Abstract
A therapy for regenerating large cartilaginous lesions within the articular surface of osteoarthritic joints remains elusive. While tissue engineering strategies such as matrix-assisted autologous chondrocyte implantation can be used in the repair of focal cartilage defects, extending such approaches to the treatment of osteoarthritis will require a number of scientific and technical challenges to be overcome. These include the identification of an abundant source of chondroprogenitor cells that maintain their chondrogenic capacity in disease, as well as the development of novel approaches to engineer scalable cartilaginous grafts that could be used to resurface large areas of damaged joints. In this study, it is first demonstrated that infrapatellar fat pad-derived stem cells (FPSCs) isolated from osteoarthritic (OA) donors possess a comparable chondrogenic capacity to FPSCs isolated from patients undergoing ligament reconstruction. In a further validation of their functionality, we also demonstrate that FPSCs from OA donors respond to the application of physiological levels of cyclic hydrostatic pressure by increasing aggrecan gene expression and the production of sulfated glycosaminoglycans. We next explored whether cartilaginous grafts could be engineered with diseased human FPSCs using a self-assembly or scaffold-free approach. After examining a range of culture conditions, it was found that continuous supplementation with both transforming growth factor-β3 (TGF-β3) and bone morphogenic protein-6 (BMP-6) promoted the development of tissues rich in proteoglycans and type II collagen. The final phase of the study sought to scale-up this approach to engineer cartilaginous grafts of clinically relevant dimensions (≥2 cm in diameter) by assembling FPSCs onto electrospun PLLA fiber membranes. Over 6 weeks in culture, it was possible to generate robust, flexible cartilage-like grafts of scale, opening up the possibility that tissues engineered using FPSCs derived from OA patients could potentially be used to resurface large areas of joint surfaces damaged by trauma or disease.
Introduction
A number of chondrocyte-based approaches have been developed for treating small focal defects within the articular surface of synovial joints.1–4 In spite of the significant advances that have been made in the field of articular cartilage tissue engineering and regenerative medicine, no cell-based therapy has been developed to treat more advanced joint diseases like osteoarthritis. This is a far greater clinical problem than the treatment of focal cartilage defects, with significant damage to the articular surface that characterizes this disease ultimately necessitating replacement of the native joint with a metal and polymer prosthesis. In recent years, there has been increased interest in the use of stem cells as part of a new treatment paradigm for osteoarthritis.5 One putative route to joint regeneration would be to replace the entire articular surface of a diseased joint with a tissue-engineered cartilaginous or osteochondral graft. Realizing such a transformative treatment option for osteoarthritis requires a number of biological and technical challenges to be overcome, in particular, the identification of a cell source suitable for use in the osteoarthritic (OA) patient population, as well as the development of novel approaches for engineering functional grafts of a geometric scale necessary to resurface an entire joint.
It has recently been demonstrated that large-scale cartilaginous grafts can be engineered by seeding human chondrocytes onto Hyaff-11 nonwoven meshes and maintaining the construct in a flow perfusion bioreactor.6 While such an approach provides a promising putative treatment option for repairing large cartilaginous defects, it remains uncertain if chondrocytes isolated from articular cartilage represent a viable cell source for the osteoarthritic patient population due to age- and disease-related declines in their chondrogenic potential.7,8 This had led to increased interest in the use of stem cells for cartilage tissue engineering applications,5 from embryonic stem cells9 to adult mesenchymal stem cells or multipotent stromal cells (MSCs) isolated from tissues, such as bone marrow (BM),10–12 adipose tissue,13–16 synovial tissue,17,18 or infrapatellar (Hoffa's) fat pad (IFP),19,20 to induced pluripotent stem cells.21 From all these options, autologous adult MSCs may represent the least challenging cell source from a regulatory perspective. While BM-derived MSCs have received particular attention for cartilage tissue engineering and regenerative medicine applications, and have been used clinically for treating focal defects,22 the observation of diminished chondrogenic potential in advanced osteoarthritis may limit their potential for treating this disease.23 IFP has been proposed as an alternative source of regenerative cells for tissue engineering19,24–34 and cartilage repair in osteoarthritis.32,35,36 Previous studies have found considerable MSC activity from IFP-derived cells.20 Furthermore, the findings that cells derived from the IFP of diseased knees possess a strong chondrogenic capacity (at least comparable to control BM-derived MSCs), even after a significant number of population doublings in vitro, suggest that this tissue contains functional MSCs even in advanced osteoarthritis.36
Identifying an autologous progenitor cell that maintains its chondrogenic potential in disease would open up the possibility of using such a cell source to engineer scaled-up cartilage grafts for the treatment of osteoarthritis. The hypothesis of this study is that human infrapatellar fat pad-derived stem cells (FPSCs) maintain their chondrogenic capacity in advanced osteoarthritis. This will be evaluated by comparing the response of FPSCs isolated from OA donors and from patients undergoing ligament reconstruction to transforming growth factor-β3 (TGF-β3) stimulation, and additionally by confirming that diseased FPSCs can respond anabolically to the application of joint-specific mechanical loading. The second phase of the study will explore the potential of such cells for engineering scaled-up cartilaginous grafts. Embedding stem cells into scaffolds or hydrogels represents one promising approach to generating such scaled-up tissues.37 However, we have previously demonstrated that hydrogel encapsulation can diminish the chondrogenic capacity of diseased human infrapatellar FPSCs compared to traditional pellet culture.38 In contrast, scaffold-free or self-assembly approaches have been shown to result in superior chondrogenesis of BM-derived MSCs compared to pellet culture.39 Furthermore, this method has been used to engineer cartilaginous grafts from multiple different cell sources39–45 and to engineer tissues of scale with healthy chondrocytes.46,47 It is therefore also hypothesized that diseased FPSCs can be used to engineer cartilaginous grafts of clinically relevant dimensions (>2 cm) for the osteoarthritic patient population using such a scaffold-free or self-assembly approach.
Materials and Methods
Cell isolation and expansion
Ethical approval for the study was obtained from the institutional review board of the Mater Misericordiae University Hospital, Dublin with infrapatellar fat pad tissue being obtained from patients either with knee osteoarthritis at joint arthroplasty or from patients undergoing ligament reconstruction without symptoms of osteoarthritis (where small biopsies of IFP tissue were removed). Informed signed consent was preobtained from each patient. Fat pad tissue was maintained in sterile phosphate buffer saline (PBS) and transferred immediately to the Trinity Centre for Bioengineering for further processing. Fibrous tissue was carefully removed from the fat pad and discarded. The remaining tissue was weighed, washed thoroughly in PBS, and diced, followed by incubation under constant rotation at 37°C with high-glucose Dulbecco's modified Eagle's medium (hgDMEM, GlutaMAXTM) (Gibco, Biosciences) containing 1% penicillin (100 U/mL)–streptomycin (100 μg/mL) and collagenase type II (4 mL solution/g tissue, 750 U/mL; Worthington Biochemical, LanganBach Services) for 4 h. Cells were filtered through serial cell sieves (Falcon) with pore size from 100, 70 to 40 μm. The isolated cells were cultured in an expansion medium [hgDMEM GlutaMax supplemented with 10% v/v fetal bovine serum (FBS), penicillin (100 U/mL)–streptomycin (100 μg/mL) (all from Gibco, Biosciences)] supplemented with 5 ng/mL fibroblast growth factor-2 (FGF-2) (Prospect-Tany TechnoGene Ltd.). Cells were expanded for two to three passages before initiation of experiments.
