Abstract
Lactoferrin (LF), a 78 kDa glycoprotein, has recently been recognized as an effector molecule in the skeleton due to its ability to decrease osteoclastogenesis and increase osteoblast proliferation, survival, and differentiation. The objective of the study is to investigate the feasibility of developing an injectable hydrogel from bovine lactoferrin (bLF) as a cell delivery vehicle. The study demonstrated the feasibility of cross-linking tyramine substituted bLF in the presence of horse radish peroxidase and hydrogen peroxide (H2O2). The gel presented a mild environment to maintain mouse bone marrow-derived stromal cell (mBMSC) viability and proliferation. Stromal cells derived from multiple gene reporter transgenic mouse (Ibsp-Topaz/Dmp1-mCherry) line showed the ability of the cells to undergo osteogenic differentiation in the hydrogel when cultured in mineralization media. The cross-linked gel supported protein phosphorylation/de-phosphorylation in the encapsulated MC3T3-E1 cells. bLF and bLF gel also showed the ability to modulate growth factor production in mBMSCs.
Introduction
Lactoferrin (LF) is an iron-binding glycoprotein with wide range of biological functions, including antimicrobial, anti-inflammatory, iron homeostasis, osteogenesis, angiogenesis, and immunomodulation.1 Interest in LF for orthopedic applications arose from recent reports indicating its ability to serve as a positive regulator of the skeleton.2–7 Our studies using recombinant human lactoferrin (rhLF) demonstrated the bioactivites of rhLF toward murine osteoblast-like cells.8 The anabolic effect of LF on osteoblasts and its potent inhibition of osteoclastogenesis in vitro suggested that it might have positive effects on bone mass in vivo.4 Bovine lactoferrin (bLF) administered over the right hemicalvaria of mice for 5 consecutive days resulted in a dramatic increase in bone area in the calvaria.6
To avoid repeated injections, bLF has been incorporated in various biomaterials to support in vivo bone regeneration. Type 1 collagen membrane was investigated as a bLF delivery vehicle and ∼27% of the loaded protein was released within the first hour.9 The bLF-loaded collagen membranes have been shown to promote calcium deposition, alkaline phosphatase activity, and osteocalcin production in MG63 human osteosarcoma cell line.9 Our recent study demonstrated the feasibility to incorporate LF in polymeric nanofibers.10 Another study investigated the efficacy of gelatin gel as a bLF delivery vehicle and demonstrated the ability of the gel to retain 10.14% of the loaded protein after 24 h. Implantation of bLF-loaded gelatin hydrogels in rat cranial defects showed improved bone regeneration compared with the control gelatin gel.11 However, very high concentration (30 mg/defect) of bLF was needed to induce statistically significant bone growth presumably due to the quick release of the protein from the gel. Being a pleiotropic factor with concentration-dependent biological activity,4,11,12 it is important to control the amount of bLF injected at the defect site since high concentrations can lead to adverse responses. A potential approach to reduce protein concentration is to develop a biomaterial wherein bLF is immobilized at concentrations appropriate to induce cellular activation. Bioactive proteins may activate cellular processes through two different phenomena: cell internalization/endocytosis or receptor-mediated signal transduction. It has been demonstrated that the low density lipoprotein receptor-related protein 1 (LRP1) serves as the mitogenic receptor for LF in osteoblastic cells and that the ligand endocytosis is not required for the activation of mitogenic signaling.13 Since internalization is not required for cell signaling, a cross-linked LF matrix may have the potential to serve as a biologically active microenvironment for the encapsulated cells.
The objective of the present study is to develop an injectable hydrogel based on bLF to serve as a cell delivery vehicle. Polymers functionalized with phenolic side groups have been shown to form cross-linked hydrogels in the presence of horse radish peroxidase (HRP) and hydrogen peroxide (H2O2). The phenolic residue of the polymers undergo one-electron oxidation and form radicals, which subsequently react with each other to form the cross-linked matrix in the presence of HRP and H2O2.14–17 The enzyme-mediated cross-linking can take place at physiological pH and temperature, making this a potential route to form injectable cell and protein delivery vehicles.18,19 The enzymatically cross-linked gels also lend versatility in terms of modulating the gelation time, and the physical and mechanical properties of gels by varying the phenolic content.14–17 In the present study, phenolic groups were introduced in bLF by reacting with tyramine.
Experimental Section
Materials
bLF, 2-(N-morpholino)ethanesulfonic acid (MES) buffer, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), tyramine hydrochloride, aqueous H2O2 solution (30% w/w), HRP, dexamethasone, and β-glycerophosphate were purchased from Sigma-Aldrich (St. Louis, MO). l-ascorbic acid was obtained from Fisher Scientific (Pittsburg, PA). Murine osteoblast-like cells (MC3T3-E1) were obtained from ATCC (Manassas, VA). The minimum essential medium-alpha (MEMα), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). Basal media consisted of the MEMα supplemented with 10% volume fraction of FBS and 1% volume fraction of penicillin/streptomycin. Differentiation media consisted of α-modified Eagles medium supplemented with 10% FBS, 1% volume fraction of penicillin/streptomycin, 50 μg/mL ascorbic acid, 8 mM β-glycerol phosphate, and 10−8 M dexamethazone.
Methods
mBMSC culture
Mouse bone marrow-derived stromal cells (mBMSC) were procured from Texas A&M University, Texas. The cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 9% fetal calf serum, 9% horse serum, 1% penicillin and streptomycin, 0.25 μL/mL amphotericin B, and 12 mM l-glutamine.
Isolation of stromal cells from Ibsp-Topaz/Dmp1-mCherry mouse
The fluorescent IBSP-Topaz/DMP1-mCherry mice were generated as previously described.20 Optically distinct fluorescent protein reporters and bacterial recombination strategies were used to create this informative and biologically relevant transgenic animal model. The stromal cells were isolated as follows. Transgenic mice were sacrificed via CO2 asphyxiation and the femoral bones were isolated. Bone marrow was flushed out of the femoral bones using 18-gauge needles. The stromal cells were then cultured in basal media (α-modified Eagles medium, 10% FBS, and 1% volume fraction of penicillin/streptomycin) for 4 days before encapsulation in the hydrogel.
