Abstract
Aims
β2-Spectrin is an actin-binding protein that plays an important role in membrane integrity and the transforming growth factor (TGF)-β signalling pathway as an adaptor for Smads. Loss of β2-spectrin in mice (Spnb2−/−) results in embryonic lethality with gastrointestinal, liver, neural, and heart abnormalities that are similar to those in Smad2+/−Smad3+/− mice. However, to date, the role of β2-spectrin in embryogenesis, particularly in heart development, has been poorly delineated. Here, we demonstrated that β2-spectrin is required for the survival and differentiation of cardiomyocytes, and its loss resulted in defects in heart development with failure of ventricular wall thickening.
Methods and results
Disruption of β2-spectrin in primary muscle cells not only inhibited TGF-β/Smad signalling, but also reduced the expression of the cardiomyocyte differentiation markers Nkx2.5, dystrophin, and α-smooth muscle actin (α-SMA). Furthermore, cytoskeletal networks of dystrophin, F-actin, and α-SMA in cardiomyocytes were disorganized upon loss of β2-spectrin. In addition, deletion of β2-spectrin in mice (Spnb2tm1a/tm1a) prevented proper development of the heart in association with disintegration of dystrophin structure and markedly reduced survival.
Conclusion
These data suggest that β2-spectrin deficiency leads to inactivation of TGF-β/Smad signalling and contributes to dysregulation of the cell cycle, proliferation, differentiation, and the cytoskeletal network, and it leads to defective heart development. Our data demonstrate that β2-spectrin is required for proper development of the heart and that disruption of β2-spectrin is a potential underlying cause of congenital heart defects.
Keywords: β2-Spectrin, TGF-β, Cytoskeleton, Cardiogenesis
1. Introduction
Spectrin, a tetrameric protein consisting of two antiparallel dimers of α- and β-subunits,1 acts as a molecular scaffold to link the plasma membrane to the actin cytoskeleton and actin cross-linking. It is known to play a key role in membrane integrity, cell shape and polarity, and cell–cell interaction.2 β2-Spectrin is ubiquitously expressed and is the most common non-erythrocytic member of the family of β-spectrin genes. Gene-targeting studies in mice have also shown that β2-spectrin regulates transforming growth factor-β (TGF-β) signalling through interactions with Smad3/4 adaptor proteins.3,4 TGF-β stimulates the translocation of Smads, which control multiple cellular processes, including cell growth, differentiation, adhesion, migration, and apoptosis. Activation of Smads increases the transcription of CDK inhibitors, inhibiting G1/S phase transition and subsequently inducing cell-cycle exit and differentiation.5 In this process, β2-spectrin plays a role in the translocation of Smads, and its loss causes G1-S phase transition through the activation of cyclin D1/CDK4 in hepatocellular carcinogenesis and angiogenesis.6
Smad signalling plays a pivotal role in heart development by stimulating the expression of bone morphogenetic protein (BMP) and TGF-β.7,8 Activated BMP and TGF-β receptors induce the phosphorylation of Smad1/5/8 and Smad2/3, respectively.9,10 The activated R-Smads associate with Smad4, and the Smad complex translocates from the cytosol to the nucleus to transactivate the target genes that induce heart development.5 Therefore, deletion of Smad4 perturbs BMP/TGF-β signalling, leading to defects in the expression of genes for several cardiac transcription factors, including Nkx2.5, GATA4, and MEF2c.11 Consequently, disruption of Smad4 in mouse embryonic cardiomyocytes results in mid-gestational lethality with defects in heart development, which manifests as a defective, hypocellular myocardial wall caused by decreased cell proliferation and increased apoptosis.11,12 Both Smad5- and Smad6-knockout mice also exhibit heart defects. Embryonic disruption of mouse Smad5 by gene targeting results in embryonic lethality with defects in heart looping due to abnormalities in left–right axis determination.10 In contrast, mice null for the negative regulator of Smads, Smad6, survive to adulthood, but exhibit hyperplasia of cardiac valves.13
In mammalian hearts, spectrin is localized to the sarcolemma, the intercalated discs and Z-bands, and is closely associated with membranes, myofibrils, and intermediate filaments.14 Loss of β2-spectrin in mice leads to embryonic lethality in mid-gestation due to multiple defects, including failure to fully develop the heart.3 Interestingly, mice with a haploinsufficiency of β2-spectrin exhibit a phenotype similar to that of Beckwith–Wiedemann syndrome (BWS), which is a hereditary stem cell cancer syndrome with abnormal appearance. In addition, dramatic decreases in β2-spectrin expression due to epigenetic silencing have been identified in BWS cell lines and non-tumour tissues generated from human patients.15 Moreover, some BWS patients show congenital heart failure with ventricular septal defects.16,17 However, the contribution of β2-spectrin to heart structure formation remains unclear.
In the present study, we report that β2-spectrin is required for proper formation of the heart during development. Using in vitro cardiomyocyte cultures, we demonstrate that multiple abnormalities occur upon loss of β2-spectrin, including dramatic alterations of associated cytoskeletal proteins, and changes in proliferation and apoptosis. In vivo, loss of β2-spectrin expression in mice caused defects in heart structure, including disintegration of dystrophin structure, and was associated with a marked reduction in survival. Our data indicate that β2-spectrin is required for proper development of the heart, by mediating TGF-β/Smad signalling, and that disruption of β2-spectrin is a potential underlying cause of congenital heart defects.