Chondrogenesis in pellet culture
To compare the chondrogenic potential of FPSCs obtained from tissue defined macroscopically as either healthy or OA in nature, cells were isolated from three healthy donors and three OA donors and cultured separately until the end of passage (P) 1 or 2. Cells from either healthy or diseased (OA) donors were then pooled together and used to form pellets. Additional pellets were also generated using FPSCs from individual (i.e., not pooled) donors. Pellets were formed by centrifuging 250,000 cells in 1.5-mL conical eppendorf tubes at 650 g for 5 min. Pellets were maintained in a chondrogenic medium, consisting of hgDMEM GlutaMax supplemented with penicillin (100 U/mL)–streptomycin (100 μg/mL) (Invitrogen), 100 μg/mL sodium pyruvate, 40 μg/mL l-proline, 50 μg/mL l-ascorbic acid-2-phosphate, 4.7 μg/mL linoleic acid, 1.5 mg/mL bovine serum albumin, 1× insulin–transferrin–selenium, 100 mM dexamethasone (all from Sigma-Aldrich), and 10 ng/mL of recombinant human TGF-β3 (Prospect-Tany TechnoGene Ltd.). Additional pellets were also supplemented with 10 ng/mL recombinant human bone morphogenic protein-6 (BMP-6; R&D Systems). Pellets were cultured at 37°C and 5% O2 for a period of 28 days. One milliliter of chondrogenic medium was supplied to each pellet, and the medium was exchanged twice weekly.
Self-assembly in transwell insert
Four million cells were resuspended in 100 μL of expansion medium and seeded onto 6.5 mm polyester (PET) transwell inserts (Corning, VWR). Each insert was allowed to sit in a six-well plate overnight to allow initial cell attachment onto the membrane (Fig. 1). The expansion medium (9 mL) was supplied for the first 2 days of culture to minimize the contraction that is mediated by the addition of chondrogenic media. Following this 2-day period, cell-seeded inserts were supplemented with the chondrogenic medium for a further 6 weeks at 20% O2. Additional self-assembled tissues were also maintained at 5% O2. Medium exchanges were performed twice weekly.
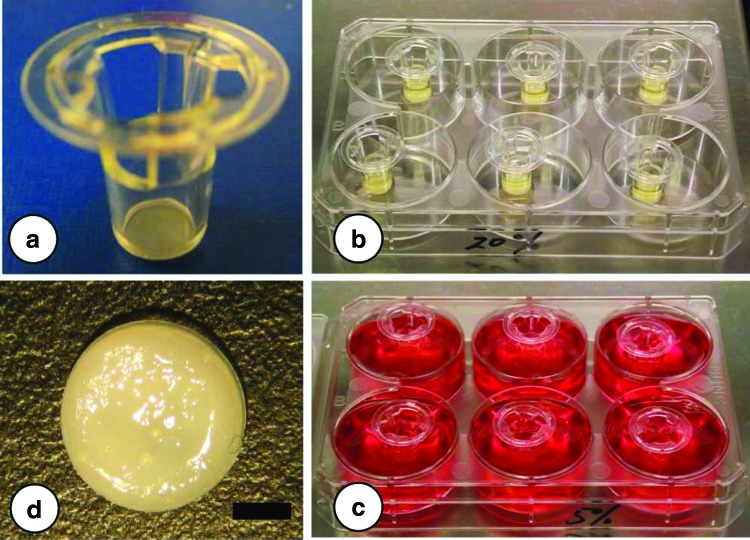
FIG. 1.
(a) Transwell inserts used to engineer cartilaginous tissues. Fat pad-derived stem cell (FPSC)-seeded inserts within a six-well plate (b) before and (c) after addition of culture media. (d) Resulting engineered tissue. Scale bar=2 mm. Color images available online at www.liebertpub.com/tea
Engineering scaled-up self-assembled constructs
To scale-up the geometry of the self-assembled construct, larger inserts generated using PLLA electrospun fiber membranes (20 mm in diameter and 50 μm in thickness; The Electrospinning Company) were wetted with 15% ethanol, washed three times in PBS, and immersed in the expansion medium. Approximately 38 million cells were resuspended in 700 μL of expansion medium and carefully seeded onto the inserts. These constructs were then cultured in the expansion medium for the first 2 days, followed by culture in a chondrogenic medium for 6 weeks at 20% O2. For the first week of culture, these constructs were kept in six-well plates. Due to volume constraints within these six-well plates, media were exchanged daily. After 1 week of culture, the constructs were released from the inserts and maintained in agarose-coated 10-cm Petri dishes, where media were changed twice weekly.
Application of cyclic hydrostatic pressure
FPSCs from three OA donors were expanded separately until P2 and were pooled together to form pellets as described above. Pellets were subjected to cyclic hydrostatic pressure (HP) at a magnitude of 10 MPa and a frequency of 1 Hz for 2 h/day and 5 days/week for the final 2 weeks of the 4-week differentiation period in a custom developed HP bioreactor.48 Pellets were transferred to sterilized heat-sealed bags filled with the chondrogenic medium for the 2-h loading period. The bags for HP loading were placed in a water-filled pressure vessel, while free swelling (FS) control groups (also contained in heat-sealed bags) were placed in an open water bath, both maintained at 37°C. After 2 h of loading, pellets were removed from the bags and transferred to an incubator maintained at 5% O2.
