimens are employed.7 Thus, even with new therapeutic options and refined CYC regimens designed to limit CYC exposure,8 disease relapses remain frequent in AAV and, consequently, so does the need for re-treatment.
Given the frequent disease relapses in AAV, it is important to determine the optimal regimen for remission re-induction and maintenance therapy. Repeat RTX administration is safe and effective in rheumatoid arthritis.9–11 Given its efficacy in remission induction in AAV, repeat RTX administration may be effective in the treatment of disease relapses in these conditions. Indeed, several retrospective studies suggest that serial RTX use was well-tolerated and effective at re-treating active disease and preventing disease relapses.12–15 However, no prospective evaluation of this strategy has been reported to date.
We report here prospective data on patients in the RAVE trial who were treated with RTX and glucocorticoids for severe disease relapse according to protocol after initial successful remission induction. For patients who were randomized initially to receive RTX, this represented the second course of RTX.
Materials and Methods
Study Design and Patients
The details of the RAVE trial design have been published.4,16 In brief, the RAVE trial enrolled ANCA-positive patients with either GPA or MPA who met criteria for severe disease and had a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) > 3 or one major disease item.17 Patients were assigned in a 1:1 fashion to RTX followed by AZA-placebo or CYC followed by AZA. Patients who experienced a severe relapse between months 6 and 18 (defined as a BVAS/WG > 3 or one major disease item or a relapse not meeting the above criteria but classified through investigator discretion as severe) were eligible to receive RTX on an open-label basis. Five patients with severe relapse between months 6 and 18 were not retreated with the open-label regimen: Three patients in the RTX group were treated according to the investigator’s best medical judgment, one withdrew due to an adverse event, and one patient in the CYC-AZA group voluntarily withdrew from the study. Patients who experienced severe relapses before the 6 month timepoint (3 RTX and 9 CYC-AZA) were eligible for blinded crossover to the opposite treatment arm and were not included in this analysis. Their outcomes have been reported previously.7 Patients with serum creatinine concentrations greater than 4.0 mg/dL or diffuse alveolar hemorrhage requiring ventilatory support were excluded from entering the RAVE trial but could receive open-label RTX at the discretion of the investigator if the event occurred after the 6 month timepoint.
Treatments
Patients initially randomized to the RTX treatment group received intravenous RTX (375 mg/m2 once weekly for four weeks) plus daily placebo-CYC followed by placebo-AZA upon remission. Patients randomized to the CYC group received RTX placebo infusions and oral CYC (2 mg/kg, adjusted for renal insufficiency) for 3–6 months followed by AZA (2 mg/kg) for a total of 18 months of therapy. Both groups received the same glucocorticoid protocol, which allowed up to three grams of intravenous methylprednisolone (1 gram/day for three days) followed by prednisone 1mg/kg. The prednisone was tapered to discontinuation over 5.5 months in all patients who achieved and maintained remission.
Patients who experienced severe relapses between months 6 and 18 were eligible to receive RTX (375 mg/m2 once weekly for four weeks) in conjunction with glucocorticoids on an open-label basis. Patients were eligible to receive pulse intravenous glucocorticoids for 1–3 days at the physician’s discretion. Oral prednisone 1mg/kg (not to exceed 80 mg/day) was then started and tapered off over 5.5 months according to the same pre-specified schedule used for initial remission induction. Investigators remained blinded to the patients’ initial treatment assignment at the time of re-treatment.
Assessments
Disease activity was assessed by the BVAS/WG.18 Disease damage was assessed by the Vasculitis Damage Index (VDI).19 Patients were assessed at 1, 2, 4, 6 and 12 months after receiving open-label RTX, then every 6 months until the trial’s common closeout date. This assessment schedule was identical to the schedule followed by patients at trial entry for the first 6 months.
ANCA measurements
ANCA type and titer were determined by ELISA.20 All ANCA measurements were performed simultaneously on the same ELISA plate at a single laboratory. ANCA rises were defined as a two-fold increase from one measurement to another or an increase to at least 20 IU if the assay had previously turned negative.