2. Methods
2.1. Animals
Spnb2tm1a(EUCOMM)Wtsi (Spnb2tm1a) mice were generated using a promoterless selection cassette for premature termination and reporter knockout of transcript.18 The live mice were kindly provided by the Welcome Trust Sanger Institute. Conditional knockouts were established by directly mating female mutant mice (C57BL/6N) with Actin-Flp mice (C57BL/6J) to delete the gene-trap cassette via FLP1 recombinase (see Supplementary material online, Figure S1).19 Mice were genotyped by polymerase chain reaction analysis using the primer pair, 5'-agc ttg ccc agt atg gtc tc-3' (forward) and 5'-gga gct taa gag cgt cat gc-3' (reverse). The amplified conditional allele displayed an electrophoretic shift-up due to the inclusion of an intronic loxP insertion site. The pregnant mice were euthanized by inhalation of carbon dioxide gas using euthanasia chamber. All animal procedures were approved by the Institutional Animal Care and Use Committee of the National Cancer Center, in accordance with the NIH Guide for the Care and Use of Animals.
2.2. Cardiomyocyte culture
Cardiomyocytes were isolated from the embryos of Spnb2 conditional mice (Spnb2co/co) at E14.5 using a conventional pre-plating method,20 with modifications. Briefly, isolated ventricles were incubated twice with enzyme solution (0.125% trypsin and 0.05% collagenase type II) for 10 min at 37 °C to completely digest the heart tissue. The digested cells were enriched for cardiomyocytes by pre-plating on a tissue culture dish twice for 40 min each to remove adherent cells. Enriched cardiomyocytes (purity >90%) were collected and plated at 100 000 cells on laminin-coated 35 mm plates (BD Biosciences). In the next day, the cells were infected with adenovirus expressing Cre-IRES-GFP (Ad-Cre; Vector Biolabs), to eliminate β2-spectrin, or, as a control, GFP (Ad-GFP; Vector Biolabs), at a 100 multiplicity of infection (MOI). Cardiomyocyte growth was assayed using an MTT-based in vitro toxicology assay kit, according to the manufacturer's instructions (Sigma). The beating of cardiomyocytes at 48 h after adenovirus infection was captured by live cell movies using a portable time-lapse microscope (JuLI, NanoEnTek) inside a CO2 incubator.
2.3. Western blot analysis, immunocytochemistry, and histology
Primary antibodies against Smad1, Smad2, Smad4, Smad5, phospho-Smad1/5 (Ser463/465), phospho-Smad2 (Ser465/467), caspase-7, cyclin D2, cyclin D3, p15, p21, p27, and phospho-histone H3 (Ser10) were obtained from Cell Signaling Technologies; antibodies against cyclin D1, CKD4, β-actin, Bcl-2, Bax, β2-spectrin, Nkx2.5, α-SMA, tropomyosin, and α-tubulin were obtained from Santa Cruz; anti-Cre was from Novagen; anti BrdU was obtained from BD Biosciences; antibody to proliferating cell nuclear antigen (PCNA) was from Pharmingen; antibody to Ki67 was from Novus; and antibody to dystrophin was prepared as described previously.21 These antibodies were used for western blotting, immunocytochemistry, and immunohistochemistry. F-actin was stained using rhodamine-conjugated phalloidin (Invitrogen). Apoptosis was monitored by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay using ApoTag Red and ApoTag Peroxidase In Situ Apoptosis Detection kits according to the manufacturer's instructions (Millipore).
2.4. Statistical analyses
All data were expressed as means ± SE. Student's t-test (http://www.physics.csbsju.edu/stats/t-test.html) was used to compare differences.
3. Results
3.1. β2-Spectrin is predominantly expressed in embryonic hearts
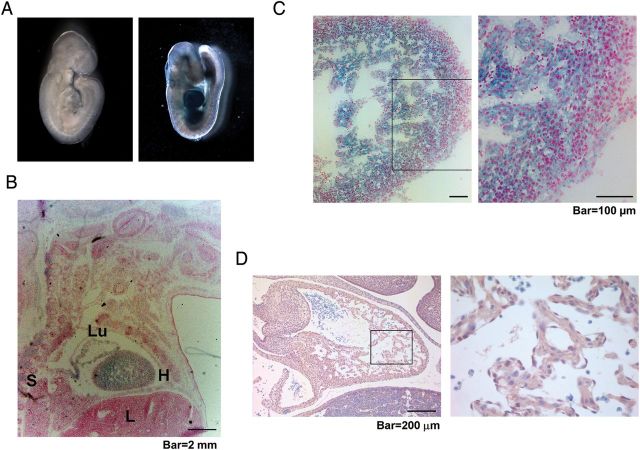
Homozygous loss of β2-spectrin in mice leads to mid-gestational death due to gastrointestinal, liver, neural, and heart defects, whereas β2-spectrin haploinsufficient mice survive until adulthood and spontaneously develop cancers in several organs.3,15 Developmental failure resulting from homozygous loss of Spnb2 suggests that β2-spectrin is essential for proper embryogenesis. To investigate the contribution of β2-spectrin in development, we examined the expression of β2-spectrin using Spnb2tm1a(EUCOMM)Wtsi (Spnb2-tm1a) mice. Spnb2-tm1a, the Spnb2-null allele used in this study, contains a reading frame-independent LacZ gene-trap cassette inserted into the intron of the Spnb2 gene.19 Using this targeting cassette enabled us to disrupt Spnb2 with an En2 splice acceptor and monitor the expression of Spnb2 by LacZ expression. An examination of the expression of β2-spectrin in E9.5 embryos by X-gal staining revealed strong LacZ expression in the hearts of Spnb2+/tm1a heterozygous embryos, whereas no signal was detected in any parts of the Spnb2+/+ embryo (Figure 1A). To determine changes in the expression pattern of β2-spectrin during development, we performed X-gal staining in sagittal sections of frozen embryos at different development stages. These experiments revealed the strongest expression of β2-spectrin in the developing heart of E16.4 embryos (Figure 1B). A magnified image of the heart showed that β2-spectrin was expressed not only in the compact layer, but also in the trabecular layer (Figure 1C). Expression of β2-spectrin was also detected in the spinal cord, liver, lung, and cerebellum (data not shown). To confirm the expression of β2-spectrin in the developing heart, we performed immunohistochemical analysis of E12.5 embryos using antibody against β2-spectrin. We observed β2-spectrin in the trabecular and compact layers of the developing heart (Figure 1D). These results demonstrate that β2-spectrin is predominantly expressed in the developing heart (see also Supplementary material online, Results).