Biochemical analysis
Pellet and self-assembled constructs were digested in papain (125 μg/mL) in 0.1 M sodium acetate, 5 mM cysteine HCl, and 0.05 M EDTA (pH 6.0) (all from Sigma-Aldrich) at 60°C under constant rotation for 18 h. Total DNA content of pellets and constructs was quantified using the Hoechst Bisbenzimide 33258 dye assay (Sigma-Aldrich). Proteoglycan content was estimated by quantifying the amount of sulfated glycosaminoglycan (sGAG) in constructs using the dimethylmethylene blue dye-binding assay (Blyscan; Biocolor Ltd.), with a chondroitin sulfate standard. Total collagen content was determined by measuring the hydroxyproline content. Samples were hydrolyzed at 110°C for 18 h in concentrated HCL (38%) and assayed using a chloramine-T assay49 with a hydroxyproline-to-collagen ratio of 1:7.69.50
Histology and immunochemistry
Pellets and constructs were fixed in 4% PFA, embedded in paraffin, and sectioned (5 μm). Sections were stained with 1% Alcian Blue 8GX (Sigma-Aldrich) in 0.1 M HCl for sGAG and Picrosirius Red for collagen. The deposition of collagen types I and II was identified through immunohistochemistry. Briefly, sections were quenched of peroxidase activity for 20 min (PBS was used to rinse sections between steps) and treated with 0.25 U/mL chondroitinase ABC (Sigma-Aldrich) in a humidified environment at 37°C for 1 h to enhance permeability of the extracellular matrix by removal of chondroitin sulfate. After incubation with 10% goat serum to block nonspecific sites, the primary antibody of mouse monoclonal anti-collagen type I diluted 1:400 or mouse monoclonal anti-collagen type II diluted 1:100 (Abcam) was applied for 1 h at room temperature. Then, the secondary antibody (anti-mouse IgG biotin conjugate; Sigma-Aldrich) was added for another hour. Color was developed using the Vectastain ABC reagent (Vectastain ABC kit: Vector Laboratories) for 45 min and exposed to the peroxidase DAB substrate kit (Vector Laboratories) for 5 min. Slides were dehydrated through ethanol and xylene and mounted with the Vectamount medium (Vector Laboratories). Human ligament and cartilage were included as controls for collagen type I and collagen type II, respectively.
Real-time polymerase chain reaction
Quantitative real-time reverse transcription–polymerase chain reaction (qRT-PCR) was used to determine the relative changes in gene expression between (1) healthy and diseased FPSCs undergoing TGF-β3-mediated chondrogenesis and (2) unloaded pellets and those subjected to HP. Total RNA was extracted from pellets following 28 days of culture. Pellet construct pooling was performed for all groups, with a total of three pellets pooled per group. Total cellular RNA was extracted from each pellet group by physical homogenization using a pestle (Sigma-Aldrich) in TRIZOL reagent (Invitrogen), followed by chloroform (Sigma-Aldrich) extraction with the PureLink™ RNA Mini Kit (Invitrogen) as per the manufacturer's instructions. To quantify mRNA expression, 50 ng of total RNA was reverse transcribed into cDNA. TaqMan gene expression assays (Applied Biosystems/Life Technologies), which contain forward and reverse primers, and a FAM-labeled TaqMan probe for human Sox9 (Hs01001343-g1), aggrecan (Hs00153936-m1), collagen type I alpha I (Hs00164004-m1), collagen type II alpha 1 (Hs01060358-m1), collagen type X alpha 1 (Hs00166657-m1), and Glyceraldehyde-three-phosphate dehydrogenase (GAPDH) (Hs02798991-g1) were used in each study. qRT-PCR was performed using an ABI 7500 Sequence Detection system (Applied Biosystems). A 20 μL volume was added to each well (5 μL of cDNA preparation, 1 μL of TaqMan probe, 10 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), and 4 μL RNase free water). Samples were assayed in triplicate in one run (45 cycles). qRT-PCR data were analyzed using relative quantification and the ΔCT-method. For the comparison of unloaded and HP-stimulated samples, the unloaded samples served as the calibrator and GAPDH as the endogenous control gene. Relative quantification values are presented as fold changes in gene expression plus/minus the standard deviation of the mean relative to the control group, which was normalized to 1.
Mechanical testing
Constructs were mechanically assessed using a protocol described previously.51 Briefly, constructs from each group were tested in unconfined compression between impermeable platens, using a standard Zwick testing machine with a 5 N load cell (Zwick Z005; Roell). A ramp and hold cycle with a ramp displacement of 1 μm/s until 10% strain was applied and maintained until equilibrium was reached (∼30 min). The equilibrium Young's modulus was calculated as the equilibrium stress divided by the applied strain.
Statistics
Numerical and graphical results are presented as mean±standard deviation (three to four samples per donor/group for each experimental arm). Statistics were performed using R (The R Foundation for Statistical Computing). Groups were analyzed for significant differences using a linear model for analysis of variance, with multiple factors and interactions between these factors also examined. Tukey's HSD test for multiple comparisons was used as post-tests. Significance was accepted at a level of p<0.05.
Results
FPSCs isolated from OA donors display a comparable chondrogenic capacity to cells from healthy donors
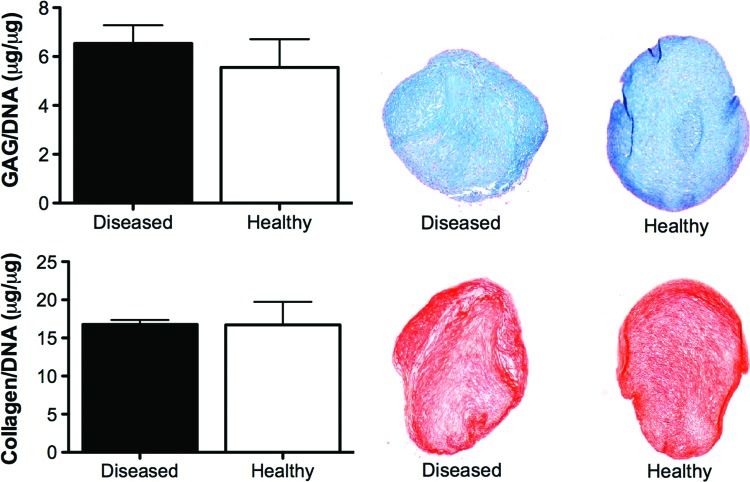
As expected, some donor-to-donor variability was observed in the chondrogenic capacity of infrapatellar FPSCs, although all donors demonstrated the ability to differentiate along the chondrogenic pathway (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). In general, the levels of glycosaminoglycan and collagen accumulation observed for these donors were comparable to previous studies from our laboratory using diseased human FPSCs.38,52 To explore if the chondrogenic capacity of FPSCs is diminished with the development of joint diseases like osteoarthritis, cartilaginous pellets were formed using cells pooled from both healthy (isolated from patients undergoing ligament reconstruction) and osteoarthritic (OA) donors. Pellets generated using either healthy or OA FPSCs had comparable DNA, sGAG, and collagen content after 4 weeks of culture in chondrogenic media (Fig. 2). Histologically, the tissue appeared cartilaginous in the healthy and diseased groups, staining positively for Alcian Blue and Picrosirius Red (Fig. 2). No significant difference was observed in the expression of Sox9, type I collagen, type II collagen, and type X collagen between healthy and OA FPSCs after 28 days of culture (Table 1).
FIG. 2.
Glycosaminoglycan (GAG) and collagen content of pellets generated using FPSCs isolated from healthy and diseased donors, as determined by biochemical assay and histological staining for Alcian Blue and Picrosirius Red. Color images available online at www.liebertpub.com/tea
Table 1.
