Abstract
During development, tissues undergo complex cellular rearrangements and changes in shape that produce a diversity of body plans and the functional organs therein. The Drosophila egg chamber has emerged as an exciting and highly tractable model in which to investigate novel mechanisms driving the elongation of tissues. Egg chambers are multicellular assemblies within flies’ ovaries that will each give rise to a single egg. Although initially spherical, these simple organ-like structures lengthen as they grow. This transformation depends on an unusual form of planar polarity in the egg chamber’s outer epithelial layer, in which arrays of linear actin bundles and fibril-like structures in the basement membrane both align perpendicular to the axis of elongation. The resulting circumferential arrangement of structural molecules is then thought to act as a “molecular corset” that directionally biases growth of the egg chamber. I will explore four fundamental questions about this system: (1) How is the circumferential pattern generated in the follicular epithelium? (2) What is the physical nature of the corset? (3) How does a corset-type mechanism lead to the cellular rearrangements necessary for the elongation of tissues? and (4) To what extent are the cellular mechanisms controlling egg chamber elongation conserved in other systems? For each topic, I will present insights gleaned from the recent literature and highlight fertile areas for future investigation.
Introduction
One of the most common morphogenetic behaviors exhibited by developing tissues is elongation. This process invariably requires precise coordination of cell shape, polarity, and adhesion across cell populations and with respect to the elongating axis. However, the morphogenetic programs that individual tissues employ can be surprisingly diverse (Keller 2006). Common mechanisms driving elongation include directed changes in cell shape, oriented cell divisions, and convergent extension. There are also fascinating cases in which a circumferential arrangement of structural molecules perpendicular to the elongating axis is used to resist an internal expansive force and promote the lengthening of tissues (Keller 2006). Here, I will discuss how studies in Drosophila are shedding new light on the way in which a novel whole-tissue rotation may synergize with these classic elongation mechanisms to transform an initially spherical organ-like unit into a highly elongated egg.
Each Drosophila egg arises from a multicellular structure within the ovary called an egg chamber (or follicle). The egg chamber itself consists of an internal cluster of germ cells comprised of 15 nurse cells and one posteriorly localized oocyte, surrounded by an epithelial monolayer of somatic follicle cells (Fig. 1A and B). This epithelium has its apical surface toward the germ cells and its basal surface in contact with a specialized extracellular matrix (ECM) called the basement membrane (Fig. 1B). The follicle cells synthesize and secrete this matrix, which envelops the egg chamber throughout its development.
Fig. 1.
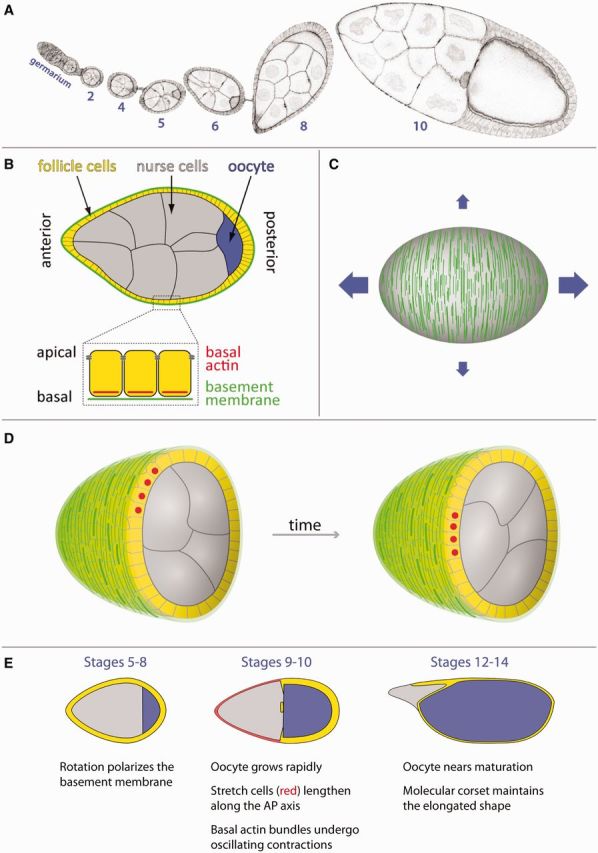
Overview of egg chamber elongation. (A) Micrograph showing a developmental array of egg chambers (ovariole) with the germarium at the anterior end. Egg chambers progressively elongate along their AP axes as they grow. Numbers indicate developmental stage. (B) Overview of the structure of an egg chamber. (C) The circumferential organization of basal actin bundles and the basement membrane is thought to function as a two-component molecular corset controlling egg chamber elongation. The vertical green lines represent basement membrane fibrils in the corset pattern on the egg chamber’s surface. The blue arrows represent how growth is preferentially channeled along the AP axis. (D) Illustrations of a rotating egg chamber that has been cut in half and tilted toward the viewer. The follicle cells collectively migrate along the inner surface of the basement membrane, which causes the entire egg chamber to rotate within the stationary matrix. Red dots mark the same four follicle cells over time. (E) The three major phases of egg chamber elongation.