B-cell kinetics
B-cells were measured by five-color flow cytometry in a commercial laboratory under contract with the Immune Tolerance Network. B-cell depletion was defined as <10 CD19+ B cells/µl, and full reconstitution as >69 CD19+ B cells/µl or return to baseline. B-cell counts between 10 and 68 CD19+ B cells/µl were categorized as detectable.
Outcomes and Disease Relapses
The primary endpoint of the retreatment analysis was complete remission, as defined by a BVAS/WG of 0 and prednisone dose of 0 at any time following retreatment with RTX and glucocorticoids. Secondary outcomes included remission, defined as a BVAS/WG of 0 at any point after RTX; complete response, defined as BVAS/WG of 0 with prednisone ≤ 10mg per day at any point after RTX; number of disease relapses, defined as an increase in the BVAS/WG of one or more points. Severe relapses were defined as an increase in BVAS/WG > 3 or one new major BVAS/WG item. Relapses not meeting criteria for severe relapse were classified as limited.
Adverse Events
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria.21
Statistical Analysis
Binary outcomes were compared using the Chi-squared or the Fisher’s exact test, depending on the cell sizes. Continuous outcomes between and within treatment groups were compared using the Wilcoxon Rank-Sum test and the Wilcoxon Signed-Rank test, respectively. All statistical tests were two-sided, and a p-value of less than 0.05 was considered to indicate statistical significance. The SAS Version 9.1 (SAS, Inc. Cary, NC) was used for all statistical analyses.
Results
Twenty-six patients received treatment with RTX for severe disease relapses between months 6 and 18. This represented the second course of RTX for 15 patients and the first RTX course for 11.
Demographics and general disease characteristics
Baseline features of the 26 patients are shown in Table 1. Twenty-one patients (81%) were PR3-ANCA-positive and 24 (92%) had diagnoses of GPA. The predominance of patients who were PR3-ANCA-positive and had GPA is similar to that of the overall trial patient population, in which 66% were PR3-ANCA-positive (p=0.12) and 75% had diagnoses of GPA (p=0.05). Both PR3-ANCA positivity and the clinical diagnosis of GPA (which correlate highly with each other) are known to be associated with disease relapse, and therefore it is not surprising that the percentages of patients with these baseline characteristics were high in this study of patients requiring re-treatment.22 Sixteen patients (62%) had relapsing disease at trial entry and 14 (54%) had received a course of CYC before entry for an earlier period of active disease.
Table 1.
Baseline characteristics of patients receiving RTX for disease relapse
| Re-treatment with RTX (initially randomized to RTX) |
First RTX course (initially randomized to CYC/AZA) |
|
|---|---|---|
| N=15 | N=11 | |
| PR3-ANCA positive | 12 (80%) | 9 (82%) |
| GPA | 13 (87%) | 11 (100%) |
| Relapsing disease at study entry | 10 (67%) | 6 (55%) |
| Received CYC prior to study entry | 8 (53%) | 6 (55%) |
| BVAS/WG at study entry (range) | 7.2 (4–13) | 9.5 (3–16) |