Fold Differences in the mRNA Levels of Different Genes (Healthy Over Diseased Donors; Calculated Using the ΔΔCT Method)
| ΔCT, healthy | ΔCT, diseased | Fold difference | |
|---|---|---|---|
| Sox9 | 9.09±1.42 | 9.36±0.86 | 1.29 |
| Aggrecan | 4.74±1.01 | 6.4±1.21 | 3.18 |
| Collagen I | −1.99±1.34 | −0.06±0.68 | 3.82 |
| Collagen II | 8.74±1.25 | 7.6±3.93 | 0.45 |
| Collagen X | 9.63±0.92 | 7.85±2.25 | 0.29 |
ΔCT=(average CT of gene of interest−the average CT of GAPDH). No significant differences were observed between healthy and diseased donors.
FPSCs from OA donors respond anabolically to the application of HP
A characteristic of chondrocytes is to respond to the application of cyclic HP with increases in cartilage extracellular matrix production.53 To confirm that chondrogenically primed FPSCs isolated from OA donors possess the capacity to respond anabolically to such a mechanical cue, cell pellets were subjected to HP stimulation for 14 days after an initial 14-day period of FS culture. The application of HP to FPSCs led to a significant increase in sGAG synthesis compared to FPSCs that were maintained in FS conditions for the entire 28-day culture period (Fig. 3A), but had no effect on DNA levels or collagen synthesis. Comparable results were obtained at the mRNA level. FPSCs from OA donors responded to the application of HP with a significant increase in the expression of aggrecan. No significant difference was observed in the expression of type I collagen, type II collagen, or type X collagen following the application of HP (Fig. 3B).
FIG. 3.
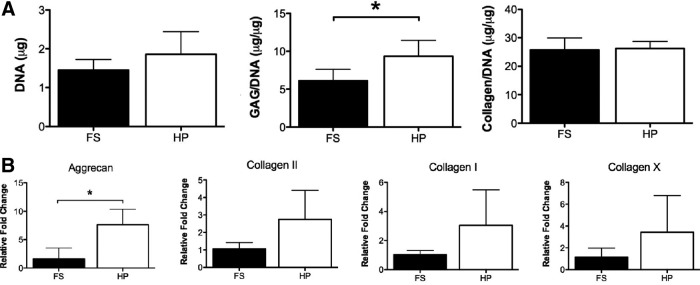
(A) DNA, GAG/DNA and collagen/DNA content of pellets maintained in free swelling (FS) conditions or subjected to cyclic hydrostatic pressure (HP). (B) Fold change in the mRNA levels of aggrecan and type I, II, and X collagen in pellets subjected to HP compared to FS controls. *indicates p<0.05.
A self-assembly approach can be used to engineer cartilaginous grafts using diseased human FPSCs
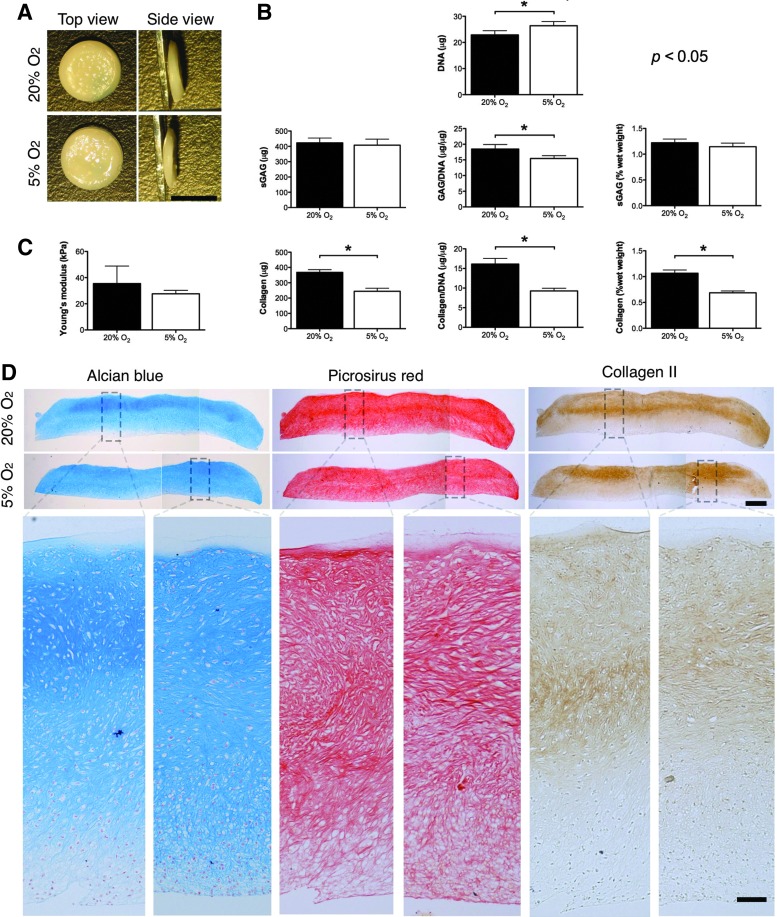
Having confirmed that FPSCs isolated from OA donors are functional for cartilage tissue engineering applications, we next sought to develop a scalable approach to engineering cartilaginous grafts using this cell source. Four million diseased FPSCs were seeded onto 6.5 mm PET transwell inserts and maintained in chondrogenic media for 6 weeks in either normoxic (20%) or low oxygen conditions (5%). The resulting engineered tissues were robust and easily handleable (Fig. 4A), with sGAG and collagen contents exceeding 1% of tissue wet weight (Fig. 4B). Grafts engineered at 5% O2 had a slightly higher DNA content, comparable sGAG content, and a lower collagen content to constructs generated in normoxic conditions (Fig. 4B). The external oxygen tension did not influence the resulting mechanical properties of the engineered tissue (Fig. 4C). The spatial accumulation of proteoglycans, as evidenced by Alcian Blue staining, was relatively homogenous, while a greater type II collagen accumulation occurred in the upper half of the engineered graft (Fig. 4D).
FIG. 4.