When an egg chamber first forms, it is 20 μm in diameter and roughly spherical. Over the next 3 days, it progresses through 14 developmental stages that transform it into an exquisitely structured egg capable of supporting embryonic development. The individual stages are largely based on morphology and are termed stage 1, stage 2, etc. During its development, the egg chamber also undergoes tremendous growth, increasing in volume almost 1000-fold. The growth is isometric for more than a day. However, during the subsequent 36 h, growth is preferentially channeled along the anterior–posterior (AP) axis. This bias produces an egg that is 2.2–2.5 times longer than it is wide, which facilitates its passage through the oviduct and likely provides an important spatial context for the molecular gradients that pattern the early embryo.
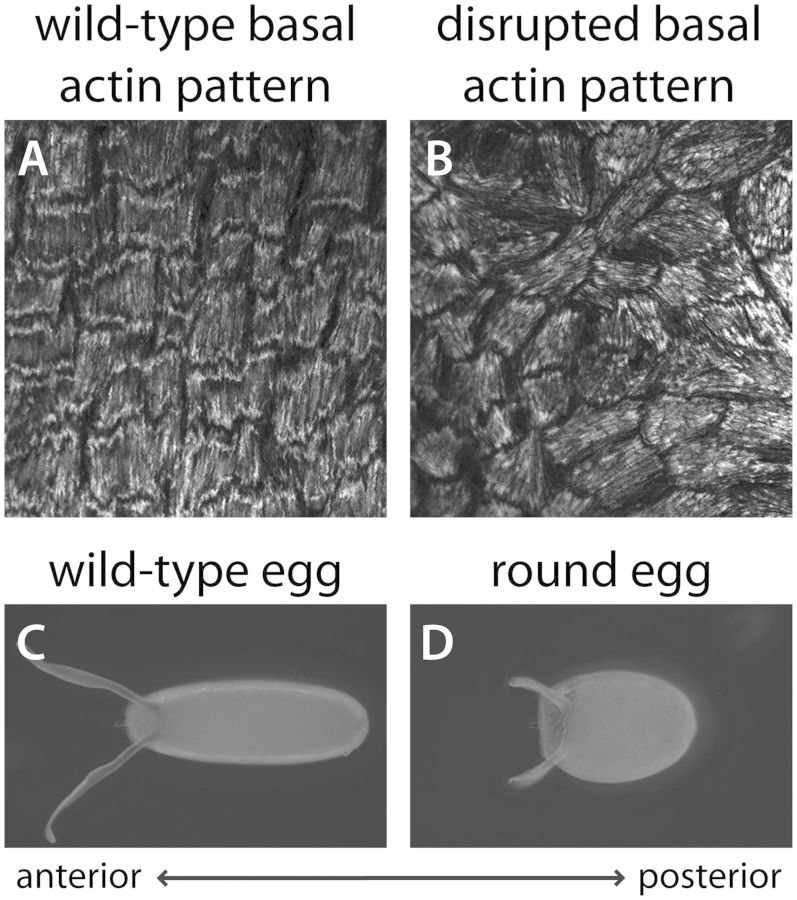
The first clue to the cellular mechanisms driving egg chamber elongation came from reports by Herwig Gutzeit in the early 1990s that the follicular epithelium displays an unusual form of planar polarity at its basal surface. This phenomenon is best seen through the organization of linear actin bundles at the basal cortex. These structures align with one another in individual follicle cells and then globally across the tissue, such that they are all oriented perpendicular to the AP axis (Figs. 2A, 3A′, and 3B) (Gutzeit 1990). Intriguingly, the basement membrane is similarly organized, with linear fibril-like arrays oriented in the same direction as the actin bundles (Figs. 1C and 3C) (Gutzeit et al. 1991). When viewed in three dimensions, this organization creates a circumferential pattern around the egg chamber’s exterior. Gutzeit further showed that this circumferential organization of the basal actin and basement membrane is lost in the female-sterile mutation kugelei, which produces eggs that are shorter and rounder than those of wild-type (Gutzeit et al. 1991). In the mutant egg chambers, the basal actin bundles are still aligned within individual cells, but their global organization is perturbed (Fig. 2A–D). The kugelei mutation was later shown to disrupt the function of the atypical cadherin Fat2 (Viktorinova et al. 2009).