Preparation of modified bLF
Standard carbodiimide-mediated coupling of amino groups of tyramine with the carboxyl groups of bLF was used to develop the modified bLF. Modified bLF was prepared as described. Briefly, 500 mg bLF was dissolved in 50 mL of 1 M MES buffer. To this solution, appropriate amounts of EDC (0.041 M), NHS (0.026 M), and tyramine hydrochloride (0.034 M) were added. The mixture was allowed to react for different time (1, 5, 15, and 24 h) under gentle stirring. The modified polymer was purified by dialysis against excess distilled and deionized water using standard regenerated cellulose dialysis tubing (MWCO 10,000) followed by lyophilization.
Characterization of modified bLF
Phenolic content of the modified bLF was determined at 275 nm using UV-Vis Spectrophotometer (Thermo Scientific Evolution 60) based on a standard curve prepared using tyramine.
Gelation time
The enzymatic gelation of modified bLF solution in HRP was initiated by adding H2O2. The effect of tyramine modification time on the gelation time of modified bLF (10 mg/mL), in the presence of HRP (10 U/mL) and 0.25% H2O2 was investigated. Gelation time was determined via vial inversion method.
Bovine LF gel morphology
The morphology of the bLF gels formed from modified bLF in the presence of 10 U HRP and 0.25% H2O2 was visualized by scanning electron microscope (SEM; Hitachi TM-1000). The gels were flash frozen by immersing in liquid nitrogen followed by lyophilization before imaging.
Real time polymerase chain reaction
mBMSCs were plated at low density and grown to 90% confluence. Cells were then treated with 100 μg/mL of bLF and or control (phosphate-buffered saline [PBS]) in osteogenic media for 7 and 14 days. Quantitative real-time PCR was employed to assess the influence of the bLF on β-catenin, IGF-1, and IGF-2 gene expression. Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Venlo, Limburg) according to the manufacturer's instructions. For cDNA synthesis, 2 μg total RNA was used as a template for Sprint RT Complete cDNA synthesis kit (Clontech, Mountain, CA) in a total volume of 20 μL. For quantitative real-time PCR, iCycler Thermal Cycler Base (Bio-Rad, Hercules, CA) and iQ Supermix (Bio-Rad), β-catenin, IGF-1, IGF-2, and GAPDH gene probes were used. The increase in reaction products during PCR was monitored by measuring the increase in fluorescence intensity caused by the binding of SYBR green to double-stranded DNA that accumulated during PCR cycles. Threshold cycle values of target genes was standardized against GAPDH expression and normalized to the expression in the control culture. The fold change in expression was calculated using the ΔΔCt comparative threshold cycle method.
Cell encapsulation in bLF gel
Modified bLF was dissolved in 10 U HRP solution in 50:50 PBS/water solution at a concentration of 10 mg/mL. The cells were resuspended in bLF/HRP solution and the solution was gelled by adding 0.25% H2O2 in PBS. The encapsulated cells were then maintained in basal or differentiation media at 37°C.
Western blot analysis
MC3T3-E1 cells (2,000,000) were encapsulated in 1 mL of bLF gels. The cells were cultured for 24 h in phenol-free basal media. Cell Lytic M and Protease Inhibitor (Sigma-Aldrich) were added to cells and then incubated at 4°C for 30 min. The gel matrix was broken down by repeated aspiration using a syringe. Protein concentrations were measured using BCA Protein Assay Kit (Thermo Scientific) and the absorbance of the samples were measured at 562 nm after 30 min incubation at 37°C. Each sample for western blotting was prepared with Laemmli Sample Buffer (Bio-Rad) and samples were boiled for 5 min. Twenty-five micrograms of each sample were run on 4–15% Tris-HCl Ready Gels (Bio-Rad) for sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis. Mini-PROTEAN Tetra System, 10× Tris/Glycine/SDS Buffer, and Precision Plus Protein Dual Color Standard (Bio-Rad) were used and gels were run at a constant 100 V. Gels were transferred at a constant 100 V for 2 h using 10× Tris/Glycine Buffer, Mini Trans-Blot Electrophoresis Transfer Cell, blot papers, and 0.2 μm Nitrocellulose Membrane. Membranes were blocked for 2 h at 4°C in 10% milk/TBS-T solution (Tris-buffered saline and 0.1% Tween-20). Membranes were washed with TBS-T solution after each incubation. Membranes were incubated overnight with primary antibodies diluted in 5% milk/TBS-T solution at 4°C and were incubated for 45 min with secondary antibody diluted in 5% milk/TBS-T solution at 4°C and then washed thrice with TBS-T. Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific) was used for signal detection on films.
Cell viability and spreading
The mBMSCs encapsulated bLF gels were incubated at 37°C in basal medium for 1 and 7 days. The cells were then stained with Live/Dead Viability/Cytotoxicity Kit (Invitrogen) and imaged by confocal microscope (Zeiss LSM 510 Confocal Microscope).
Cell differentiation in bLF gel
Stromal cells isolated from IBSP-Topaz/DMP1-mCherry mouse were encapsulated in bLF gels (500,000 cells/mL) and cultured in mineralization media. The cells were imaged at 0, 3, 6, 10, and 20 days postencapsulation using confocal microscopy (Zeiss LSM 510 Confocal Microscope).
Cell encapsulation in gelatin gel
Tyramine-modified gelatin was prepared according to the reported protocol17 and used as the three-dimensional (3D) reference matrix. Similar to modified bLF, tyraminated gelatin was dissolved in 10 U/mL HRP solution in 50:50 PBS/water solution at a concentration of 10 mg/mL. Cell pellet was resuspended in gelatin/HRP solution and gelled by adding 0.25% H2O2 in PBS. The encapsulated cells were then maintained in basal media at 37°C for immunocytochemical analysis.