(A) Macroscopic image of tissues engineered on transwell inserts at 5% pO2 or 20% pO2. Scale bar=5 mm. (B) DNA, GAG, collagen, GAG/DNA, collagen/DNA, GAG (% wet weight [ww]), collagen (% ww) content of tissues engineered at 5% pO2 or 20% pO2. (C) Young's modulus of tissues engineered at 5% pO2 or 20% pO2. (D) Histological staining of engineered tissue for Alcian Blue, Picrosirius Red, and immunostaining for type II collagen. Scale bar in lower magnification image=500 μm. Scale bar in higher magnification image=100 μm. *indicates p<0.05. Color images available online at www.liebertpub.com/tea
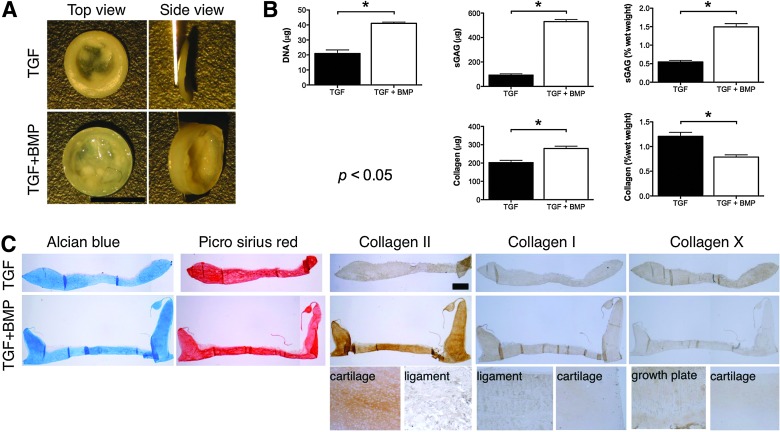
BMP-6 enhances the development of self-assembled cartilaginous grafts
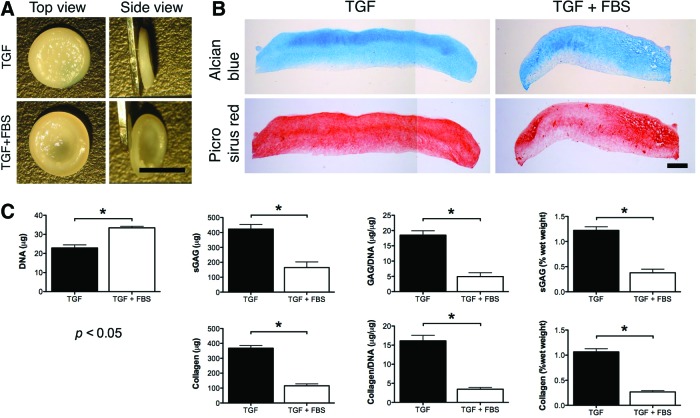
We next sought to explore how different media supplementation conditions, specifically those that have previously been shown to be beneficial for cartilage tissue engineering applications, would influence the development of grafts generated using diseased FPSCs using a self-assembly approach (it should be noted that these experiments were performed independently using FPSCs from different donors). Withdrawal of TGF-β from the media after 2 weeks of culture, which has been shown to enhance the development of cartilaginous tissues engineered using primary, juvenile bovine chondrocytes embedded into agarose hydrogels,54 led to the development of inferior grafts (Supplementary Fig. S2). Similarly, supplementation with 10% FBS, which we have previously demonstrated enhances the development of cartilaginous tissues engineered using diseased human FPSCs encapsulated in agarose hydrogels,38 negatively impacted the development of the engineered graft (Fig. 5). In contrast, supplementation with BMP-6 was found to enhance the accumulation of both sGAG and collagen (when measured in μg) within the engineered tissue, at least in part, by promoting the proliferation of FPSCs as evident by the higher DNA content within these tissues (Fig. 6B). Tissues engineered in the presence of BMP-6 also stained strongly for type II collagen (Fig. 6C). Comparable staining for type I and type X collagen was observed in the two groups.
FIG. 5.
(A) Macroscopic image of tissues engineered on transwell inserts in the presence or absence of fetal bovine serum (FBS). Scale bar=5 mm. (B) Histological staining of engineered tissue for Alcian Blue and Picrosirius Red. Scale bar=500 μm. (C) DNA, GAG, collagen, GAG/DNA, collagen/DNA, GAG (% ww), collagen (% ww) content of tissues engineered in the presence or absence of fetal bovine serum (FBS). *indicates p<0.05. Color images available online at www.liebertpub.com/tea
FIG. 6.
(A) Macroscopic image of tissues engineered on transwell inserts in the presence or absence of bone morphogenic protein-6 (BMP-6). Scale bar=5 mm. (B) DNA, GAG, collagen, GAG/DNA, collagen/DNA, GAG (% ww), collagen (% ww) content of tissues engineered in the presence or absence of BMP-6. (C) Histological staining of engineered tissue for Alcian Blue, Picrosirius Red, and immunostaining for collagen types I, II, and X. Ligament, cartilage, and growth plate tissue were used as either a positive or negative control for specific immunostaining. Scale bar=500 μm. *indicates p<0.05. Color images available online at www.liebertpub.com/tea
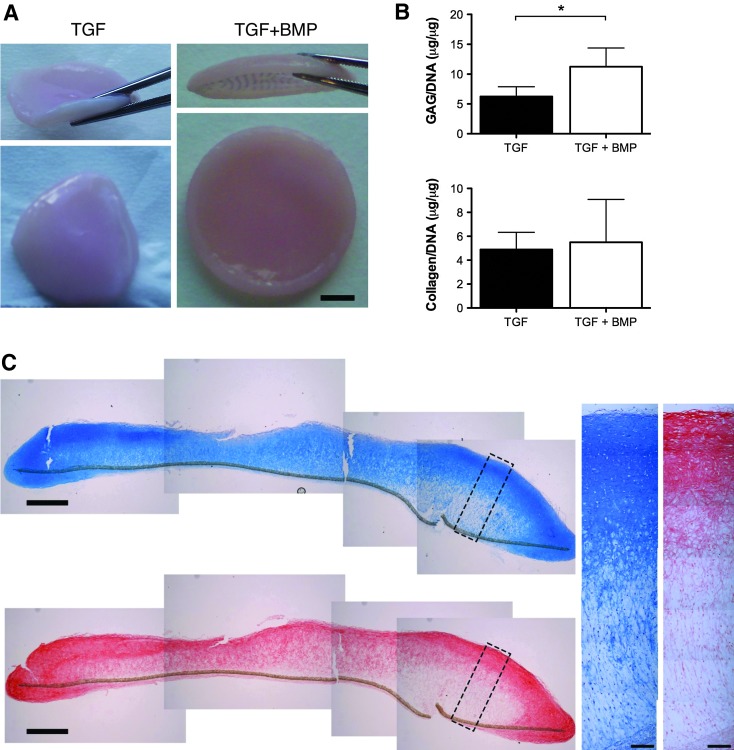
Grafts of clinically relevant dimensions can be engineered by self-assembly of human FPSCs on electrospun PLLA fibers
In a final proof-of-principal study, we sought to determine if the self-assembly approach could be scaled-up to engineer cartilaginous grafts of clinically relevant dimensions. To this end, ∼40 million FPSCs derived from OA donors were seeded onto electrospun PLLA fiber membranes 2 cm in diameter. This led to the development of robust, flexible cartilaginous grafts (Fig. 7A). Supplementation with BMP-6 again enhanced the development of these engineered tissues (Fig. 7B). The tissues stained reasonably homogenously for Alcian Blue, although certain regions were stained less intensely for proteoglycans toward the bottom of the graft (Fig. 7C). Collagen accumulation generally appeared greater toward the surface of the engineered grafts, as evident by Picrosirius Red staining and collagen type II immunohistochemistry (Fig. 7C).
FIG. 7.