Fig. 2.
Relationship between the basal actin pattern and final egg shape. (A–B) Organization of contractile actin bundles at the basal surface of the follicular epithelium at stage 12. The F-actin is visualized with rhodamine phalloidin. (A) In a wild-type epithelium, the basal actin bundles are oriented perpendicular to the AP axis, which creates a circumferential pattern around the egg chamber. (B) Under certain mutant conditions, the basal actin bundles are still largely aligned within individual cells, but their tissue-level organization is lost. (C–D) Dorsal views of mature eggs. (C) A wild-type, elongated egg. (D) The rounded egg shape that arises from the disrupted tissue pattern shown in (B).
Fig. 3.
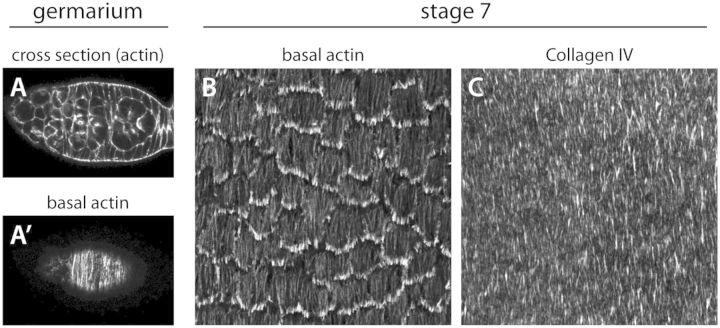
Organization of the basal actin and basement membrane at early stages. (A) The basal actin bundles first show a circumferential organization in the germarium (A) optical cross-section through a germarium. (A′) Basal view of the same germarium showing the actin pattern. (B) The basal actin bundles show a robust circumferential organization during the period that the egg chamber is rotating. (A–B) The F-actin is visualized with rhodamine phalloidin. (C) Fibril-like structures in the basement membrane show a similar organization as that of the basal actin. The basement membrane in visualized with Collagen IV-GFP.
Together, these observations led to the model that prevails today—that the egg chamber elongates through a “molecular corset” mechanism. In this model, the striking circumferential organization of the actin bundles and basement membrane at the basal epithelial surface promotes AP elongation by mechanically constraining isometric expansion (Fig. 1C). Here, I will first provide a general overview of oogenesis, highlighting major events in the elongation program. I will then explore four fundamental questions about this system: (1) How is the circumferential pattern generated in the follicular epithelium? (2) What is the physical nature of the corset? (3) How does a corset-type mechanism lead to the cellular rearrangements that elongate the tissue? and (4) To what extent are the cellular mechanisms controlling egg chamber elongation conserved in other systems? For each question, I will review what is currently known and then highlight fertile areas for future investigation.