Immunocytochemical analysis
For two-dimensional (2D) study, 100,000 BMSCs were plated on 24-well plate (n=3) and cultured for 24 h. Cells were then serum starved for 18 h and treated with 100 μg/mL of bovine LF and untreated (PBS control) for 24 h. For 3D study, BMSCs (500,000/mL) were encapsulated in bLF gels and gelatin gel and cultured for 24 h in basal media (n=3).
All samples were fixed with ice-cold methanol for 15 min and then washed in PBS. Ten percent normal blocking serum in PBS was then added to each sample for 20 min to suppress nonspecific binding of IgG. Samples were then incubated in primary antibody diluted (1:100) in 1% normal blocking serum for 60 min and used in accordance with the manufacturer's instructions. After PBS washing, samples were incubated for 45 min in dark chamber with FITC-conjugated secondary antibody (ABcam) diluted 1:200 in 1% normal blocking serum. Samples were mounted using Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA) with propidium iodide (nuclear staining) (Sigma-Aldrich) and imaged using confocal microscopy (Zeiss LSM 510 Confocal Microscope).
Results and Discussion
Development of injectable hydrogel from modified bLF
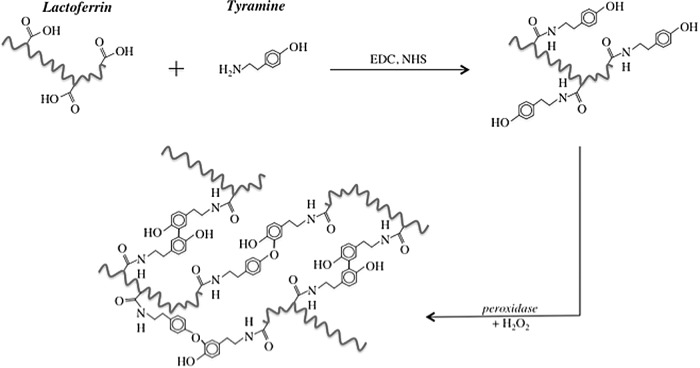
Being a polypeptide, bLF contains several amino acids carrying carboxyl groups such as aspartic acid and glutamic acid and amino acids with phenolic groups such as tyrosine. Upon activation with H2O2, the phenolic groups in polymers act as the preferred substrates for HRP to mediate polymer cross-linking. Despite having some tyrosine residues, bLF was unable to undergo enzymatic cross-linking in the presence of HRP and H2O2, indicating its phenolic content is lower than what is needed to develop an effectively cross-linked matrix at the concentration studied. Therefore, attempts were made to increase the phenolic content of the bLF by chemically substituting the carboxyl groups of bLF with tyramine using standard carbodiimide chemistry (Fig. 1). Figure 2A shows the change in phenolic content of bLF as a function of reaction time. As can be seen, the extent of tyramine substitution increases with the reaction time. The phenolic content of the unmodified bLF is presumably due to the tyrosine residues. The modified bLF after 24 h of reaction showed a phenolic content ∼1.5 times higher than the unmodified bLF.
FIG. 1.
Schematic showing the enzymatic cross-linking of modified bovine lactoferrin (bLF).
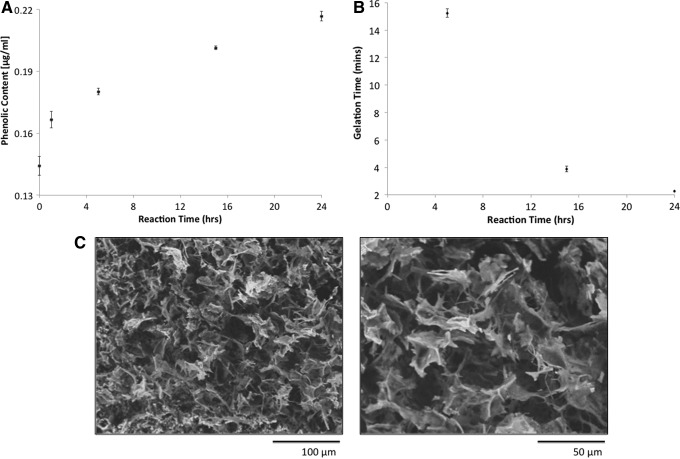
FIG. 2.
(A) Effect of tyramine modification time on bLF phenolic content; (B) Effect of tyramine modification time on bLF gelation time. Each data point represents the average of triplicate samples. (C) Scanning electron microscope analysis of flash-frozen and freeze-dried bLF hydrogel. Modified bLF (25 mg/mL) was dissolved in 10 U horse radish peroxidase (HRP) solution and treated with 0.25% hydrogen peroxide (H2O2) to form the gel.
For clinical applications, a gelation time of 1–2 min is considered to be ideal for injectable hydrogels as cell or protein delivery vehicle.21 Therefore, gelation time was used to determine the appropriate phenolic content of modified bLF. Figure 2B shows the gelation time of bLF reacted with tyramine for various periods of time. The gelation time of the modified bLF solution was found to depend on the reaction time. The 1 h reacted bLF behaved similar to that of unmodified bLF and was unable to undergo gelation under the tested reacted conditions. The 5 h reacted bLF underwent gelation in ∼15 min and the gelation time significantly decreased in the case of 15 h reacted bLF. The 24 h reacted bLF underwent gelation in about 2 min, which falls in the clinically feasible injection time and was therefore used for the rest of the study.
The morphology of the gels was investigated using SEM. Figure 2C shows the morphology of the gel at two different magnifications. The gels presented an open irregular porous structure indicating the potential for nutrient diffusion and ability to support 3D cell growth. The flaky appearance of bLF gel was found to be different from the more defined pore structure observed in the case of rhLF gel developed by the similar enzymatic cross-linking process.22 Even though both human and bovine LFs have shown to have biological activities toward musculoskeletal cells, there is only 69% species homology between bLF and hLF.23,24 Further studies are required to understand the effect of protein composition on the gel microstructure.