(A) Macroscopic image of tissues engineered on 2 cm diameter electrospun PLLA fiber membranes in the presence or absence of BMP-6. Scale bar=5 mm. (B) GAG/DNA and collagen/DNA content of scaled-up tissues engineered in the presence or absence of BMP-6. (C) Histological staining of scaled-up engineered tissue for Alcian Blue and Picrosirius Red. Scale bar in lower magnification image=1 mm. Scale bar in higher magnification image=100 μm. *indicates p<0.05. Color images available online at www.liebertpub.com/tea
Discussion
Perhaps the greatest challenge in orthopedic tissue engineering and regenerative medicine is the development of novel therapies for the treatment of osteoarthritis. If this is to be realized using an autologous cell source, it will be first necessary to identify a cell source that maintains its chondrogenic capacity following the onset and progression of this disease. The results of this study suggest that FPSCs maintain their functionality for cartilage tissue engineering applications in disease. Similar to previous studies using chondrocytes53,55–57 and FPSCs from healthy porcine donors,58 we also found that diseased FPSCs can respond anabolically to the application of cyclic HP. Furthermore, it is demonstrated that such cells can self-assemble a cartilage-like matrix when seeded onto electrospun PLLA fiber membranes, leading to the development of engineered tissues of a scale that could be considered for use in joint resurfacing. To the best of our knowledge, this is the first study to report engineering cartilaginous grafts of such scale using diseased human stem cells.
Previous studies have suggested that stem cells may display a diminished chondrogenic capacity in osteoarthritis.23 MSCs isolated from BM aspirates, obtained from the tibia or femur of patients undergoing joint replacement surgery for arthritis, have been shown to have a diminished chondrogenic potential compared to cells from normal donors.23 In contrast, a more recent study observed no difference in the chondrogenic potential of MSCs isolated from the BM of knee osteoarthritis patients compared to those isolated from patients presenting with a femoral fracture.59 Another study found a higher number of MSCs in the synovial fluid of OA joints compared to healthy joints,60 with both populations of MSCs demonstrating the ability to undergo chondrogenesis, although certain functional differences between the two cell populations were observed.61 Despite the inflammatory environment present in the osteoarthritic joint space, we found that FPSCs isolated from diseased donors did not display a diminished chondrogenic capacity in pellet culture compared to cells isolated from patients undergoing ligament reconstruction. In general, FPSCs from OA donors appear to be able to generate cartilaginous pellets with at least a comparable glycosaminoglycan content to that reported for BM-derived MSCs from diseased patients.23 In fact, previous studies directly comparing these two cell sources have reported that 80% of fat pad-derived cultures are more chondrogenic than control BM-derived MSCs.36
Having confirmed that FPSCs maintain their chondrogenic capacity in disease, we next sought to identify the optimal approach for engineering functional cartilaginous grafts using this cell source. The scaffold-free approach has previously been used to generate cartilaginous grafts using chondrocytes, BM- and synovial membrane-derived MSCs.39,40,43,46,62 Furthermore, it has been demonstrated that such scaffold-free transwell cultures provide conditions more conducive to MSC chondrogenesis compared to the well-established pellet culture system.39 While we did not directly compare the chondrogenesis of FPSCs in the transwell and pellet systems, by comparing across the experiments performed in this article we do not find any strong evidence for enhanced chondrogenesis in the transwell inserts. This may be due to our decision to use a very high cell seeding density for transwell inserts (4 million cells per insert compared to 0.5 million cells per insert used by Murdoch et al.39), which may lead to regions of nutrient and/or oxygen depletion within the engineered tissue, thereby potentially negating the putative benefits of this culture system in terms of enhanced mass transport properties.39 Our decision to use such high numbers of FPSCs was motivated by previous studies demonstrating that the self-assembly approach benefits from high seeding densities, by facilitating the formation of more uniform and functional cartilaginous tissues.42,45
In contrast to our own previous findings using the pellet culture system38 and in fibrin hydrogels,31 we found that low oxygen conditions did not enhance chondrogenesis of FPSCs in the transwell system. Murdoch et al. also reported that preliminary experiments at a lowered oxygen tension (5%) did not enhance chondrogenic differentiation of BM-derived MSCs in transwell cultures.39 The high cell density and associated levels of oxygen consumption may be leading to the development of anoxic conditions toward the bottom of the transwell generated grafts, similar to that reported in hydrogels seeded at high MSC densities,63 which may suppress the chondrogenic capacity of MSCs.64 Such anoxic conditions, if they indeed exist, would be exacerbated by culture at a low oxygen tension. These gradients can potentially be overcome by the use of perfusion culture at a flow rate that maintains a spatially homogeneous oxygen supply, which in turn has been shown to support the development of uniform engineered cartilage of clinically relevant thicknesses.65 Regardless of any nutrient transport issues, it should be noted that the composition of these engineered tissues, in terms of sGAG content, is comparable to that generated using culture-expanded human chondrocytes cultured on porous scaffolds within perfusion bioreactors.6 This provides further support for the use of FPSCs from diseased joints for clinical cartilage tissue engineering applications.
A potential concern with the grafts generated using this approach was that the mechanical stiffness of the engineered tissues was an order of magnitude lower compared with native articular cartilage. It remains an open question as to how mechanically functional an engineered tissue needs to be before implantation, although in silico66 and in vivo67 studies provide evidence to support the concept that implantation of a more mechanically functional graft will enhance articular cartilage regeneration. Should improvements in the mechanical properties of such grafts be deemed necessary before implantation, a number of potential strategies are available. For example, given that we have demonstrated that the application of cyclic HP to diseased FPSCs leads to increases in cartilage matrix-specific matrix production, it may be possible to use such bioreactor stimulation protocols to improve the functionality of the graft before implantation.48,53,68–75
In agreement with our previous studies using human FPSCs embedded into agarose hydrogels, we found that supplementation with BMP-6 leads to increases in the absolute DNA, sGAG, and collagen content of the engineered tissues.76 In subcutaneous fat-derived stem cells, BMP-6 treatment has been shown to promote TGF-β receptor expression, with the combined application of TGF-β3 and BMP-6 enhancing their chondrogenic capacity.77 Such enhanced extracellular matrix deposition is clearly beneficial for cartilage tissue engineering applications, although it should be noted that grafts generated in the presence of BMP-6 appear more geometrically inhomogeneous than those generated in the presence of TGF-β3 only. We believe that this may be due to the side walls of the transwell insert (Fig. 1a) providing a surface to preferentially guide the migration and/or deposition of extracellular matrix within this region of the insert, which is particularly prevalent in the presence of BMP-6, where significant cell proliferation is observed. Such inhomogeneity is clearly a challenge for cartilage tissue engineering applications.