Overview of egg chamber elongation
Egg chambers are assembled in an anterior ovarian region called the germarium (Figs. 1A and 3A). Here, two populations of stem cells give rise to the germ cells and follicle cells, respectively, which then come together in their concentric organization (Spradling et al. 2008). Interestingly, the basal actin bundles are already present and circumferentially organized within the follicle cell precursors located in the posterior half of the germarium (Frydman and Spradling 2001) (Fig. 3A′). However, this tissue-level organization is lost during stages 1–4 when the egg chamber is spherical; the actin bundles are still aligned within each cell, but are no longer globally oriented. Although the follicle cells are always in contact with their basement membrane, starting from the somatic stem cells that give rise to the tissue (O’Reilly et al. 2008), the matrix lacks obvious structure during these early stages.
The egg chamber begins to elongate at stage 5 (Fig. 1E). Concurrent with this morphogenesis, the basal actin bundles once again become globally aligned and the fibril-like structures begin to form in the basement membrane (Fig. 3B–C). Importantly, between stages 5 and 8, the egg chamber also displays a surprising behavior. The follicle cells undergo a dramatic collective migration perpendicular to the AP axis, where their basal surfaces crawl along the inside of the basement membrane (Fig. 1D). Because the apical surfaces of the follicle cells are attached to the germ cells through cadherin-based adhesions, this collective movement causes the entire egg chamber to rotate within its surrounding matrix, which remains largely stationary throughout the process (Haigo and Bilder 2011).
During stages 9 and 10, the egg chamber continues to elongate, and the oocyte grows dramatically due to the uptake of yolk, until it occupies half the total volume of the egg chamber’s interior (Fig. 1E). To accommodate this internal expansion, the epithelium must also increase its surface area. Thus, about 50 anterior follicle cells, called stretch cells, disassemble their basal actin bundles and dramatically flatten over the nurse cells. The remaining follicle cells contact the oocyte and adopt a columnar morphology. Within the columnar cells, the circumferential basal actin pattern is strongest in a band of cells near the boundary with the stretch cells. Increasing tissue-level disorder is then seen toward the posterior pole (Gutzeit 1990). During this period, the basal actin bundles also undergo oscillating myosin-mediated contractions (discussed in more detail below) (He et al. 2010).
After stage 10, growth has stopped and the active phase of egg chamber elongation is complete. However, the egg chamber’s elliptical shape must now be maintained throughout the final four stages of oogenesis. At stage 11, the nurse cells transfer their cytoplasm to the oocyte, which rapidly expands to fill the egg chamber’s interior. In response, the remaining follicle cells flatten to maintain epithelial continuity over the oocyte. During this transition, the basal actin-bundle array in each cell transiently reorients by 90° and adopts a fan-shaped morphology with the fan’s base toward the egg chamber’s posterior pole (Delon and Brown 2009). During stages 12–14, these structures return to their normal orientation, which restores the circumferential actin pattern over the entire egg chamber’s surface (Fig. 1E).
When oogenesis is complete, the egg’s elliptical shape appears to be stabilized by the rigid eggshell that surrounds the mature oocyte. The follicle cells begin secreting the eggshell proteins at stages 9 and 10 (Waring 2000). However, at stage 12, this structure has still not matured to the point that it can maintain the elongated shape of the oocyte (Haigo and Bilder 2011). The exact stage at which the eggshell becomes competent to perform this function is unknown.
Generation of the circumferential epithelial pattern
The circumferential pattern associated with the molecular corset is a type of epithelial planar polarity. However, the Frizzled/Strabismus and Fat/Dachsous signaling pathways that specify planar polarity in many tissues are not required for its generation (Viktorinova et al. 2009). Instead, this organization primarily depends on a complex series of cell–ECM interactions. To learn about the growing list of proteins that have been implicated in producing this pattern, I recommend an excellent recent review (Gates 2012). Here, I will highlight the key role that egg chamber rotation plays in this process, focusing first on the basement membrane and then on the basal actin bundles.
In the original paper describing egg chamber rotation, Haigo and Bilder (2011) elegantly showed that this unusual motion helps to build the fibril-like structures in the basement membrane. The fibrils are only generated during the period that the egg chamber is rotating and they are always oriented in the same direction as the migration path. Moreover, mutant conditions that block rotation also disrupt the remodeling of the matrix (Haigo and Bilder 2011; Lerner et al. 2013; Lewellyn et al. 2013). Once the basement membrane becomes circumferentially polarized in this way, this architecture persists throughout the rest of oogenesis, although the fibrils become more dispersed as the egg chamber grows (Haigo and Bilder 2011).