| Mean time to OLR (range in days) | 381 (225–556) | 319 (197–537) |
| BVAS/WG at OLR (range) | 5.3 (3–11) | 5.3 (2–12) |
| Off prednisone at relapse | 10 (67%) | 8 (73%) |
| Mean prednisone dose at OLR (range in mg) | 2.8 (0–15mg) | 2.0 (0–10mg) |
| Mean prednisone dose at RTX treatment if dose > 0mg (range in mg) | 8.5 (3–15mg) | 7.5 (5–10mg) |
| Organ involvement | ||
| Constitutional signs or symptoms | 10 (67%) | 6 (55%) |
| Cutaneous involvement | 1 (7%) | 2 (18%) |
| Mucous membranes and eyes | 1 (7%) | 0 (0%) |
| Ear, nose and throat | 7 (47%) | 7 (64%) |
| Pulmonary involvement | 6 (40%) | 7 (64%) |
| Nodules/cavities | 3 (20%) | 1 (9%) |
| Endobronchial involvement | 0 (0%) | 4 (36%) |
| Alveolar hemorrhage | 1 (7%) | 2 (18%) |
| Other | 3 (20%) | 1 (9%) |
| Renal involvement† | 6 (40%) | 2 (18%) |
| Hematuria | 4 (27%) | 2 (18%) |
| RBC casts | 2 (13%) | 0 (0%) |
| Rise in creatinine | 3 (20%) | 1 (9%) |
| Serum creatinine (range in mg/dL) | 1.3 (0.7–4.1) | 1.2 (0.7–2.5) |
| Neurologic involvement | 3 (20%) | 2 (18%) |
Severe renal disease was defined as the presence of RBC casts or a rising creatinine
The mean BVAS/WG at the time of relapse leading to treatment with RTX was lower than the trial entry BVAS/WG for the same patient subset (5.3 versus 8.2; P < 0.001). Five patients had a higher BVAS/WG at re-treatment than at trial entry. Eighteen patients were off prednisone at the time of relapse, while the remaining 8 patients were receiving an average of 8 mg of prednisone daily (range 3–15 mg). The mean time from the date of randomization to treatment or re-treatment with RTX for severe disease relapse was 355 days: 319 days [range 195–537 days] for the CYC/AZA group and 381 days [range 225–556] for the RTX group (patients who relapsed before month six were not eligible for open-label RTX and are not included). After receiving RTX for treatment of disease relapse, patients were followed for an average of 311 days (range 29–427 days). There were no significant differences with respect to baseline characteristics between patients initially randomized to RTX as compared to those initially randomized to CYC/AZA in this cohort of patients.
Re-treatment with rituximab and prednisone
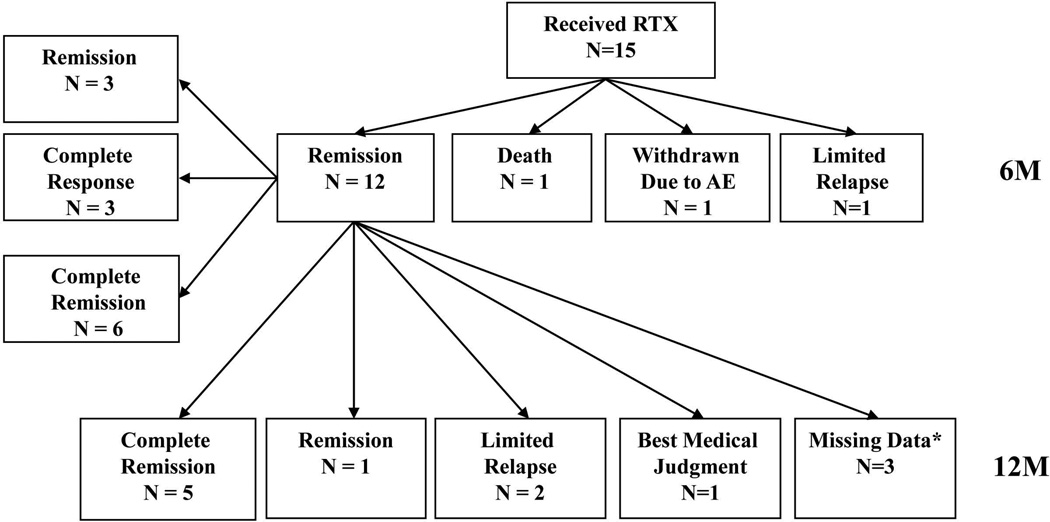
Fifteen patients initially randomized to RTX received RTX and prednisone (mean starting dose 68 mg/day) as a second treatment course during the study period. The outcomes of these patients at the 6 and 12 month timepoints after re-treatment are represented in Figure 1. Treatment with this regimen led to remission in 13 patients (87%) by a median of 30 days. Ten patients (67%) achieved remission and were able to decrease prednisone dose to 10 mg or less. Of these, 6 patients (40%) were able to taper off prednisone (complete remission) (Table 2). Of the two patients who did not reach remission, one died from progressive diffuse alveolar hemorrhage despite re-treatment with RTX. Another patient reached a BVAS/WG of 1 before suffering a limited relapse 12 months after re-treatment with RTX. Two patients suffered limited relapses after reaching remission.