The final part of this study demonstrated that it is possible to engineer grafts of clinically relevant dimensions (≥2 cm) using diseased FPSCs seeded onto electrospun PLLA fiber membranes. To overcome the issue discussed above of preferential tissue growth around the periphery of the transwell insert, the engineered grafts were carefully removed from the inserts after 1 week of culture, which lead to the development of a more geometrically uniform tissue. The composition of cartilaginous tissues generated on these larger PLLA membranes was reasonably similar to that generated on the smaller PET inserts, although the larger tissues were perhaps slightly more inhomogeneous in their spatial composition, with certain regions of the grafts staining less intensely for collagen and proteoglycans (Fig. 7). Previous studies that have attempted to engineer grafts of such scale have demonstrated that the use of flow perfusion bioreactor systems can lead to the generation of more homogenous grafts.6 The use of such bioreactor systems will be explored in future studies in an attempt to improve the composition, organization, and functionality of cartilage grafts engineered using human FPSCs isolated from OA donors.
In conclusion, the results of this study demonstrate that stem/progenitor cells isolated from the infrapatellar fat of the knee—a large, easily accessible tissue that yields high numbers of such cells,36 can be used to tissue engineer clinically sized cartilaginous grafts. This opens up the possibility of using such grafts to treat large defects associated with trauma or diseases such as osteoarthritis. Of course, there are still a number of outstanding questions that need to be addressed (in addition to the other challenges that have been highlighted above) before this can become a clinical reality. Many of these relate to any stem cell-based therapy proposed for the treatment of osteoarthritis,5 but outstanding questions specific to the proposed approach include concerns related to the in vivo phenotypic stability of grafts engineered using FPSCs78 and how such tissues will adopt and respond to joint specific environmental cues following implantation into load-bearing defects. Notwithstanding these concerns, the results of this study motivate the continued investigation of such tissue engineering strategies, as these grafts may ultimately be used to resurface entire joint surfaces and hopefully delay or prevent the need for total joint replacement prostheses.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Irish Research Council for Science, Engineering and Technology under enterprise partner scheme with the Sports Surgery Clinic Dublin (IRCSET-SSC-2010-01), a European Research Council Starter Grant (StemRepair–Project number: 258463), and a Science Foundation Ireland President of Ireland Young Researcher Award (08/Y15B1336). The authors would like to thank Richard Downey for help with the collection of biological tissue.
Disclosure Statement
No competing financial interests exist.
References
- 1.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., and Peterson L.Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331,889, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Peterson L., Minas T., Brittberg M., Nilsson A., Sjögren-Jansson E., and Lindahl A.Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 212,2000 [DOI] [PubMed] [Google Scholar]
- 3.Nehrer S., Dorotka R., Domayer S., Stelzeneder D., and Kotz R.Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years' follow-up. American J Sports Med 37(Suppl 1),81S, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Kon E., Gobbi A., Filardo G., Delcogliano M., Zaffagnini S., and Marcacci M.Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 37,33, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Diekman B.O., and Guilak F.Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol 25,119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro R., Olivares A.L., Brans G., Wirz D., Longinotti C., Lacroix D., et al. Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing. Biomaterials 31,8946, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Tallheden T., Bengtsson C., Brantsing C., Sjögren-Jansson E., Carlsson L., Peterson L., et al. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther 7,R560, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbero A., Grogan S., Schäfer D., Heberer M., Mainil-Varlet P., and Martin I.Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12,476, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Terraciano V., Hwang N., Moroni L., Park H.B., Zhang Z., Mizrahi J., et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25,2730, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U.In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238,265, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Yoo J.U., Barthel T.S., Nishimura K., Solchaga L., Caplan A.I., Goldberg V.M., et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80,1745, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 7,211, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Erickson G.R., Gimble J.M., Franklin D.M., Rice H.E., Awad H., and Guilak F.Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290,763, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad H.A., Wickham M.Q., Leddy H.A., Gimble J.M., and Guilak F.Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25,3211, 2004 [DOI] [PubMed] [Google Scholar]
- 17.De Bari C., Dell'Accio F., Tylzanowski P., and Luyten F.P.Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44,1928, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Nishimura K., Solchaga L.A., Caplan A.I., Yoo J.U., Goldberg V.M., and Johnstone B.Chondroprogenitor cells of synovial tissue. Arthritis Rheum 42,2631, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Dragoo J.L., Samimi B., Zhu M., Hame S.L., Thomas B.J., Lieberman J.R., et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br 85,740, 2003 [PubMed] [Google Scholar]
- 20.Wickham M.Q., Erickson G.R., Gimble J.M., Vail T.P., and Guilak F.Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res 412,196, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Diekman B.O., Christoforou N., Willard V.P., Sun H., Sanchez-Adams J., Leong K.W., et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A 109,19172, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakitani S., Nawata M., Tensho K., Okabe T., Machida H., and Ohgushi H.Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med 1,74, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Murphy J.M., Dixon K., Beck S., Fabian D., Feldman A., and Barry F.Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 46,704, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Khan W.S., Adesida A.B., and Hardingham T.E.Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther 9,R55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan W.S., Tew S.R., Adesida A.B., and Hardingham T.E.Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther 10,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurgens WJFM, Van Dijk A., Doulabi B.Z., Niessen F.B., Ritt MJPF, Van Milligen F.J., et al. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy 11,1052, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Vinardell T., Buckley C.T., Thorpe S.D., and Kelly D.J.Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med 5,673, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Buckley C.T., Vinardell T., Thorpe S.D., Haugh M.G., Jones E., McGonagle D., et al. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech 43,920, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Buckley C.T., and Kelly D.J.Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J Mech Behav Biomed Mater 11,102, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Buckley C.T., Vinardell T., and Kelly D.J.Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage 18,1345, 2010 [DOI] [PubMed] [Google Scholar]
- 31.O'HEireamhoin S., Buckley C.T., Jones E., McGonagle D., Mulhall K.J., and Kelly D.J.Recapitulating aspects of the oxygen and substrate environment of the damaged joint milieu for stem cell-based cartilage tissue engineering. Tissue Eng Part C Methods 19,117, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Lopa S., Colombini A., de Girolamo L., Sansone V., and Moretti M.New strategies in cartilage tissue engineering for osteoarthritic patients: infrapatellar fat pad as an alternative source of progenitor cells. J Biomater Tissue Eng 1,40, 2011 [Google Scholar]
- 33.Ahearne M., Liu Y., and Kelly D.J.Combining freshly isolated chondroprogenitor cells from the infrapatellar fat pad with a growth factor delivery hydrogel as a putative single stage therapy for articular cartilage repair. Tissue Eng Part A 20,930, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahearne M., and Kelly D.J.A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed Mater 8,035004, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Toghraie F.S., Chenari N., Gholipour M.A., Faghih Z., Torabinejad S., Dehghani S., et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 18,71, 2011 [DOI] [PubMed] [Google Scholar]