To understand how fibrils form in the basement membrane, we must first better define their composition. Basement membranes are assembled from four primary proteins: Type IV collagen (Col IV), Laminin, Perlecan (or other heparan sulfate proteoglycans), and Nidogen (Yurchenco 2011). Importantly, Col IV is a network-forming collagen, which means that the inter-molecular interactions between protomers favor the formation of a planar matrix (Myllyharju and Kivirikko 2004; Khoshnoodi et al. 2008). Drosophila entirely lack fibril-forming collagens such as Types I–III, as well as the fibril-forming protein fibronectin (Broadie et al. 2011). It is therefore essential not to confuse the egg chamber’s polarized basement membrane with the true fibrillar ECMs found in other organisms. Given that Col IV, Laminin, and Perlecan have all been shown to become linearly organized during egg chamber elongation (Gutzeit et al. 1991; Schneider et al. 2006; Haigo and Bilder 2011), it is likely that the “fibrils” in this system are simply linear aggregates of basement membrane material.
Although this model awaits experimental validation, it suggests two non-mutually exclusive hypotheses for how fibril-like structures might form within a basement membrane. One possibility is that traction forces exerted by the migrating follicle cells could rearrange portions of the existing planar network into linear arrays. The more intriguing possibility, however, is that the fibrils arise de novo from newly secreted protein. Clonal expression of Col IV-GFP (Green Fluorescent Protein) has shown that the follicle cells actively secrete new matrix as they migrate (Haigo and Bilder 2011). Moreover, the intracellular machinery that secretes the basement membrane shows an intriguing localization at the basal trailing edge of each migrating follicle cell (Lerner et al. 2013), which suggests that the mechanisms that circumferentially pattern the epithelium may also feed into the process of fibril formation. Whether the fibril-like structures form within the secretory pathway or through modified assembly of the matrix in the extracellular space will require further investigation. In either case, the rotational motion provides an elegant mechanism for orienting the fibrils in the matrix with respect to the axis of elongation.
Although it is easy to intuit how egg chamber rotation could polarize the basement membrane, the relationship between rotation and global alignment of the basal actin bundles has been harder to discern. Given their similarity to ventral stress fibers (Vallenius 2013), these structures almost assuredly contribute to the motility of follicle cells by mediating cell–matrix interactions. It therefore seems likely that they would have to be circumferentially oriented prior to the onset of rotation. However, recent studies of the Ste20-family kinase Misshapen (Msn) have suggested that the opposite is true—that rotation globally aligns the basal actin bundles (Lewellyn et al. 2013). Msn’s primary role in this system is to promote the motility of individual follicle cells by reducing integrin-based adhesion at each cell’s trailing edge. Interestingly, loss of Msn function from a small subset of follicle cells does not block the migration of wild-type cells. Under this condition, rotation proceeds and the basal actin bundles become circumferentially oriented across the tissue. In contrast, when the percentage of msn mutant cells is high enough to block bulk epithelial movement, the basal actin pattern is globally perturbed. A similar all-or-nothing link between the ability of the egg chamber to rotate and the circumferential actin pattern appears to occur in kugelei mosaic epithelia (Viktorinova et al. 2009; Viktorinova and Dahmann 2013). Recent studies have shown that forces exerted by epithelial morphogenesis or by tension from neighboring tissues can contribute to the planar polarization of other epithelia (Aigouy et al. 2010; Olguin et al., 2011; Lee et al. 2012). These data suggest that the tissue-level alignment of the basal actin bundles may have a similar morphogenetic basis. Future studies will be required to determine how epithelial motility induces this organization.