Figure 1.
β2-Spectrin is prominent in the developing heart. (A) X-gal staining of a whole-mount Spnb2+/tm1a embryo (right) at E9.5 revealed the expression of β2-spectrin. (B) X-gal staining of a frozen section of a Spnb2+/tm1a embryo at E16.5. H, heart; L, liver; Lu, lung; S, spinal cord. (C) At E16.5, expression of β2-spectrin was detected in both compact and trabecular areas of the heart. (D) Normal heart from a wild-type embryo at E12.5 was stained with the antibody against β2-spectrin. The right panels represent magnifications of the boxed area. Scale bars, 2 mm (B); 100 μm (C); 200 μm (D).
3.2. Disruption of β2-spectrin results in structural defects of the developing heart
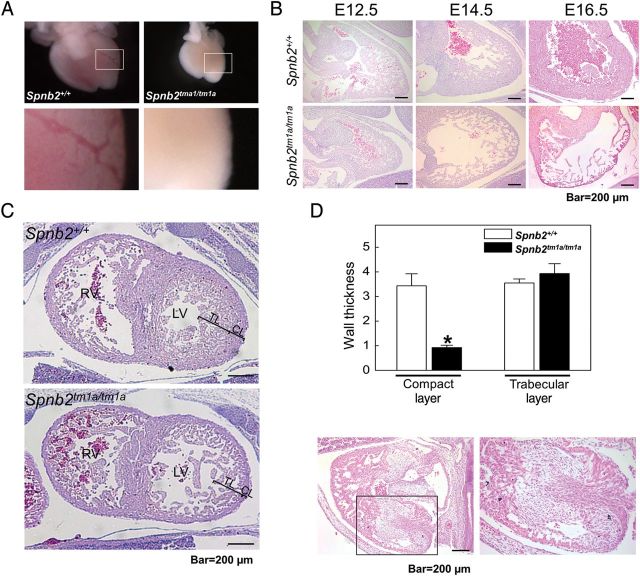
To determine the contribution of β2-spectrin to heart development, we investigated embryonic heart development in the presence and absence of β2-spectrin. A morphological examination revealed that hearts from homozygous mutant embryos at E15.5 were smaller than those of wild-type littermate controls (Figure 2A, upper panel). In addition, a clearly evident, thick blood vessel was observed in Spnb2+/+ embryonic hearts, but not in the hearts of Spnb2tm1a/tm1a embryos (Figure 2A, lower panels). To characterize the heart malformation upon loss of β2-spectrin in detail, we performed a histological analysis of the heart over the course of embryogenesis. The size and appearance of hearts were not significantly different through E12.5. However, after E14.5 the hearts from Spnb2tm1a/tm1a embryos showed a failure of the ventricular wall to thicken when compared with Spnb2+/+ hearts (Figure 2B). To determine whether the loss of β2-spectrin caused the failure of heart wall thickening, we examined the thickness of the compact and trabecular layers in the left ventricle at E14.5. We found a significant difference in the thickness of the compact layer between wild-type and Spnb2tm1a/tm1a embryonic hearts. In contrast, the thickness of the trabecular layer was not significantly different between the two genotypes (Figure 2C and D). However, the density of cells in the trabecular layer of Spnb2tm1a/tm1a hearts was lower than that in wild-type hearts. Interestingly, thickening of the heart wall, along with a fibrous pattern, was observed in 3 of 11 hearts from mutant E11.5 embryos, suggesting that β2-spectrin expression in the heart is dependent on its developmental stage (Figure 2E). These results suggest that the loss of β2-spectrin induces a statistically significant alteration of ventricular wall thickening, resulting in a defect in heart development.
Figure 2.
Developing hearts of Spnb2tm1a/tm1a embryos exhibit structural defects. (A) The morphologies of hearts at E15.5 are shown. The hearts of Spnb2tm1a/tm1a embryos were smaller than those of their wild-type littermate controls. Magnified images of hearts (boxed area in upper panels) are shown in lower panels. Blood vessels are evident in the heart of the Spnb2+/+ embryo, but not in the mutant heart. (B) Sagittal sections of hearts were stained with H&E at various developmental stages. The ventricle wall of mutant embryo hearts failed to thicken after E14.5. (C) Embryonic hearts at E14.5 were frontally sectioned and stained, and the thicknesses of the compact layer (CL) and trabecular layer (TL) of the left ventricle were measured. LV, left ventricle; RV, right ventricle; TL, trabecular layer; CL, compact layer. (D) Quantification of the wall thickness of embryonic hearts (*P < 0.05). (E) The heart of a Spnb2tm1a/tm1a embryo at E11.5, showing thickened myocardial fibres. Magnified image of the heart (boxed area of the left panel) is shown in the right panel. Scale bar, 200 μm.