Figure 1. Outcomes of patients initially randomized to RTX receiving RTX for severe disease relapse.
Remission – BVAS/WG = 0
Complete response – BVAS/WG = 0 and prednisone ≤ 10mg per day
Complete remission – BVAS/WG = 0 and prednisone = 0mg per day
*Missing data denotes patients who did not have a 12 month visit but continued to be followed in the study until the common close out date.
Table 2.
Outcomes of patients receiving RTX for disease relapse
| Re-treatment with RTX (initially randomized to RTX) |
First RTX course (initially randomized to CYC/AZA) |
|
|---|---|---|
| Mean follow-up time after re-treatment | 302 (35–427) | 324 (29–377) |
| Average prednisone dose to treat relapse (mg) | 67.8 | 69.1 |
| Solumedrol pulse × 1 | 11 (73%) | 6 (55%) |
| Solumedrol pulse × 2 | 0 (%) | 1 (9%) |
| Solumedrol pulse × 3 | 1 (7%) | 2 (18%) |
| Remission* | 13 (87%) | 10 (91%) |
| Time to remission (days) | 56 (27–181) | 36 (27–60) |
| Complete response† | 10 (67%) | 9 (82%) |
| Time to complete response (days) | 133 (95–186) | 130 (112–182) |
| Complete remission‡ | 6 (40%) | 7 (64%) |
| Time to complete remission (days) | 166 (121–184) | 171 (124–189) |
| Limited relapses after re-treatment with RTX | 3 (20%) | 1 (9%) |
| Severe relapses after re-treatment with RTX | 0 (0%) | 2 (18%) |
| BVAS/WG at relapse after re-treatment with RTX | 2.7 (2–3) | 6 (1–11) |
| Time to relapse after re-treatment with RTX (median days) | 349 (121–428) | 328 (121–364) |
| Baseline VDI | 2.1 (0–7) | 1.1 (0–5) |
| VDI at re-treatment with RTX | 3.2 (0–8) | 2.0 (0–6) |
| VDI at 12 mos post re-treatment with RTX | 4.6 (0–10) | 3.7 (1–7) |
Remission – BVAS/WG = 0
Complete response – BVAS/WG = 0 and prednisone ≤ 10mg per day
Complete remission – BVAS/WG = 0 and prednisone = 0mg per day
Treatment with RTX and prednisone after initial randomization to CYC/AZA
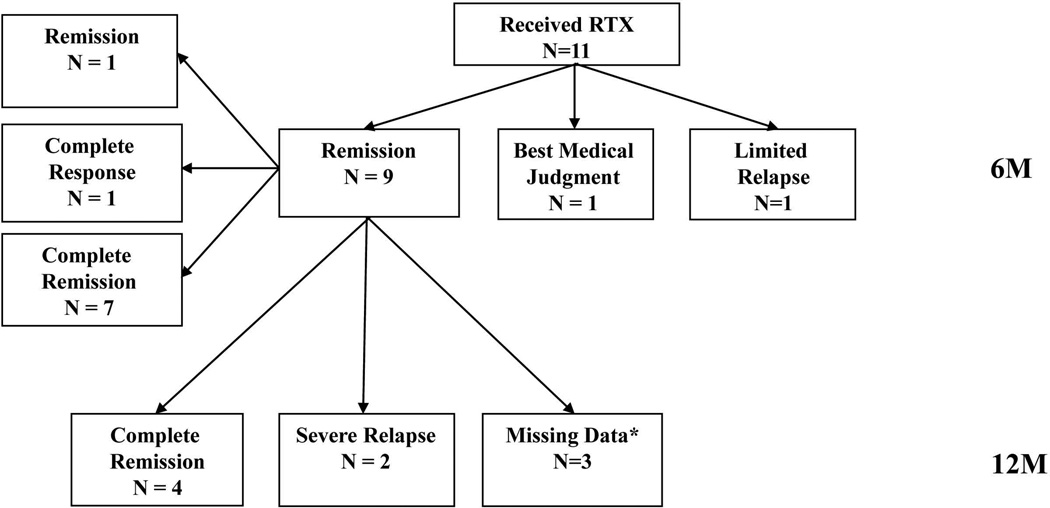
The outcomes of patients who received RTX and prednisone for disease relapse following initial randomization to CYC/AZA were similar to those who received RTX following initial assignment to the RTX group (Table 2 and Figure 2). Ten patients (91%) reached remission and, of these, 7 (64%) achieved complete remission. Among the 11 patients treated with RTX for the first time, 3 experienced relapses of which 2 were severe.