Cellular interactions with bLF and bLF gel
LF is considered as a bone anabolic molecule and previous studies demonstrated the ability of bLF to improve osteoblast survival, proliferation, and differentiation, even though the molecular mechanism behind bLF's anabolic effect is not completely understood.2,25 Bone regeneration is a coordinated cascade of spatiotemporal events regulated by an array of cytokines and growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), bone morphogenetic protein (BMP), and Wnts.25 The ability of LFs to increase growth factors and cytokine production in cells has been recognized as a key factor for its pleotropic effects. Our group, along with others, have demonstrated the ability of LF to upregulate the synthesis of growth factors such as VEGF and FGF in MC3T3-E1 preosteoblast cells.8,26,27 Another potential anabolic molecule that has been shown to play a key role in cell survival, proliferation, and differentiation is IGF. Studies have shown the increased bone turnover via increased osteoblast number and function can be directly and/or indirectly caused by local IGF-1 and IGF-2 production.28 The ability of bLF to bind to insulin-like growth factor-binding protein-3 (IGFBP-3) suggests that bLF might also be involved in the regulation of the IGF system.29 The ability of human primary osteoblast cells to significantly upregulate IGF-1 mRNA in the presence of LF has recently been reported.12 Using rhLFs of different iron concentrations, we recently demonstrated the ability of LF to stabilize β-catenin and regulate several key upstream proteins and transcription factors of β-catenin in MC3T3-E1 cells.8,22 β-catenin plays major roles during the development of multicellular organisms. It serves as a downstream effector of the canonical Wnt signaling cascade, which is implicated in skeletal regeneration and to cadherins and is required for the correct assembly and function of adherent junctions.30 In the present study, we investigated the ability of bLF to modulate the gene expression of β-catenin, IGF-1, and IGF-2 in mBMSCs during osteogenic differentiation using real time polymerase chain reaction (RT-PCR) (Fig. 3). At day 7, significant upregulation of β-catenin, and IGF-1 were observed in bLF-treated mBMSCs compared with control. At day 14, significant upregulation of both IGF-1 and IGF-2 was observed in bLF-treated cells compared with control. The data demonstrate the potential of bLFs to modulate growth factor production in mBMSCs.
FIG. 3.
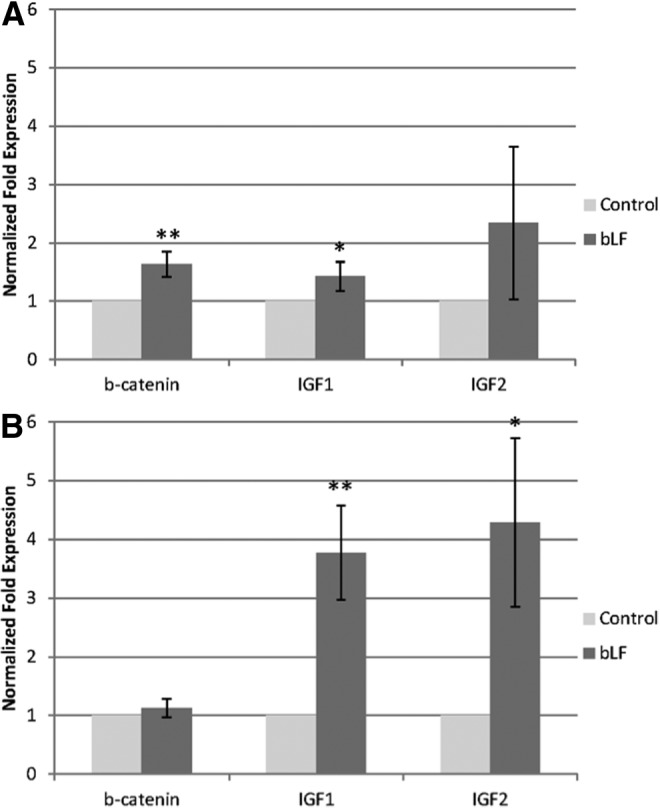
Real time polymerase chain reaction data showing the mRNA expression of β-catenin, IGF-1, and IGF-2 by mouse bone marrow-derived stromal cells (mBMSCs) at (A) 7 and (B) 14 days in culture. Cells were cultured in mineralization media and fed with 100 μg/mL bLF and equal volume of phosphate-buffered saline was added to the control group. Statistically significant differences were evaluated by t-test and are indicated (*p<0.05 and **p<0.01).
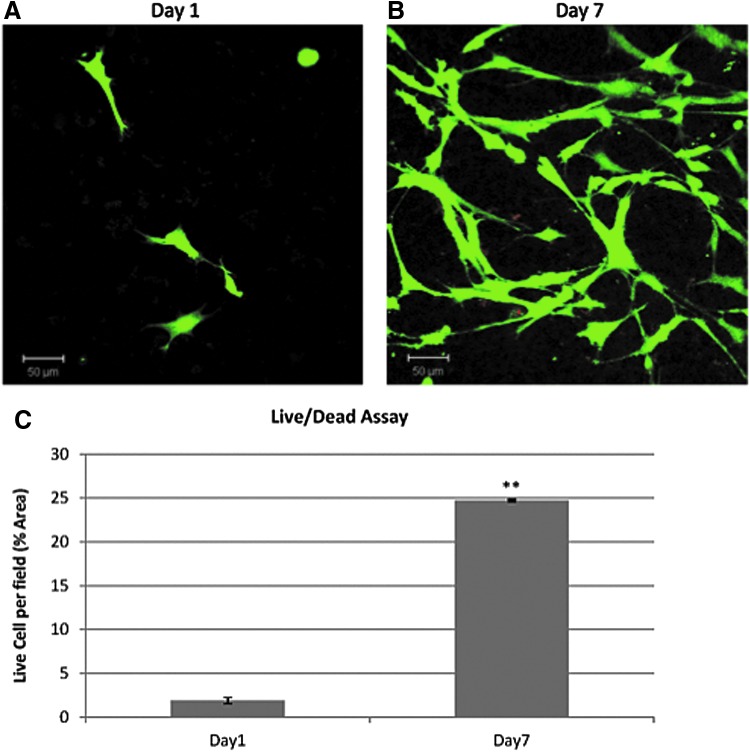
Figure 4 shows the cell viability as followed by LIVE/DEAD fluorescent staining of mBMSCs encapsulated in bLF gels. Figure 4A and B show the viability of cells cultured in basal media after 1 and 7 days respectively. As can be seen from the green fluorescence, the HRP-mediated enzymatic cross-linking process presents a mild environment to maintain the viability of encapsulated cells and the cells started spreading within 24 h of encapsulation. At day 7, the viability of the cells was maintained. Moreover, the cell number was found to be significantly higher than that at day 1 (Fig. 4C). Previous studies have demonstrated the significant mitogenic effect of bLFs toward a variety of cell types.6–8 The bLFs mitogenic activity is reported to be at least partially modulated by LRP1 receptors by inducing phosphorylation of MAP kinase signaling transduction cascade.13 The data demonstrated the nontoxicity of enzymatically cross-linked bLF gels and its ability to maintain the viability of encapsulated mBMSCs and support cell proliferation. It remains to be clarified if the bLF gel induce LRP1-mediated MAP kinase signaling pathways in the encapsulated mBMSCs.