- 36.English A., Jones E.A., Corscadden D., Henshaw K., Chapman T., Emery P., et al. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology 46,1676, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Buckley C.T., Meyer E.G., and Kelly D.J.The influence of construct scale on the composition and functional properties of cartilaginous tissues engineered using bone marrow-derived mesenchymal stem cells. Tissue Eng Part A 18,382, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., Buckley C.T., Downey R., Mulhall K.J., and Kelly D.J.The role of environmental factors in regulating the development of cartilaginous grafts engineered using osteoarthritic human infrapatellar fat pad-derived stem cells. Tissue Eng Part A 18,1531, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murdoch A.D., Grady L.M., Ablett M.P., Katopodi T., Meadows R.S., and Hardingham T.E.Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells 25,2786, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Hu J.C., and Athanasiou K.A.A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12,969, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Elder S.H., Cooley A.J., Jr., Borazjani A., Sowell B.L., To H., and Tran S.C.Production of hyaline-like cartilage by bone marrow mesenchymal stem cells in a self-assembly model. Tissue Eng Part A 15,3025, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Revell C.M., Reynolds C.E., and Athanasiou K.A.Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng 36,1441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ando W., Tateishi K., Hart D.A., Katakai D., Tanaka Y., Nakata K., et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 28,5462, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Brehm W., Aklin B., Yamashita T., Rieser F., Trüb T, Jakob R.P., et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage 14,1214, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Mesallati T., Buckley C.T., and Kelly D.J.A comparison of self-assembly and hydrogel encapsulation as a means to engineer functional cartilaginous grafts using culture expanded chondrocytes. Tissue Eng Part C Methods 20,52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estrada L.E., Dodge G.R., Richardson D.W., Farole A., and Jimenez S.A.Characterization of a biomaterial with cartilage-like properties expressing type X collagen generated in vitro using neonatal porcine articular and growth plate chondrocytes. Osteoarthritis Cartilage 9,169, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Kim M., Kraft J.J., Volk A.C., Pugarelli J., Pleshko N., and Dodge G.R.Characterization of a cartilage-like engineered biomass using a self-aggregating suspension culture model: molecular composition using FT-IRIS. J Orthop Res 29,1881, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer E.G., Buckley C.T., Steward A.J., and Kelly D.J.The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater 4,1257, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Kafienah W., and Sims T.J.Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol Biol 238,217, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Ignat'eva NY, Danilov N.A., Averkiev S.V., Obrezkova M.V., Lunin V.V., and Sobol E.N.Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem 62,51, 2007 [Google Scholar]
- 51.Buckley C.T., Thorpe S.D., O'Brien F.J., Robinson A.J., and Kelly D.J.The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J Mech Behav Biomed Mater 2,512, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., Buckley C.T., Mulhall K.J., and Kelly D.J.Combining BMP-6, TGF-β3 and hydrostatic pressure stimulation enhances the functional development of cartilage tissues engineered using human infrapatellar fat pad derived stem cells. Biomater Sci 1,745, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Elder B.D., and Athanasiou K.A.Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev 15,43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byers B.A., Mauck R.L., Chiang I.E., and Tuan R.S.Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14,1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toyoda T., Seedhom B.B., Kirkham J., and Bonass W.A.Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology 40,79, 2002 [PubMed] [Google Scholar]
- 56.Toyoda T., Seedhom B.B., Yao J.Q., Kirkham J., Brookes S., and Bonass W.A.Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum 48,2865, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Hansen U., Schünke M., Domm C., Ioannidis N., Hassenpflug J., Gehrke T., et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech 34,941, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Vinardell T., Rolfe R.A., Buckley C.T., Meyer E.G., Ahearne M., Murphy P., et al. Hydrostatic pressure acts to stabilise a chondrogenic phenotype in porcine joint tissue derived stem cells. Eur Cell Mater 23,121–32; discussion 33–34, 2012. [DOI] [PubMed] [Google Scholar]
- 59.García-Álvarez F., Alegre-Aguarón E., Desportes P., Royo-Cañas M., Castiella T., Larrad L., et al. Chondrogenic differentiation in femoral bone marrow-derived mesenchymal cells (MSC) from elderly patients suffering osteoarthritis or femoral fracture. Arch Gerontol Geriatr 52,239, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Jones E.A., Crawford A., English A., Henshaw K., Mundy J., Corscadden D., et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum 58,1731, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Krawetz R.J., Wu Y.E., Martin L., Rattner J.B., Matyas J.R., and Hart D.A.Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One 7,e43616, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novotny J.E., Turka C.M., Jeong C., Wheaton A.J., Li C., Presedo A., et al. Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng 12,2755, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Thorpe S.D., Nagel T., Carroll S.F., and Kelly D.J.Modulating gradients in regulatory signals within mesenchymal stem cell seeded hydrogels: a novel strategy to engineer zonal articular cartilage. PLoS One 8,e60764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cicione C., Muiños-López E., Hermida-Gómez T., Fuentes-Boquete I., Díaz-Prado S., and Blanco F.J.Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int 2013,232896, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wendt D., Stroebel S., Jakob M., John G.T., and Martin I.Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions. Biorheology 43,481, 2006 [PubMed] [Google Scholar]
- 66.Nagel T., and Kelly D.J.The composition of engineered cartilage at the time of implantation determines the likelihood of regenerating tissue with a normal collagen architecture. Tissue Eng Part A 19,824, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Jin C.Z., Cho J.H., Choi B.H., Wang L.M., Kim M.S., Park S.R., et al. The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect. Tissue Eng Part A 17,3057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elder B.D., and Athanasiou K.A.Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One 3,e2341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elder B.D., and Athanasiou K.A.Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A 15,1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogawa R., Mizuno S., Murphy G.F., and Orgill D.P.The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A 15,2937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kraft J.J., Jeong C., Novotny J.E., Seacrist T., Chan G., Domzalski M., et al. Effects of hydrostatic loading on a self-aggregating, suspension culture-derived cartilage tissue analog. Cartilage 2,254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Correia C., Pereira A.L., Duarte ARC, Frias A.M., Pedro A.J., Oliveira J.T., et al. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A 18,1979, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puetzer J., Williams J., Gillies A., Bernacki S., and Loboa E.G.The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose-and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng Part A 19,299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Safshekan F., Tafazzoli-Shadpour M., Shokrgozar M.A., Haghighipour N., Mahdian R., Hemmati A.Intermittent hydrostatic pressure enhances growth factor-induced chondroinduction of human adipose-derived mesenchymal stem cells. Artif Organs 36,1065, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Carroll S.F., Buckley C.T., and Kelly D.J.Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad. J Biomech. DOI: 10.1016/j.jbiomech.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Buckley C.T., Mulhall K., and Kelly D.J.Combining BMP-6, TGF-ß3 and hydrostatic pressure stimulation enhances the functional development of cartilage tissues engineered using human infrapatellar fat pad derived stem cells. Biomater Sci 1,745, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Hennig T., Lorenz H., Thiel A., Goetzke K., Dickhut A., Geiger F., et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211,682, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Vinardell T., Sheehy E.J., Buckley C.T., and Kelly D.J.A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18,1161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.