Figure 2. Outcomes of patients initially randomized to CYC/AZA receiving RTX for severe disease relapse.
Remission – BVAS/WG = 0
Complete response – BVAS/WG = 0 and prednisone ≤ 10mg per day
Complete remission – BVAS/WG = 0 and prednisone = 0mg per day
*Missing data denotes patients who did not have a 12 month visit but continued to be followed in the study until the common close out date.
ANCA and B-cells at relapse and after re-treatment with RTX
Twenty patients (77%) had a rising ANCA titer at the time of severe relapse that led to treatment with open-label RTX. Two patients had a persistently positive ANCA and four patients were ANCA-negative. Twenty-two of 23 patients with samples collected at time of relapse (96%) had detectable or reconstituted B-cells (Table 3). The patient who died due to progressive alveolar hemorrhage had both detectable B-cells and a rising ANCA titer at the time of disease relapse. Only one patient had both a negative ANCA and depleted B-cells. Of the six patients who had a flare after re-treatment with open-label RTX, only one had depleted B-cells at the time of re-treatment.
Table 3.
ANCA and B-cell status at severe relapse and six months after re-treatment with RTX
| Re-treatment with RTX (initially randomized to RTX) |
First RTX course (initially randomized to CYC/AZA) |
|
|---|---|---|
| N=15 | N=11 | |
| ANCA at severe relapse | ||
| Rising | 12 (80%) | 8 (73%) |
| Positive | 1 (7%) | 1 (9%) |
| Negative | 2 (13%) | 2 (18%) |
| ANCA 6 months after re-treatment | ||
| Rising | 1 (7%) | 0 (0%) |
| Positive | 5 (33%) | 7 (64%) |
| Negative | 9 (60%) | 4 (36%) |
| B-cells at relapse leading to re-treatment | ||
| Depleted | 1 (7%) | 0 (0%) |
| Detectable | 3 (20%) | 5 (46%) |
| Reconstituted | 11 (73%) | 3 (27%) |
| Missing data | 0 (0%) | 3 (27%) |
| B-cells 6 months after re-treatment | ||
| Depleted | 10 (67%) | 8 (73%) |
| Detectable | 1 (7%) | 0 (0%) |
| Reconstituted | 1 (7%) | 2 (18%) |
| Missing data | 3 (20%) | 1 (9%) |
Although these data might suggest utility of B-cell concentration measurements and ANCA titers in predicting disease flares, they must be contrasted with the number of patients in the trial overall who had detectable B cells and positive ANCA titers after remission but did not experience flares over the course of follow-up. Two-thirds of the patients in the RTX group had detectable or reconstituted B-cells without suffering a relapse up to the 18 month timepoint (B-cell depletion was more prolonged in the CYC-AZA group). Similarly, two-thirds of patients in both groups had a positive or rising ANCA titer without suffering a relapse within 18 months of trial entry.22
Six months after re-treatment with RTX, 18 patients (82%) had depleted B-cells (4 had missing data). There were no significant differences between patients receiving their first or second course of RTX with respect to B-cell depletion or reconstitution following RTX treatment. ANCA had become negative at 6 months after re-treatment in 13 patients (9 RTX, 4 CYC/AZA) (p=0.43).