Rotational motion clearly plays a central role in creating the circumferential pattern in the follicular epithelium; however, many questions remain about events that occur both before and after the developmental window when the egg chamber is rotating. The observation that basal actin bundles are present and already globally aligned within the follicle cell precursors in the germarium is puzzling. What is the significance of this early pattern? Why is it lost in young egg chambers only to be re-established by the rotational motion at stage 5? The later dynamics in the pattern of the basal actin bundle are also mysterious, particularly the 90° shift in their orientation at stage 11. Why does this transition occur, and how is the circumferential pattern re-established? In the latter case, it is interesting to speculate that the actin bundles may return to their corset-like orientation by “reading” the polarity information that is stored in the fibrillar basement membrane. Identifying the mechanisms at play during these lesser-studied stages will be essential for our eventual understanding of this morphogenetic system.
Physical nature of the molecular corset
Perhaps the biggest challenge moving forward will be to determine how the circumferential patterning of the follicular epithelium actually drives elongation. The idea of a molecular corset functioning at the basal surface seems quite intuitive at first. However, we know very little about the underlying mechanics, which are likely to be complex and to vary at different oogenic stages. Here, I will highlight some key questions about how the global alignment of the basal actin bundles and polarized basement membrane actually provide the corset function.
As mentioned previously, the basal actin bundles appear to function as stress fibers that mechanically couple the basal cortex to the basement membrane through integrin-based adhesions (Bateman et al. 2001; Delon and Brown 2009). Importantly, studies of cultured cells have shown that the internal organization and molecular dynamics of stress fibers vary between migrating and non-migrating cells (Pellegrin and Mellor 2007; Naumanen et al. 2008; Deguchi and Sato 2009; Vallenius 2013). Consistent with this notion, the basal actin bundles become increasingly dense post-rotation (compare Figs. 2A and 3B), and change the composition of their anchoring focal adhesions (Delon and Brown 2009). During stages 9 and 10, they also undergo oscillating myosin-mediated contractions that cause the basal surfaces of individual cells to temporarily shorten in a pulsatile manner. How are these structural changes in the basal actin bundles regulated and how does their presumed corset function change over developmental time?
The unusual fibrillar organization of the basement membrane raises even more questions. Do the fibrils increase the stiffness of the matrix, promote epithelial migration, direct the tissue-level alignment of the basal actin bundles or provide some combination of these functions? Answering these questions will require the identification of a condition that selectively eliminates the fibrils, without blocking rotation or changing the concentration of one or more proteins in the matrix. Interestingly, the basement membrane appears to play a particularly important role in maintaining the shape of the egg chamber at late oogenic stages. Treatment with the actin-depolymerizing drug Latrunculin A does not affect the shape of the egg chamber at stage 12. In contrast, treatment with collagenase, which partially digests the basement membrane and disrupts the basal actin bundles, leads to rapid rounding of the egg chamber (Haigo and Bilder 2011).
Importantly, little work has been done to characterize the expansive force that is thought to come from the growth of germ cells. Orientation of the follicular epithelium and experimental evidence both indicate that this force is first detected at the apical surface (Wang and Riechmann 2007). What are the key mechanical features of the epithelium as a whole that allow this force to be resisted by a presumed stiffening of the basal surface?
Finally, when thinking about a corset-type mechanism for elongation, it is hard to ignore the fact that egg chambers develop within a tubular sheath of muscle that continuously pushes the developing eggs toward the oviduct (Hudson et al. 2008). Interestingly, the muscle fibers are primarily oriented perpendicular to the AP axis, which raises the possibility that this tissue could provide a compressive force that helps to shape the egg chamber (Gill 1964). One argument against this model comes from an elegant set of experiments, in which a germarium, or young egg chamber, is isolated from its muscle sheath and then surgically transplanted into the abdominal cavity of a host fly (Srdic and Jacobs-Lorena 1978; Montell et al. 1991; Lin and Spradling 1993; Willard et al. 2004). These tissues can often fully recapitulate oogenesis, including the formation of stage-14 egg chambers with relatively normal morphology. Thus, the muscle is not strictly required for elongation. However, the classic “round egg” mutations that block epithelial rotation reduce egg chamber elongation, but do not eliminate it. It will be interesting to explore whether the encircling muscle partially compensates for the loss of the epithelium-based corset under these conditions.