FIG. 4.
Live/Dead fluorescent staining showing the viability of the mBMSCs encapsulated in bLF gel. Green color showing live cells and red color showing dead cells. Cells were cultured in basal media (A) after 1 day in culture and (B) after 7 days in culture. (C) Percentage of live cells per field was calculated for the mBMSCs encapsulated in bLF gel after 1 and 7 days. Statistically significant differences were evaluated by t-test and are indicated (**p<0.01). Color images available online at www.liebertpub.com/tea
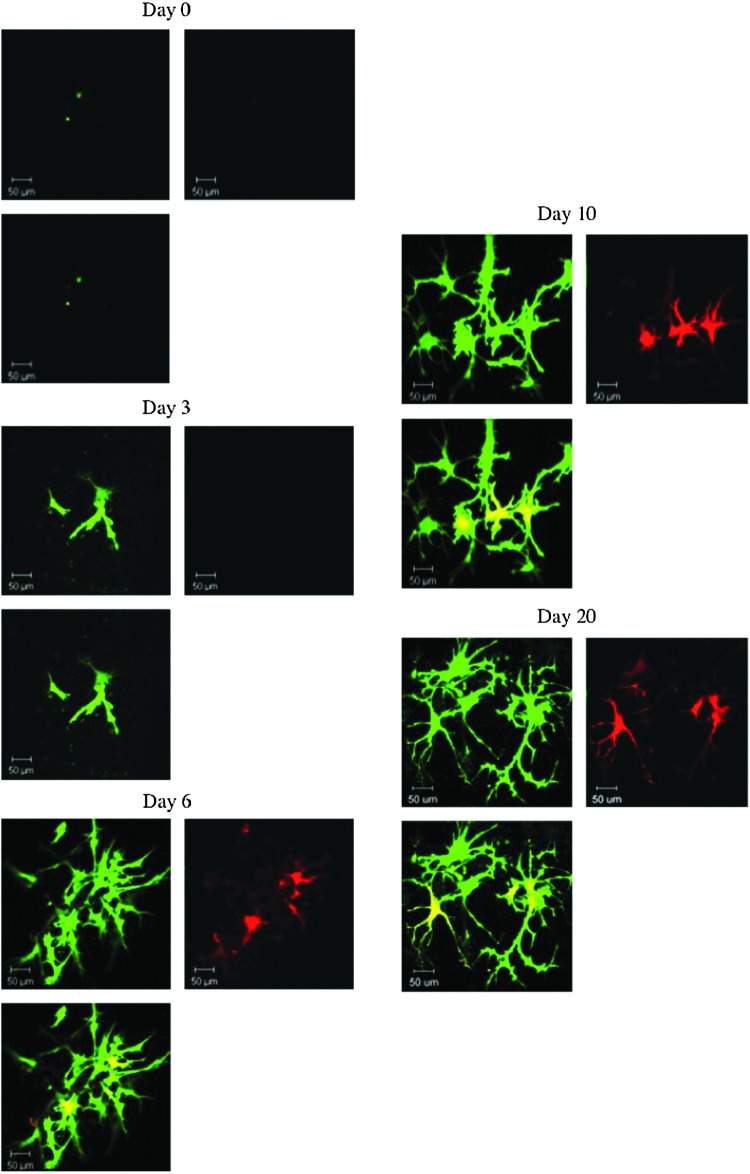
Following the phenotypic changes in the encapsulated cells via live cell fluorescent imaging is an attractive tool to understand cell behavior in 3D culture. In situ characterization techniques are particularly of importance in the case of chemically cross-linked hydrogels, wherein cell isolation from the cross-linked hydrogel poses significant challenges. We investigated the feasibility of using transgenic osteoblast reporter mouse-derived stromal cells to follow the sequence of cell differentiation upon encapsulation in the gel. IBSP-Topaz/DMP1-mCherry mouse is a multi-fluorescent protein reporter animal selectively expressing spectrally distinct fluorescent proteins based on regulatory elements derived from Ibsp and Dmp1, which are highly expressed in osteocytes and osteoblasts respectively. Ibsp is an early marker of osteoblast differentiation and Dmp1 is a noncollagenous matrix protein present in dentin and bone. The Ibsp indicated by green fluorescence served as an indicator for early stage and Dmp1 indicated by red fluorescence as a late stage marker for osteogenic differentiation. Figure 5 shows the fluorescent photomicrographs of stromal cells isolated from IBSP-Topaz/DMP1-mCherry mouse encapsulated in bLF gel as a function of culture time. Figure 5A shows the lack of fluorescence in the encapsulated cells indicating the absence of Ibsp or Dmp1 expression in the cells indicating a rather undifferentiated cell population. At day 3 of the culture, the cells showed the presence of green fluorescence indicating the expression of Ibsp by the cells (Fig. 5B). The absence of red fluorescence denotes the lack of expression of Dmp1 at that time point indicating the early differentiation stage of the cells. Figure 5C shows the fluorescent image 6 days postencapsulation. An increase in green fluorescence at day 6 compared to day 3 indicates an increase in Ibsp expression. Moreover, at day 6, red fluorescence was also observed indicating the expression of Dmp1 by the cells. The cells maintained the green and red fluorescence on subsequent time points (days 10 and 20), indicating the osteogenic lineage of the encapsulated cells. The study demonstrated the ability of bLF gel to support the differentiation of stromal cells when cultured in mineralization media as evidenced by the temporal expression of the osteogenic proteins. Since the in situ fluorescent staining shows only the cells that are differentiating, further studies are required to understand the percent of the total population of cells that are undergoing osteogenic differentiation. A recent study demonstrated the favorable effect of LF on human adipose stem cell differentiation.31 However, the effect of bLF on mBMSCs has not been investigated. Further studies are also required to understand whether bLF can promote the osteogenic differentiation of mBMSCs. Future studies will focus on comparing the temporal expression of Ibsp and Dmp1 proteins in bLF gel compared to cells encapsulated in other standard gels to understand whether bLF gel can favorably modulate the osteogenic differentiation of mBMSCs.