Adverse events in patients treated with RTX and prednisone for disease relapse
There were a total of 156 AEs in patients who received open-label RTX for severe disease relapse (85 in 14 patients receiving a second course of RTX and 71 in 9 patients receiving a first RTX course) (Table 4). Compared to all patients over the initial six months of the trial, there were fewer AEs over the first six months after re-treatment in patients who were re-treated with RTX for disease relapse (8.4 AEs/patient year vs. 24.1 AEs/patient year) (p<0.001). This difference persisted until the end of follow-up after re-treatment as compared to 18 months after initial randomization (4.7 AEs/patient year vs. 11.8 AEs/patient year) (p<0.001). There was only one episode of grade 1 leukopenia and no cases of severe neutropenias reported in this cohort. There were 13 infections, of which 10 (77%) involved the ears, nose and upper respiratory tract. Other infections included viral gastroenteritis, influenza, and a urinary tract infection (one each). There were two infections of grade 3 severity (gastroenteritis and sinusitis).
Table 4.
Adverse events after RTX for disease relapse
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Death | Total | |
|---|---|---|---|---|---|---|
| Patients receiving RTX at 6 mos | 78 | 18 | 5 | 0 | 1 | 102 |
| Patients receiving RTX AEs/pt year at 6 mos | 6.4 | 1.5 | 0.4 | 0 | 0.1 | 8.4 |
| Patients receiving RTX at end of follow-up | 113 | 34 | 8 | 0 | 1 | 156 |
| Patients receiving RTX AEs/pt year at end of follow-up |
3.4 | 1.0 | 0.2 | 0 | 0.0 | 4.7 |
| All other patients at 6 mos | 1,404 | 316 | 88 | 11 | 3 | 1,822 |
| All other patients AEs/pt year at 6 mos | 18.5 | 4.2 | 1.2 | 0.1 | 0.0 | 24.1 |
| All other patients at 18 mos | 1,809 | 429 | 111 | 12 | 3 | 2,364 |
| All other patients AEs/pt year at 18 mos | 9.0 | 2.1 | 0.6 | 0.1 | 0.0 | 11.8 |
Disease damage in patients treated with RTX and prednisone for disease relapse
Average VDI scores rose in both treatment groups over the course of the study. In patients initially randomized to RTX, the VDI rose from an average of 2.1 at study entry to 3.2 at re-treatment with RTX and 3.9 at end of follow-up. Similarly, in patients initially randomized to CYC/AZA, the VDI rose from 1.1 at study entry, to 2.0 at re-treatment with RTX and 2.8 at end of follow-up. The VDI increase was attributable to both direct disease damage and glucocorticoid-related toxicity (data not shown).
Discussion
We have analyzed the outcomes of patients in the RAVE trial who were treated with RTX and prednisone for disease relapse. Our results demonstrate that a majority of patients who received a second course of RTX and prednisone achieved clinical remission without an increase in the number of adverse events compared to patients in the RAVE trial overall. This series is the first prospective report to date on the use of repeat RTX in patients with AAV who experience disease relapses.
The prospective design of this study and the collection of data within the confines of a label-enabling clinical trial offers certain advantages over retrospective examinations of serial RTX treatment.11–14 First, patients in the RAVE trial were re-treated with RTX only for severe relapses of the underlying disease, and the definitions of remission, complete remission, and severe and limited disease relapses were defined in advance by the protocol. In contrast, a significant proportion of patients in the retrospective studies received RTX on a scheduled basis (every 4 or 6 months), regardless of the presence or absence of AAV symptoms.11–12 Second, no concomitant immunosuppression aside from prednisone was allowed in our trial. By comparison, most of the retrospective studies describing serial RTX treatment allowed concomitant immunosuppressive medications in addition to glucocorticoids during at least a portion of the study, making it difficult to ascertain the impact of RTX and prednisone.11,12,14
The remission rate after a second course of RTX (87%) in our cohort compares favorably to the remission rate observed within initial RTX use for remission induction in the RAVE trial (86%) and with remission rates in retrospective series of serial RTX treatment. The 87% figure also compares favorably to historical success rates in remission induction of AAV.5,23–25 Numerically more subjects initially randomized to CYC/AZA achieved complete remission after re-treatment with RTX as compared to those initially randomized to RTX and then re-treated (7 of 11 [64%] versus 6 of 15 [40%] p=0.42), but time to remission and relapse rates were similar between the two groups. This suggests that the safety and efficacy of re-treatment with RTX is not dependent on whether prior treatments included RTX or CYC-based therapy.