3.3. Disruption of β2-spectrin causes abnormalities of Smad signalling, proliferation, and apoptosis in differentiating cardiomyocytes
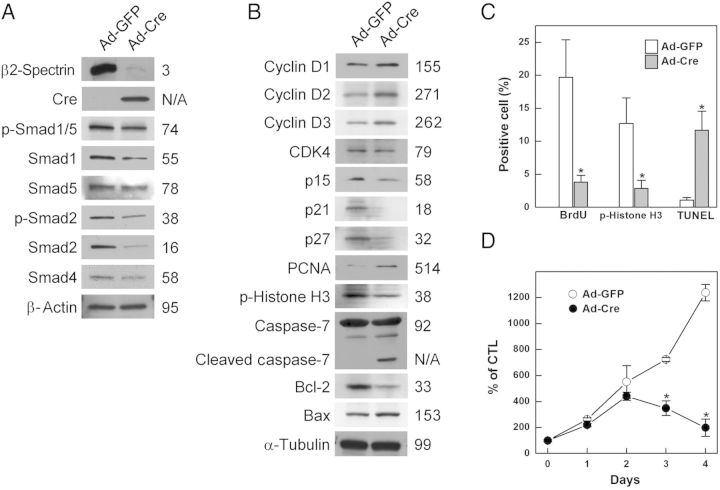
Previous reports have shown that β2-spectrin is involved in TGF-β signalling through its association with and translocation of Smad3 and Smad4.3 Moreover, the loss of TGF-β signalling causes congenital heart defects, indicating that the TGF-β/Smad signalling pathway is necessary for normal development of the heart.9 To test whether heart defects caused by the loss of β2-spectrin are mediated by abnormalities in Smad signalling pathways, we investigated the expression pattern of Smad proteins upon loss of β2-spectrin by infecting cardiomyocytes generated from conditional knockout embryos with Cre recombinase-containing adenovirus. Loss of β2-spectrin decreased the levels of expression of Smad1, Smad2, and Smad4, as well as down-regulating the phosphorylation of Smad1/5 and Smad2, suggesting that the reduction in the expression of Smad proteins suppresses their phosphorylation (Figure 3A). Collectively, these results suggest that the loss of β2-spectrin not only blocks the transmission of TGF-β/Smad signalling, but also prevents the expression and activation of its signalling components.
Figure 3.
Alteration in β2-spectrin expression suppresses Smad signalling, deregulates the cell cycle, and induces apoptosis in cardiomyocytes. Cardiomyocytes were isolated from the ventricles of Spnb2 conditional mouse embryos at E14.5. β2-Spectrin in cardiomyocytes was deleted by infecting cells with an adenovirus carrying the Cre recombinase and EGFP (Ad-Cre); control cells were infected with Ad-GFP expressing EGFP. (A) The expression patterns of Smad proteins were analysed by repeated western blotting. (B) Cell cycle regulation and survival of cardiomyocytes was evaluated by measuring the levels of the indicated proteins by western blotting using specific antibodies under the same conditions. The numbers indicate relative amounts of each protein (%) after Cre recombination for β2-spectrin deletion. β-Actin and α-tubulin were used as loading controls. (C) Cardiomyocytes positive for BrdU incorporation, p-histone H3 expression, and TUNEL positivity rates were counted 48 h after Ad-GFP or Ad-Cre infection. The numbers represent means ± SE from at least 300 cells, with statistically significant differences (P < 0.05) indicated by asterisks. (D) Survival of infected cardiomyocytes at indicated times, as estimated by MTT assays. Each number represents the means ± SE of quadruplicate determinations, with statistically significant differences (P < 0.01) indicated by asterisks.
We previously showed that a β2-spectrin deficiency leads to cyclin D1/CDK4 activation and contributes to dysregulation of the cell cycle, cellular proliferation and apoptosis, and promotes carcinogenesis.22 Moreover, haploinsufficiency of cdk4 in Spnb2+/− mice results in a dramatic decline in liver cancer formation compared with that observed in Spnb2+/− mice, suggesting that changes in cyclin D/CDK4 result from alterations in β2-spectrin expression.22 We therefore assessed whether the levels of the expression of D-type cyclins, CDK4, and CDK inhibitors in cardiomyocytes were altered in the absence of β2-spectrin. We found that the levels of the D-type cyclins D1, D2, and D3 were increased to 155, 271, and 262%, respectively, of controls, whereas the levels of the CDK inhibitors p15, p21, and p27 were decreased to 58, 18, and 32%, respectively, of controls after removal of β2-spectrin by adenoviral-Cre infection, suggesting that the G1/S checkpoint is weakened in β2-spectrin deleted cardiomyocytes. However, despite the induction of G1/S transition, removal of β2-spectrin did not result in the level of proliferation observed in control cardiomyocytes at this time. Thus, to test whether the loss of β2-spectrin altered another checkpoint in cardiomyocytes, we measured the level of phospho-histone H3 in these cells. The level of phospho-histone H3 in these cells was 38% of control, suggesting that mitotic activity is decreased following removal of β2-spectrin from cardiomyocytes. We also found that deletion of β2-spectrin increased the expression of the pro-apoptotic protein Bax and cleaved caspase-7, indicating a deregulation of the cell cycle in cardiomyocytes undergoing apoptosis (Figure 3B). To assess the impact of β2-spectrin deficiency on cell-cycle progression and apoptosis, we assessed DNA synthesis, mitosis, and DNA fragmentation in cultured cardiomyocytes at 48 h after adenoviral-Cre infection. We observed statistically significant reductions in BrdU incorporation (19% of control) and phospho-histone H3 expression (23% of control), as well as an 11-fold increase in the induction of TUNEL signalling (Figure 3C and Supplementary material online, Figure S2). Interestingly, β2-spectrin deletion induced regulators of G1/S transition but failed to initiate DNA synthesis. We also performed an MTT assay to determine whether deregulation of the cardiomyocyte cell cycle altered their proliferation. Although cardiomyocytes infected with empty virus showed increased proliferation throughout the culture period, cells from which β2-spectrin had been deleted showed maximum proliferation on Day 2 and decreased thereafter, with statistically significant differences from control (Figure 3D). Taken together, these data demonstrate that disruption of β2-spectrin reduces Smad signalling, dysregulates the cell cycle, and induces apoptosis, altering the proliferation of differentiating cardiomyocytes.