Elongating the egg chamber
No matter where the morphogenetic forces originate, the lengthening of the egg chamber along its AP axis will ultimately require changes in the shape and/or relative positions of the cells within the follicular epithelium. One possibility is that the individual follicle cells lengthen along their AP axes. This process appears to contribute to egg chamber elongation at stage 9, which will be discussed below. Alternatively, the ratio of the number of follicle cells along the AP axis versus the number of follicle cells along the circumferential axis could increase. A topological change of this type can arise through two non-mutually exclusive mechanisms: (1) convergence and extension, where the cells exchange neighbors in a direction perpendicular to the AP axis or (2) oriented cell divisions, where the cells preferentially divide along the AP axis.
Although the egg chamber lengthens nearly two-fold during egg chamber rotation (stages 5–8), how the follicular epithelium changes its shape during this period is mysterious. The individual cells do not lengthen along their AP axes. Moreover, live imaging of the central domain of the migrating epithelium has suggested that the follicle cells rarely exchange neighbors (Haigo and Bilder 2011; Lewellyn et al. 2013). This observation is consistent with the recent discovery that groups of follicle cells remain physically connected to one another through cytoplasmic bridges following division, which likely inhibits large-scale rearrangements of the tissue (Airoldi et al. 2011; McLean and Cooley 2013).
Whether oriented cell divisions contribute to egg chamber elongation has not yet been examined; however, it is reasonable to believe that they might. During epiboly movements in zebrafish, anisotropic tension along the animal–vegetal axis of the surface epithelium is sufficient to orient cell divisions (Campinho et al. 2013). The resulting cellular rearrangements then release the tension and elongate the tissue. It is possible that resistance from the molecular corset, or the migration itself, might induce similar tension that orients follicle cell divisions. At best, however, polarized divisions can only account for part of the elongation during the rotational period, as the follicle cells stop dividing at the end of stage 6. Thus, the follicle cells must also be exchanging neighbors in some subtle way. For example, the exchange of neighbors might be restricted to the egg chamber poles which traditionally have been more difficult to image. Automated cell tracking across the entire epithelium will ultimately be required to tease apart the relative contributions of oriented cell divisions versus other cellular rearrangements during these stages.
The cellular mechanisms driving egg chamber elongation are better understood during stages 9 and 10, when the basal actin bundles undergo oscillating contractions (He et al. 2010). The contractions first occur near the egg chamber’s center at stage 9, and then exclusively over the oocyte at stage 10. Given its timing and location, this fascinating behavior may function specifically as a corset for the rapidly expanding oocyte, thereby channeling its growth toward the egg chamber’s anterior pole. In support of this notion, drugs that create a hypo-contractile or hyper-contractile state within the follicle cells lead to the predicted changes in shape of the oocyte (He et al. 2010). The expansion of this posterior-most germ cell then pushes the nurse cells toward the anterior. Importantly, as the stretch cells flatten to cover the nurse cells, they preferentially lengthen in the AP direction (Grammont 2007; Kolahi et al. 2009), which likely accounts for the dimensional changes in the epithelium during this period.
Conservation of mechanisms
Although egg chamber elongation has been most intensively studied in Drosophila melanogaster, circumferential organization of the follicular epithelium has been observed in egg chambers from a diverse array of insects. The first report came from studies of the gall midge (Heteropeza pygmaea) and the American cockroach (Periplantea americana) (Tucker and Meats 1976). In both cases, a circumferential array of microtubules was seen at the basal epithelial surface, perpendicular to the egg chamber’s long axis. This pattern has now also been described in Drosophila (Viktorinova and Dahmann 2013). Circumferential epithelial arrangements have also been noted for basal actin bundles and the basement membrane in the honey bee (Apis mellifera), and for basal actin bundles and microtubules in the cotton bug (Dysdercus intermedius) (Fleig et al. 1991). These non-Drosophila species represent four distantly related insect orders, whose mode of oogenesis, follicular epithelium, and shape of egg vary widely. Whether the circumferential epithelial organization seen in these diverse species represents a corset-type mechanism controlling egg shape or performs some other function remains an open question.