FIG. 5.
Ibsp & Dmp1 reporter expression of mouse stromal cells isolated from transgenic Ibsp-Topaz/Dmp1-mCherry mice encapsulated in 10 mg/mL bLF gel as a function of time. The upper left column shows the expression of Ibsp in green, the upper right column shows the expression of Dmp1 in red, and the bottom left column shows the merge of the two colors. Color images available online at www.liebertpub.com/tea
To investigate the ability of bLF gels to modulate signaling pathways in encapsulated cells, MC3T3-E1 cells were used, since our previous studies demonstrated the ability of soluble LF to significantly activate β-catenin and increase Akt phosphorylation in MC3T3-El cells.8 Phosphorylated Akt is known to play a critical role in cell survival (anti apoptosis) and its function has also been reported to play an important role in glycogen metabolism and cell growth.32,33 LF is known to induce the phosphorylation of Akt, however, it has been reported that the anti-apoptotic activity of bLF toward osteoblast cells is not dependent on the phosphorylation of Akt implicating potential other functions.5 Our recent study demonstrated the involvement of Wnt5a in LF-mediated cell survival in MC3T3-El cells.22 Figure 6 shows the expression of phosphorylated/dephosphorylated proteins in MC3T3-E1 cells after 24 h in culture in the presence of bLF. Figure 6 (lane 2) is the western blot showing the expression of phosphorylated Akt by MC3T3-E1 cells when cultured on TCPS (2D culture) in the presence of 100 μg/mL bLF compared to the untreated 2D control (Fig. 6, lane 1). Cells treated with bLF showed positive expression for pAkt. Since soluble bLF can significantly stimulate phosphorylated Akt in MC3T3-E1 cells after 24 h in culture, the protein was used as a bioactive marker to test the bioactivity of injectable bLF gel. Figure 6 (lane 3) is the western blot showing the expression of phosphorylated Akt by MC3T3-E1 cells encapsulated in injectable bLF gel after 24 h in culture indicating the potential of bLF gel to retain some of the biological activities of the protein. Equivalent levels of tubulin in all the lanes confirmed equal protein loading in the above experiments. Similar to pAkt, significant β-catenin de-phosphorylation or activation was seen in MC3T3-E1 cells when treated with 100 μg/mL bLF compared with control culture (Fig. 6, lane 2 vs. lane 1). Figure 6 (lane 3) is the western blot showing the positive expression of active β-catenin by cells encapsulated in bLF gels. Since the de-phosphorylation (activation) and subsequent accumulation of β-catenin is a key step in cellular signaling pathway, the ability of the bLF gel to support the activation of β-catenin in the encapsulated cells implies its potential biological activity. Studies have shown that the 3D culture34 and the physical and mechanical properties of the carriers35 can significantly affect cellular functions. Further studies are required to delineate the effect of matrix properties and matrix-induced receptor-mediated signaling in the encapsulated cells to understand the biological activity of the gel.
FIG. 6.
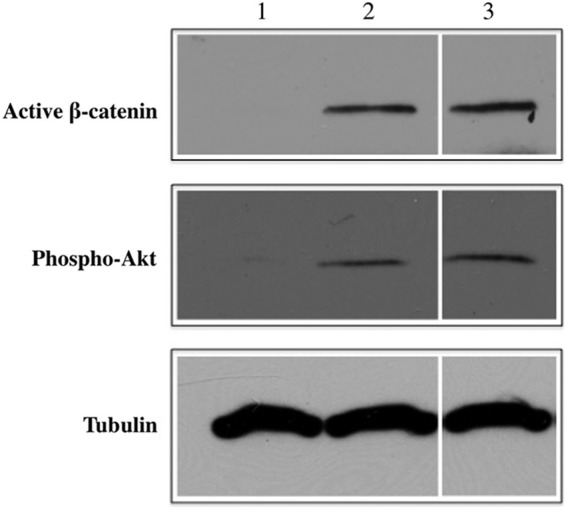
Increased stabilization of β-catenin and phosphorylation of Akt in MC3T3-E1 cells after 24 h treatment in two-dimensional (2D) culture; lane 1: untreated control; lane 2: cells exposed to 100 μg/mL of bLF; lane 3: cells encapsulated in 10 mg/mL bLF gel. Similar to cells cultured in the presence of soluble bLF, cells encapsulated in bLF gel showed expression of β-catenin and phosphorylated Akt.