3.4. Disruption of β2-spectrin suppresses cardiomyocyte differentiation and myofibril formation
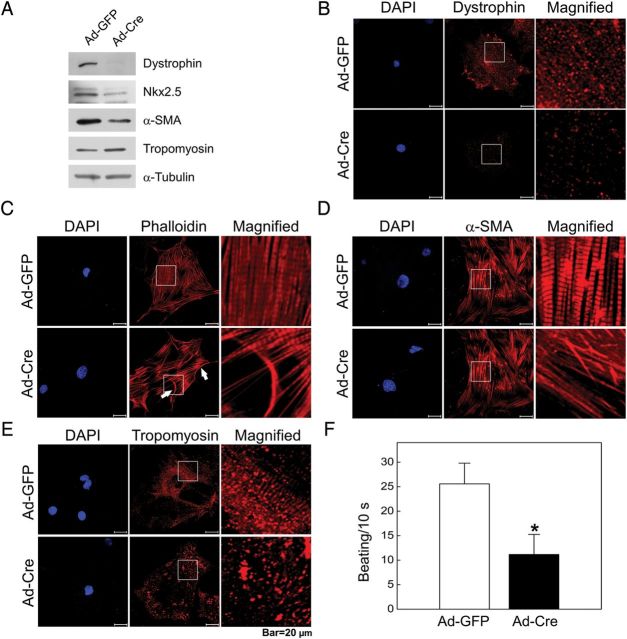
To assess whether β2-spectrin is responsible for the proper differentiation of cardiomyocytes, we examined the differentiation status of normal and β2-spectrin deleted cardiomyocytes. As shown in Figure 4A, the expression of the muscle differentiation markers, dystrophin, Nkx2.5, and α-SMA was remarkably down-regulated in adenovirus-Cre-infected cells, indicating that β2-spectrin is required for the differentiation of cardiomyocytes. In differentiated cardiomyocytes and cardiac muscle, dystrophin is localized at the inner surface of the membrane where it connects the cytoskeleton of a muscle fibre to the surrounding extracellular matrix through the cell membrane.23 Thus, we investigated the contribution of β2-spectrin to the distribution of dystrophin in cultured cardiomyocytes in the presence or absence of β2-spectrin. Upon deletion of β2-spectrin in cardiomyocytes, dystrophin was barely detectable, but was present in the membrane and cytosol with a striped pattern in control cardiomyocytes (Figure 4B). To examine the characteristic structure of cardiomyocytes, we next investigated the localization of F-actin and α-SMA. Unlike control cells, in which this structure was well developed, the striated pattern of F-actin fibres and α-SMA distribution was not detected in β2-spectrin deleted cardiomyocytes (Figure 4C and D). Tropomyosin, an actin-binding protein that regulates actin mechanics, has also been shown to interact with β2-spectrin.3 Although tropomyosin expression was slightly increased in β2-spectrin deleted cardiomyocytes, its localization became scattered in contrast to that in control cardiomyocytes, which exhibited a typical striated distribution pattern (Figure 4E). Next, we tested whether β2-spectrin deleted cardiomyocytes retained their function by assessing their contractile activity. A comparison of the spontaneous beating rate of cultured cardiomyocytes showed that the beating rate of β2-spectrin deleted cardiomyocyte was only 44% that of controls, a reduction that was statistically significant (Figure 4F). These results demonstrate that β2-spectrin is required for the differentiation and localization of cytoskeletal proteins as well as for the contractile function of cardiac muscle.
Figure 4.
Loss of β2-spectrin suppresses cardiomyocyte differentiation. (A) Cardiomyocyte differentiation markers and cytoskeletons were detected by western blotting following deletion of β2-spectrin. The expression of differentiation markers decreased in β2-spectrin deleted cardiomyocytes. (B) The distribution of dystrophin in cardiomyocytes was determined by immunostaining with a dystrophin-specific antibody and viewing under a laser-scanning confocal microscope. Dystrophin was present predominantly in the cytosol with a stripe-like pattern and in the membranes in control cardiomyocytes, but was barely detectable in β2-spectrin deleted cardiomyocytes. The distribution of F-actin (C), α-SMA (D), and tropomyosin (E) in cardiomyocytes was investigated by staining with rhodamine-conjugated phalloidin (F-actin), and by immunostaining using primary antibodies against α-SMA or tropomyosin and rhodamine-conjugated secondary antibodies. The myofibrillar organization and typical striated distribution pattern of cytoskeletons are clearly evident in control cardiomyocytes. However, these structures were altered after β2-spectrin deletion. (F) The graph depicts the spontaneous beating rate of cardiomyocytes. Spontaneous beatings of cardiomyocytes were counted under a phase-contrast microscope in three independent experiments. The beating rate significantly decreased in β2-spectrin deleted cardiomyocytes compared with normal cardiomyocytes (*P < 0.05). Scale bar, 20 μm.