ANCA titers and B-cell counts did not predict disease relapse in this cohort. Even though most patients had a positive or rising ANCA titer and detectable or reconstituted B-cells at the time of relapse, two-thirds of patients in the RAVE trial with a positive ANCA or detectable B-cells did not relapse during the first 18 months of the study. Thus, the return of B cells and the presence of a positive or rising ANCA titer does not signal an imminent disease flare in the majority of patients. Conversely, relapse was unlikely in the presence of a negative ANCA and depleted B-cells between the 6 and 18 month timepoints.22 Prior to the 6 month timepoint, however, the occurrence of disease relapses in the setting of a negative ANCA assay and depleted B-cells is not uncommon.7
We observed a lower rate of AEs among patients after treatment of relapse with RTX than in the RAVE trial overall (4.7 versus 11.8 adverse events per patient year respectively). One possible explanation for this finding is that the rate of AEs is related to disease severity rather than to treatment, as patients treated with RTX for disease relapse had lower disease activity scores at time of re-treatment compared to study entry. Late-onset neutropenia has been reported after treatment with RTX,26 but was not observed in this study. Infections following re-treatment with RTX were most frequently caused by viral etiologies and were primarily of only mild to moderate severity.
Disease damage as measured by the VDI continued to increase throughout the study in patients treated with RTX for disease relapse. This was attributable to disease activity (e.g., hearing loss, peripheral neuropathy) and glucocorticoid-related toxicity (e.g., osteoporosis, hypertension and diabetes, among others). A detailed analysis of disease damage in the RAVE trial is currently under study. The progression of damage as measured by the VDI throughout this trial raises the important question of whether or not progressive damage in AAV might be halted by regularly scheduled re-treatment with RTX instead of waiting for clinically-evident disease relapses before instituting RTX again.
Our study has several limitations. The majority of patients treated with RTX for disease relapse were PR3-ANCA-positive. Thus, generalizability of these findings to patients with MPO-ANCA is unclear. However, the data we are able to provide on PR3-ANCA-positive patients address the subset of patients that is most likely to experience disease relapses and is therefore the subset for whom re-treatment with RTX is most important to consider. Patients experiencing a disease relapse after initially achieving remission were not randomized to different treatment approaches in our study, and therefore there was no true comparison group for patients re-treated with RTX. However, we attempted to provide a measure of comparison by examining patients who received RTX for the first time after initial randomization to CYC/AZA. There remains some concern in the literature regarding the possibility of hypogammaglobulinemia following serial RTX treatment. We do not address this point in the manuscript because a complete analysis of hypogammaglobulinemia in the RAVE trial is currently under study. Because the trial used the four weekly 375mg/m2 RTX doses regimen, our results are not necessarily generalizable to the 1g given twice RTX regimen commonly employed in rheumatoid arthritis, even though these two regimens appear to be approximately equivalent in their ability to deplete circulating B cells.10 Finally, the sample size of patients with disease relapses who received RTX is small, limiting subgroup comparison and detection of rare events.
In conclusion, this first prospective analysis of the re-treatment of AAV patients who are experiencing disease relapses appears to confirm the observations from retrospective studies that re-treatment with RTX and glucocorticoids is safe and effective in this setting. Treatment strategies comparing “on-demand” treatment with RTX and scheduled RTX in AAV deserve further study. Further insights into the pathophysiology of AAV, better biomarkers, and a more complete understanding of which patients are at risk for disease relapse and the timing of such relapses may lead to rational applications of B-cell depletion therapy as prophylactic treatment.