The RT-PCR results (Fig. 3) showed significant gene upregulation of IGF-1 and IGF-2 by mBMSCs and previous studies have demonstrated the ability of bLF to increase the expression of VEGF in MC3T3-E1 cells.21,27 We next investigated the expression of these proteins in mBMSCs cultured on TCPS (2D culture) for 24 h in basal media in the presence and absence of 100 μg/mL of bLF (Fig. 7). As can be seen from the fluorescent photomicrographs, bLF increased the protein expression of IGF-2 and VEGF-α in mBMSCs compared with the untreated control after 24 h in culture. VEGF is a major angiogenic factor that has shown to be essential during early stages of bone healing by promoting angiogenesis.36 Previous studies have shown the ability of bLF to enhance the expression of VEGF in MC3T3-E1 cells mediated by p44/p42 MAP kinase pathway.27 The p44/p42 MAP kinase pathway is mediated by LRP1 cell surface receptors in osteoblast cells.13 Since soluble bLF treatment increased VEGF-α expression in mBMSC, immunocytochemical analysis was used to follow the expression of VEGF in mBMSCs encapsulated in bLF hydrogel (Fig. 8). Cells encapsulated in gelatin gel prepared using the same enzymatic cross-linking process was used as a 3D reference matrix to evaluate the differences in expression of VEGF-α by the encapsulated cells. mBMSCs encapsulated in bLF hydrogel showed an increase in VEGF-α expression (as indicated by the green fluorescence) compared with cells encapsulated in gelatin hydrogel supporting the potential biological activity of bLF gel.
FIG. 7.
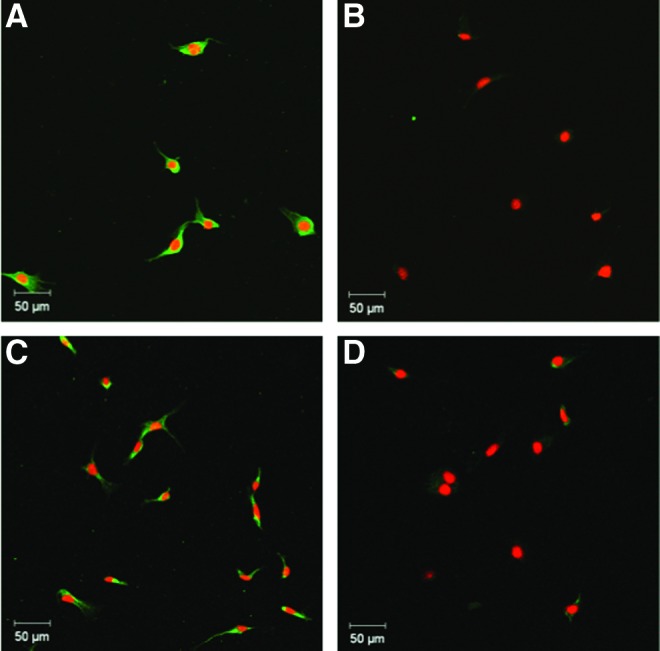
Fluorescent photomicrographs of bLF (100 μg/mL)-treated mBMSCs stained for (A) IGF-2 and (C) vascular endothelial growth factor (VEGF)-α and untreated mBMSCs (control) stained for (B) IGF-2 and (D) VEGF-α after 24 h in 2D culture. Nuclei were stained using propidium iodide (red) and protein expression was detected using FITC-labeled (green) secondary antibodies. The data demonstrate the increased expression of both IGF-2 and VEGF-α by bLF-treated cells compared with the untreated control. Color images available online at www.liebertpub.com/tea
FIG. 8.
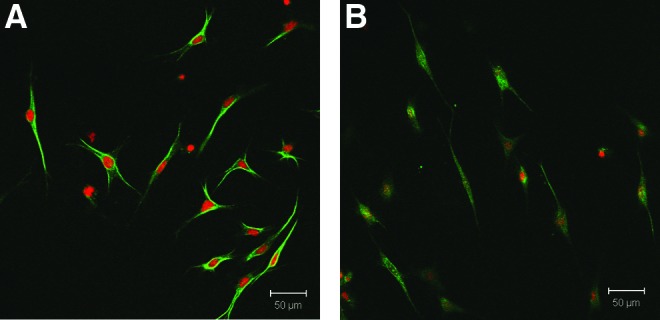
Fluorescent photomicrographs of mBMSCs encapsulated in (A) bLF gel (10 mg/mL) (B) gelatin gel (10 mg/mL) cultured in basal media for 24 h immunostained for VEGF-α. Nuclei were stained using propidium iodide (red) and protein expression was detected using FITC-labeled (green) secondary antibodies. The data indicate the increased expression of VEGF-α by cells in bLF gel compared with cells encapsulated in gelatin gel. Color images available online at www.liebertpub.com/tea
Conclusions
This study demonstrated the feasibility of developing enzymatically cross-linkable injectable hydrogel from bLF in the presence of HRP and H2O2. The mild enzymatic cross-linking process presented a conducive microenvironment to support mBMSC viability. Temporal expression of Ibsp and Dmp1 proteins in mouse stromal cells encapsulated in bLF gel showed that the cells can undergo osteogenic differentiation when cultured in the gel in osteogenic media. The cells encapsulated in bLF gel showed the expression of active β-catenin and phophorylated Akt, two proteins known to be significantly upregulated by LFs. The study also demonstrated the ability of bLF and bLF gel to modulate growth factor production in mBMSCs. Being a pleiotropic factor, LF and modified LF might elicit a variety of biological activity in the presence of multiple cell types. This aspect needs to be comprehensively studied to appreciate the effectiveness of the protein to support tissue regeneration. Furthermore, the effect of the local retention of a biologically active gel for prolonged time in vivo needs to be investigated to understand the potential of this biomaterial as a cell delivery vehicle.
Acknowledgments
The authors greatly acknowledge the funding support from the Department of Defense (W81XWH-10-1-0947), NIH AR-061575, Raymond and Beverly Sackler Center for Biomedical, Biological, Physical, and Engineering Sciences, and Mr. Eric James for the SEM images.
Disclosure Statement
The authors do not have commercial associations that might create a conflict of interest in connection with this article.