3.5. Loss of β2-spectrin alters apoptosis, proliferation, and differentiation in embryonic hearts
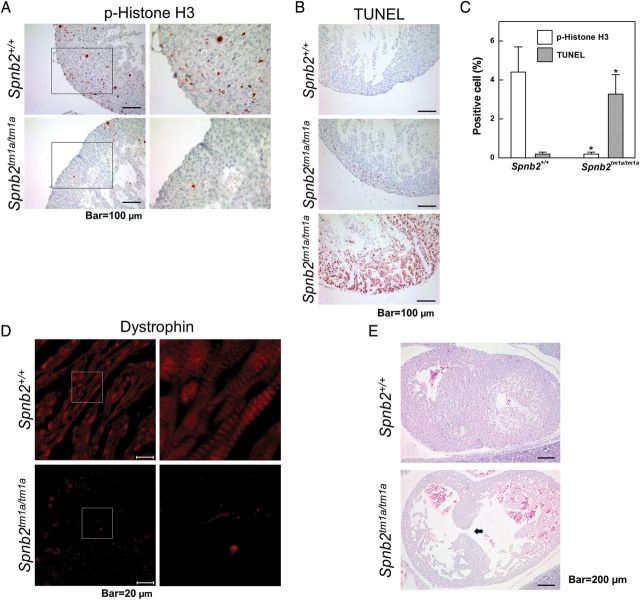
As noted above (Figure 2), the hearts of Spnb2tm1a/tm1a embryos failed to increase in size and their walls did not thicken properly compared with those of Spnb2+/+ embryos. Therefore, we examined the proliferation and degree of apoptosis in embryonic hearts, especially in the compact layer. An immunohistochemical analysis using an antibody against phospho-histone H3 showed that G2/M transiting cells were barely detectable in Spnb2tm1a/tm1a hearts at E16.5 (Figure 5A). Similar results were obtained by staining for the proliferation marker Ki-67 (Supplementary material online, Figure S3). An evaluation of apoptosis in the hearts of Spnb2+/+ and Spnb2tm1a/tm1a embryos using TUNEL assays revealed that apoptotic cells were readily detected in the hearts of Spnb2tm1a/tm1a embryos but not in those of Spnb2+/+ embryos. Comparisons of these signals in embryonic hearts showed that the G2/M transiting signal in Spnb2tm1a/tm1a hearts was only 5% that of controls, while the apoptotic signal was 16-fold higher, with both of these alterations being statistically significant (Figure 5C). These results suggest that the differences in heart size and ventricle wall thickness between Spnb2+/+ and Spnb2tm1a/tm1a embryos reflect prevention of cell proliferation and induction of apoptosis caused by the loss of β2-spectrin.
Figure 5.
Loss of β2-spectrin alters proliferation, apoptosis, and differentiation in embryonic hearts. (A) The proliferation of cardiomyocytes was evaluated immunohistochemically in wild-type and mutant embryonic hearts at E16.5 using an antibody against phospho-histone H3. Positive cells in ventricles were reduced in Spnb2tm1a/tm1a hearts. (B) Apoptotic cells in embryonic hearts at E16.5 were detected using TUNEL assays. Apoptotic cells were more readily detected in Spnb2tm1a/tm1a hearts than in Spnb2+/+ hearts. (C) Quantification of phospho-histone H3- and TUNEL-positive cells. After staining of hearts from wild-type (n = 6) and Spnb2tm1a/tm1a (n = 5) embryos, at least 200 heart cells were counted in each and positivity rates calculated (*P < 0.01). The most severely apoptotic heart (bottom panel of Figure 5A) was excluded in counting. (D) Immunohistochemical localization of dystrophin using an anti-dystrophin antibody. Myofibril formation was absent from mutant hearts. Magnified images of boxed area in left panels are shown on the right. (E) Wild-type and Spnb2tm1a/tm1a hearts at E14.5 were frontally sectioned after H&E staining. The arrow indicates a ventricular septum defect. Scale bars, 100 μm (A and B); 20 μm (C); 200 μm (E).
Dystrophin, whose loss causes Duchenne muscular dystrophy (DMD), is concentrated along with β-spectrin in three distinct domains at the sarcolemma.24 In this study, we found that the loss of β2-spectrin in cultured cardiomyocytes led to down-regulation and mislocalization of dystrophin. An extension of this analysis to an immunohistochemical examination of dystrophin distribution in embryonic hearts yielded the same results as were obtained with isolated cardiomyocytes. Unlike wild-type embryos, in which these proteins were distributed in the striped pattern typical of myofibrils, the distribution of dystrophin was disrupted in Spnb2tm1a/tm1a embryos (Figure 5D). Interestingly, 6 out of 19 mutant embryos also displayed a ventricular septal defect (VSD)—a defect in the wall dividing the left and right ventricles of the hearts that is also found in BWS patients with congenital heart disease (Figure 5E and Supplementary material online, Figure S4). Taken together, these observations indicate that the loss of β2-spectrin not only altered cardiomyocyte survival, it also influenced the architecture of cardiac muscle, consequentially leading to heart defects during embryogenesis.
4. Discussion
TGF-β/Smad signalling regulates fundamental cell responses, including proliferation, migration, and apoptosis, across a wide range of cell types. As a consequence, it also controls morphogenesis. In response to TGF-β treatment, Smad2 and Smad3 C-termini are phosphorylated by the TGF-β receptor, forming heteromeric complexes with Smad4 that undergo nuclear translocation and subsequently regulate the expression of target genes, including those involved in inhibition of cell growth, cell migration, and differentiation. Precise control of these cellular events is essential for accurate development of the heart.9 The gene-targeted mouse model has proved useful for many of these studies, because genetic control of heart morphogenesis occurs similarly in mice and humans. These approaches have shown that loss of TGF-β responsiveness results in deregulation of these processes, providing overwhelming evidence showing that correct functioning of the TGF-β signalling pathway is crucial to these events.9,10
β2-Spectrin, a Smad adaptor protein, has been shown to play a critical role in localizing Smads and facilitating the tumour-suppressor functions of TGF-β/Smad signalling. A deficiency of β2-spectrin protein has been shown to result in mislocalization of Smad3 and Smad4 as well as a loss of the TGF-β-dependent transcriptional response; these defects could be rescued by restoration of β2-spectrin.3 In terms of contribution to development, the loss of β2-spectrin in mice results in a phenotype similar to that observed in Smad-deficient mice: embryonic lethality; gastrointestinal, liver, neural, and heart defects; and abnormal angiogenesis.3,6 We show here that the loss of β2-spectrin in cardiogenesis causes defects in heart development with abnormalities in cardiomyocyte differentiation and cytoskeletal formation. The developmental defects in β2-spectrin deleted hearts are considered to result from blocking Smad signalling. The phenotype of Spnb2tm1a/tm1a hearts was similar to the abnormalities observed in Smad4 or TGF-β mutant embryonic hearts: ventricular hypoplasia, down-regulation of proliferation, and a ventricular septal defect.11,25 Moreover, loss of β2-spectrin suppressed both phosphorylation and transcription of Smad proteins, suggesting that β2-spectrin is required for the transmission and expression of TGF-β/Smad signalling. Thus, since β2-spectrin is an important mediator and effector of TGF-β/Smad signalling, deletion of its expression in embryos results in heart abnormalities similar to those resulting from defects in TGF-β/Smad signalling.