Acknowledgments
Funding:
This research was performed as a project of the Immune Tolerance Network (NIH Contract N01-AI-15416; Protocol number ITN021AI), an international clinical research consortium headquartered at the University of California San Francisco and supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation; Genentech, Inc. and Biogen-Idec, Inc. At the Mayo Clinic, the trial was supported by Clinical and Translational Science Award (CTSA) Grant Number 1 UL1 RR024150-01 (National Center for Research Resources; NCRR). At Johns Hopkins, the trial was supported by UL1 RR 025005 (NCRR) and by grants K24 AR049185 (Dr. Stone) and K23 AR052820 (Dr. Seo). At Boston University, the trial was supported by CTSA grant number UL1RR 025771, NIH M01 RR00533, K24 AR02224 (Dr. Merkel), and an Arthritis Foundation Investigator Award (Dr. Monach). ANCA ELISA kits were provided by EUROIMMUN AG (Lübeck, Germany).
References
- 1.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39(9):1475–1482. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 2.Boumpas DT, Austin HA, 3rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119(5):366–369. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis A 20-year followup study. Arthritis Rheum. 1995;38(8):1120–1127. doi: 10.1002/art.1780380815. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolatemofetilvs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Flossmann O, Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(2):542–548. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 7.Miloslavsky EM, Specks U, Merkel PA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2013;65(9):2441–2449. doi: 10.1002/art.38044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 9.Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56(12):3896–3908. doi: 10.1002/art.23059. [DOI] [PubMed] [Google Scholar]
- 10.Emery P, Deodhar A, Rigby WF, et al. Efficacy and safety of different doses and re-treatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69(9):1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keystone E, Fleischmann R, Emery P, et al. Multiple courses of rituximab produce sustained efficacy in patients with rheumatoid arthritis with an inadequate response to one or more TNF inhibitors. Arthritis and Rheumatism. 2010;62(Suppl. 10):321. [Google Scholar]
- 12.Rhee EP, Laliberte KA, Niles JL. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol. 2010;5(8):1394–1400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RB, Ferraro AJ, Chaudhry AN, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60(7):2156–2168. doi: 10.1002/art.24637. [DOI] [PubMed] [Google Scholar]
- 14.Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): A single-center ten-year experience. Arthritis Rheum. 2012;64(11):3770–3778. doi: 10.1002/art.34584. [DOI] [PubMed] [Google Scholar]
- 15.Smith RM, Jones RB, Guerry MJ, et al. Rituximab for remission maintenance in relapsing ANCA-associated vasculitis. Arthritis Rheum. 2012;64(11):3760–3769. doi: 10.1002/art.34583. [DOI] [PubMed] [Google Scholar]
- 16.Specks U, Merkel PA, Hoffman GS, et al. Design of the Rituximab in ANCA-associated Vasculitis (RAVE) Trial. Open Arthritis J. 2011;4:1–18. [Google Scholar]
- 17.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’sgranulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–380. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 20.Damoiseaux J, Dahnrich C, Rosemann A, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2009;68:228–233. doi: 10.1136/ard.2007.086579. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v.3. doi: 10.1200/JOP.2015.006106. http://ctepcancergov/forms/CTCAEv3pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. EUVAS (European Vasculitis Study Group) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. for the European Vasculitis Study Group A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 25.The Wegener's Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus Standard Therapy for Wegener's Granulomatosis. N Engl J Med. 2005;352:351–361. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 26.Tesfa D, Ajeganova S, Hägglund H, et al. Late-onset neutropenia following rituximab therapy in rheumatic diseases: association with B lymphocyte depletion and infections. Arthritis Rheum. 2011;63(8):2209–2214. doi: 10.1002/art.30427. [DOI] [PubMed] [Google Scholar]