References
- 1.Gonzalez-Chavez S.A., Arevalo-Gallegos S., and Rascon-Cruz Q.Lactoferrin: structure, function and applications. Int J Antimicrob Agents 33,301e1, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Cornish J., and Naot D.Lactoferrin as an effector molecule in the skeleton. Biometals 23,425, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Cornish J., Palmano K., Callon K.E., Watson M., Lin J.M., Valenti P., Naot D., Grey A.B., and Reid I.R.Lactoferrin and bone; structure-activity relationships. Biochem Cell Biol 84,297, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Naot D., Grey A., Reid I.R., and Cornish J.Lactoferrin—a novel bone growth factor. Clin Med Res 3,93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grey A., Zhu Q., Watson M., Callon K., and Cornish J.Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol Cell Endocrinol 251,96, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cornish J.Lactoferrin promotes bone growth. Biometals 17,331, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cornish J., Callon K.E., Naot D., Palmano K.P., Banovic T., Bava U., Watson M., Lin J.M., Tong P.C., Chen Q., Chan V.A., Reid H.E., Fazzalari N., Baker H.M., Baker E.N., Haggarty N.W., Grey A.B., and Reid I.R.Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 145,4366, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Amini A.A., and Nair L.S.Evaluation of the bioactivity of recombinant human lactoferrin towards murine preosteoblast cells. Tissue Eng Part A 19,1047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takayama Y., and Mizumachi K.Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J Biosci Bioeng 107,191, 2009 [DOI] [PubMed] [Google Scholar]
- 10.James E., and Nair L.S.Development and characterization of lactoferrin loaded poly(e-caprolactone) nanofibers. J Biomed Nanotechnol 10,500, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Takaoka R., Hikasa Y., Hayashi K., and Tabata Y.Bone regeneration by lactoferrin released from a gelatin hydrogel. J Biomater Sci Polym Ed 22,1581, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Naot D., Chhana A., Matthews B.G., Callon K.E., Tong P.C., Lin J.M., Costa J.L., Watson M., Grey A.B., and Cornish J.Molecular mechanisms involved in the mitogenic effect of lactoferrin in osteoblasts. Bone 49,217, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Grey A., Banovic T., Zhu Q., Watson M., Callon K., Palmano K., Ross J., Naot D., Reid I.R., and Cornish J.The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol 18,2268, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kurisawa M., Chung J.E., Yang Y.Y., Gao S.J., and Uyama H.Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem Commun (Camb) 34,4312, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S., Uyama H., and Kimura S.Enzymatic polymerization. Chem Rev 101,3793, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Jin R., Hiemstra C., Zhong Z., and Feijen J.Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials 28,2791, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Amini A.A., and Nair L.S.Enzymatically crosslinked gelatin gel as osteoblast delivery vehicle. J Bioact Compat Polym 27,342–355, 2012 [Google Scholar]
- 18.Lee F., Chung J.E., and Kurisawa M.An injectable hyaluronic acid-tyramine hydrogel system for protein delivery. J Control Release 134,186, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Sakai S., Yamada Y., Zenke T., and Kawakami K.Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J Mater Chem 19,230, 2009 [Google Scholar]
- 20.Maye P., Stover M.L., Liu Y., Rowe D.W., Gong S., and Lichtler A.C.A BAC-bacterial recombination method to generate physically linked multiple gene reporter DNA constructs. BMC Biotechnol 9,20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amini A.A., and Nair L.S.Evaluation of the osteogenic activity of injectable bovine lactoferrin gel. Proceedings of the 2011 Annual Meeting of the Society for Biomaterials Soc Biomater 2011 [Google Scholar]
- 22.Amini A.A., and Nair L.S.Characterization of recombinant human lactoferrin gel as an injectable osteogenic biomaterial. Proceedings of the TERMIS-NA annual conference, 2011. Tissue Eng Int Regen Med Soc 2011 [Google Scholar]
- 23.van Berkel P.H., Welling M.M., Geerts M., van Veen H.A., Ravensbergen B., Salaheddine M., Pauwels E.K., Pieper F., Nuijens J.H., and Nibbering P.H.Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat Biotechnol 20,484, 2002 [DOI] [PubMed] [Google Scholar]
- 24.van Berkel P.H., van Veen H.A., Geerts M.E., and Nuijens J.H.Characterization of monoclonal antibodies against human lactoferrin. J Immunol Methods 267,139, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Amini A.A., and Nair L.S.Lactoferrin: a biologically active molecule for bone regeneration. Curr Med Chem 18,1220, 2011 [DOI] [PubMed] [Google Scholar]
- 26.James E., Xiao L., Hurley M.M., and Nair L.S.Lactoferrin is a regulator of FGF expression in osteoblast-like MC3T3 cells. Proceedings of the 57th ORS annual meeting Orthop Res Soc 2011 [Google Scholar]
- 27.Nakajima K.I., Kanno Y., Nakamura M., Gao X.D., Kawamura A., Itoh F., and Ishisaki A.Bovine milk lactoferrin induces synthesis of the angiogenic factors VEGF and FGF2 in osteoblasts via the p44/p42 MAP kinase pathway. Biometals 24,847, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Bouillon R.Growth hormone and bone. Horm Res 36Suppl 1,49, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Baumrucker C.R., Gibson C.A., and Schanbacher F.L.Bovine lactoferrin binds to insulin-like growth factor-binding protein-3. Domest Anim Endocrinol 24,287, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Arnsdorf E.J., Tummala P., and Jacobs C.R.Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4,e5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ying X., Cheng S., Wang W, lin Z, Chen Q., Zhang W., Kou D., Shen Y., Cheng X., Peng , Lei. , Xu H.Z., and Lu C.Z.Effect of lactoferrin on osteogenic differentiation of human adipose stem cells. Int Orthop 36,647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajduch E., Litherland G.J., and Hundal H.S.Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett 492,199, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Nave B.T., Ouwens M., Withers D.J., Alessi D.R., and Shepherd P.R.Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344Pt 2,427, 1999 [PMC free article] [PubMed] [Google Scholar]
- 34.Lo A.T., Mori H., Mott J., and Bissell M.J.Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia 17,103, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Cohen D.M., and Chen C.S.Mechanical control of stem cell differentiation. StemBook 2008. DOI: 10.3824/stembook.1.26.1 [DOI] [PubMed] [Google Scholar]
- 36.Amini A.A., and Nair L.S.Injectable hydrogels for bone and cartilage repair. Biomed Mater 7,024105, 2011 [DOI] [PubMed] [Google Scholar]