We previously demonstrated that loss of β2-spectrin induces cell cycle deregulation through activation of cyclin D1/CDK4 and weakening of G1/S arrest in liver carcinogenesis.22,26 We therefore tested whether a β2-spectrin deficiency in primary mouse cardiomyocytes also causes similar abnormalities. We found that the loss of β2-spectrin decreased expression of CDK inhibitors and increased expression of D-type cyclins and PCNA, indicating a disruption of the proper G1/S checkpoint and uncontrolled cell proliferation. However, mice with cardiac-specific up-regulation of cyclin D1 or cyclin D2 exhibited developmental hyperplasia with increased heart size.27,28 In contrast, embryos with deletion of D-type cyclins displayed a similar cardiac phenotype to Spnb2tm1a/tm1a embryos, including severely thinned walls of the ventricles, mainly affecting the compact zone, and VSD.29 Despite increases in G1/S transition signalling, the β2-spectrin deletion did not cause hyperplastic phenotypes in cultured cardiomyocytes and embryonic hearts, except for thickened ventricle walls at E11.5. In contrast, β2-spectrin-deficient cardiomyocytes exhibited reduced levels of phospho-histone H3 expression and BrdU incorporation, and embryonic hearts of Spnb2 mutants showed decrements in phospho-histone H3 and Ki67 signals. Apoptosis was enhanced in cell cycle deregulated cardiomyocytes resulting from β2-spectrin deletion, as evidenced by the induction of cleaved caspase-7/Bax proteins, and increases in TUNEL positivity. In addition to these in vitro results, our in vivo experiments showed that Spnb2tm1a/tm1a embryonic hearts failed to accumulate cardiomyocytes, with extensive apoptosis during later stages of heart development (E16.5). Thus, these studies suggested that the hypoplastic appearance of the developing heart resulting from loss of β2-spectrin is caused by inductions of cell cycle arrest and apoptosis.
Several lines of evidence have shown that a failure of blood vessel formation prevents heart development in association with apoptosis. Abnormal angiogenesis caused by endothelial cell-specific targeting of surviving not only caused embryonic lethality in gestation with prominent and diffuse haemorrhaging, but it was also accompanied by strikingly abnormal heart development.30 In contrast, coexpression of vascular endothelial growth factor (VEGF) and angiopoietin-1 was shown to promote angiogenesis and cardiomyocyte proliferation, and reduce apoptosis in myocardial-infarcted porcine hearts.31 We previously demonstrated that embryonic disruption of β2-spectrin resulted in a failure of normal vasculature development and significantly decreased endothelial cell differentiation in the developing yolk sac.6 Notably, our morphological analysis showed that Spnb2tm1a/tm1a hearts were paler than wild-type hearts and their blood vessels were barely detectable. An insufficient blood supply resulting from abnormal blood vessel formation in the heart may be an indirect cause of heart developmental defects.
Muscle fibre comprises myofibrils arranged in sarcomeres containing a number of proteins, including actin and myosin, which are the major components of the filaments. The structure and function of muscle fibres are maintained by the surrounding extracellular matrix and membrane-associated proteins. To provide further insight into the relationship between β2-spectrin and muscle structure, we analysed cardiomyocyte cytoskeleton structure and survival. Among the cytoskeletal elements examined was dystrophin, an actin-binding protein-like spectrin and a key component of the plasma membrane-associated cytoskeleton of muscle cells, including cardiac muscle cells.32 A mutation in the dystrophin protein causes DMD and Becker muscular dystrophy (BMD), which are the most common muscular dystrophies. Most DMD/BMD patients (∼90%) show cardiac defects.33 Dystrophin exhibits a diffuse distribution in mouse tibialis anterior muscle transfected with siRNA against ankyrin-B,34 which interacts with β2-spectrin in neonatal cardiomyocytes.35 In addition, β2-spectrin is required for ankyrin-B localization and function at skeletal muscle costameres.34 In β2-spectrin-deficient cardiomyocytes and embryonic hearts, there was a dramatic reduction and abnormal distribution of dystrophin. We also found that β2-spectrin deleted primary cardiac muscle cells exhibited an abnormal pattern of F-actin, tropomyosin, and α-SMA, and their beating rate was significantly decreased. Thus, β2-spectrin is required to sustain cellular and functional architecture, and its loss in the heart prevents proper expression and localization of cytoskeletal components.
Congenital heart defects are the most prevalent of all birth defects and are a leading cause of death in the first year of life. Genetic studies have revealed the importance of TGF-β signalling in organogenesis, including cardiac development.9 Importantly, we provide evidence that β2-spectrin, a mediator of TGF-β/Smad signalling, is required for heart development, and its loss prevents cardiomyocyte differentiation, proliferation, and cytoskeletal network formation, and leads to apoptosis of cardiac muscle cells. A recent report examining affected non-tumour tissues as well as cell lines showed that β2-spectrin is epigenetically silenced in individuals with BWS.15 The primordial germ cells, embryo, and foetus are also highly susceptible to epigenetic dysregulation by environmental chemicals, which can thereby exert multiple adverse effects.36 Indeed, prenatal exposure to diverse environmental chemicals dysregulates the foetal epigenome, with potential consequences for subsequent developmental disorders and diseases manifesting in childhood, throughout life, or even transgenerationally. In view of the present findings, we suggest that β2-spectrin likely plays an important role in cardiac development as a survival modulator and structural organizer in cardiac muscle cells, and further suggest that alteration of β2-spectrin is among the potential causes of congenital heart defects.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the grants from the National Cancer Center of Korea (NCC -1310660); the Korea Healthcare Technology R&D Project (A111334); and the National Research Foundation of Korea (2010-0021822).
Acknowledgments
Conflict of interest: none declared.
References
- 1.Viel A, Branton D. Spectrin: on the path from structure to function. Curr Opin Cell Biol. 1996;8:49–55. doi: 10.1016/s0955-0674(96)80048-2. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 4.Thenappan A, Li Y, Kitisin K, Rashid A, Shetty K, Johnson L, et al. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology. 2010;51:1373–1382. doi: 10.1002/hep.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Baek HJ, Lim SC, Kitisin K, Jogunoori W, Tang Y, Marshall MB, et al. Hepatocellular cancer arises from loss of transforming growth factor beta signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128–1137. doi: 10.1002/hep.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li TS, Komota T, Ohshima M, Qin SL, Kubo M, Ueda K, et al. TGF-beta induces the differentiation of bone marrow stem cells into immature cardiomyocytes. Biochem Biophys Res Commun. 2008;366:1074–1080. doi: 10.1016/j.bbrc.2007.12.095. [DOI] [PubMed] [Google Scholar]
- 8.Pal R, Khanna A. Role of smad- and wnt-dependent pathways in embryonic cardiac development. Stem Cells Dev. 2006;15:29–39. doi: 10.1089/scd.2006.15.29. [DOI] [PubMed] [Google Scholar]
- 9.Arthur HM, Bamforth SD. TGFbeta signaling and congenital heart disease: insights from mouse studies. Birth Defects Res A Clin Mol Teratol. 2011;91:423–434. doi: 10.1002/bdra.20794. [DOI] [PubMed] [Google Scholar]
- 10.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 11.Qi X, Yang G, Yang L, Lan Y, Weng T, Wang J, et al. Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev Biol. 2007;311:136–146. doi: 10.1016/j.ydbio.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Song L, Yan W, Chen X, Deng CX, Wang Q, Jiao K. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101:277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 14.Isayama T, Goodman SR, Zagon IS. Localization of spectrin isoforms in the adult mouse heart. Cell Tissue Res. 1993;274:127–133. doi: 10.1007/BF00327993. [DOI] [PubMed] [Google Scholar]
- 15.Yao ZX, Jogunoori W, Choufani S, Rashid A, Blake T, Yao W, et al. Epigenetic silencing of beta-spectrin, a TGF-beta signaling/scaffolding protein in a human cancer stem cell disorder: Beckwith-Wiedemann syndrome. J Biol Chem. 2010;285:36112–36120. doi: 10.1074/jbc.M110.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taweevisit M, Thorner PS. Hydrops fetalis in the stillborn: a series from the central region of thailand. Pediatr Dev Pathol. 2010;13:369–374. doi: 10.2350/09-12-0771-OA.1. [DOI] [PubMed] [Google Scholar]
- 17.Drut R, Quijano G, Altamirano ME, Jones MC, Maffessoli OB. Vascular malformation and choroid plexus adrenal heterotopia: new findings in Beckwith-Wiedemann syndrome? Fetal Pediatr Pathol. 2006;25:191–197. doi: 10.1080/15513810601015704. [DOI] [PubMed] [Google Scholar]
- 18.Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, et al. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis. 2004;38:151–158. doi: 10.1002/gene.20012. [DOI] [PubMed] [Google Scholar]
- 19.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers LS, Schnurr DC, Broka D, Camenisch TD. An improved protocol for the isolation and cultivation of embryonic mouse myocytes. Cytotechnology. 2009;59:93–102. doi: 10.1007/s10616-009-9197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Froehner SC, Murnane AA, Tobler M, Peng HB, Sealock R. A postsynaptic Mr 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J Cell Biol. 1987;104:1633–1646. doi: 10.1083/jcb.104.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, Zouhairi ME, et al. Transforming growth factor-beta adaptor, beta2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology. 2011;53:1676–1684. doi: 10.1002/hep.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaprielian RR, Severs NJ. Dystrophin and the cardiomyocyte membrane cytoskeleton in the healthy and failing heart. Heart Fail Rev. 2000;5:221–238. doi: 10.1023/A:1009805419285. [DOI] [PubMed] [Google Scholar]
- 24.Porter GA, Dmytrenko GM, Winkelmann JC, Bloch RJ. Dystrophin colocalizes with beta-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol. 1992;117:997–1005. doi: 10.1083/jcb.117.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 26.Kim SS, Shetty K, Katuri V, Kitisin K, Baek HJ, Tang Y, et al. TGF-beta signaling pathway inactivation and cell cycle deregulation in the development of gastric cancer: role of the beta-spectrin, ELF. Biochem Biophys Res Commun. 2006;344:1216–1223. doi: 10.1016/j.bbrc.2006.03.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 29.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Zwerts F, Lupu F, De Vriese A, Pollefeyt S, Moons L, Altura RA, et al. Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood. 2007;109:4742–4752. doi: 10.1182/blood-2006-06-028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A. 2011;108:2064–2069. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson SA, Cullen MJ, Rothery S, Coppen SR, Severs NJ. High-resolution en-face visualization of the cardiomyocyte plasma membrane reveals distinctive distributions of spectrin and dystrophin. Eur J Cell Biol. 2005;84:961–971. doi: 10.1016/j.ejcb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 34.Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, Bennett V. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem. 2011;286:7370–7378. doi: 10.1074/jbc.M110.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 36.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]