The argument for functional conservation is strongest for Heteropeza, which shares the Dipteran order with Drosophila. In the late 1970s, D.F. Went performed live imaging on Heteropeza egg chambers and reported that they displayed a sustained rotational motion around their AP axes over the course of 30–40 h of development in culture (Went 1977; Fux et al. 1978). He even speculated that the rotation occurred within a static basement membrane, and noted that egg chambers that stopped rotating prematurely had a rounded morphology. However, these papers went largely uncited until the rotational motion was re-discovered independently in Drosophila some 30 years later (Haigo and Bilder 2011). Work on another Dipteran, the fungus gnat (Bradysia tritici), showed that treatment with collagenase, which presumably partially digested the basement membrane, caused rapid rounding of the egg chamber (Gutzeit and Haas-Assenbaum 1991). Thus, the mechanisms controlling egg shape in Drosophila may, in fact, be well conserved within this order.
To date, the exact mode of elongation that shapes flies’ eggs has not been seen in other tissues and organs. However, the basal surfaces and basement membranes of developing epithelia have traditionally received far less attention than the apical and junctional regions. Thus, a circumferential tissue organization at the interface between the cells and the basement membrane may be more prevalent than we know. Moreover, although the structures of the tissues vary, a molecular corset-type mechanism is used to elongate and straighten the notochord in fish and amphibians and to lengthen the AP axis of the nematode Caenorhabditis elegans (Keller 2006).
The feature of egg chamber elongation that most captures the imagination is rotational motion. To what extent can we expect this process to be conserved? Recent work on cultured mammalian cells has suggested that coherent rotational motion may be an intrinsic property of epithelia when they are constrained in certain geometries (reviewed by Rorth [2012]). Epithelial cells cultured on micro-patterned circular ECMs will spontaneously and collectively migrate in either a clockwise or counterclockwise direction (Brangwynne et al. 2000; Huang et al. 2005; Doxzen et al. 2013). Likewise, spherical epithelial cysts show a sustained rotational behavior when at least partially embedded in a three-dimensional ECM (Zeng et al. 2006; Ferrari et al. 2008; Guo et al. 2008; Marmaras et al. 2010; Tanner et al. 2012; Wang et al. 2013). It is therefore possible that the ability of epithelial cells to self-organize for rotational migration might be used in other evolutionary contexts to organize a tissue for morphogenesis.
One place where similar migration dynamics may occur is in the zebrafish’s kidney. This bilaterally symmetric organ consists of two glomeruli fused at the midline and two pronephric tubules that extend posteriorly from this central domain. During development, the tubular epithelial cells collectively migrate toward the glomeruli and create hairpin-shaped bends in the tubules near their attachment sites (Vasilyev et al. 2009). Similar to the follicle cells, the migrating pronephric epithelia lack a free leading edge and the basal surface of each cell crawls along an ensheathing basement membrane. The primary difference is that the pronephric cells migrate along the tissue’s long axis as opposed to perpendicular to it. Strikingly, this anterior migration depends on posteriorly directed flow of fluid within the tubule’s lumen. When this flow is blocked, the epithelial cells switch to a circumferential migration and the tubules rotate. This observation suggests that rotational motion may be the default state for this epithelium, which is then co-opted and redirected by the mechanical stimulus provided by the flowing fluid.
Looking toward the future
Compared with more established morphogenetic models, the study of egg chamber elongation is in its infancy—and yet, it has already highlighted the importance of two novel tissue-shaping behaviors, epithelial rotation and oscillating contractions of the basal cortex. The ease of genetic manipulation has long made the follicle cells a premier model for the study of epithelial biology, and forward genetic approaches have begun to identify the protein networks that shape the egg (Vlachos and Harden 2011; Horne-Badovinac et al. 2012). Going forward, it is likely that a combination of genetic and proteomic approaches will systematically identify the key molecular players. The speed and flexibility of the new CRISPR/Cas9 genome-editing system will allow precise manipulation of the endogenous proteins to reveal the temporal, spatial, and regulatory contexts in which they function. Importantly, because most of the critical events occur near the egg chamber’s surface at the cell–basement membrane interface, these molecular studies can be further enhanced by the use of total internal reflection fluorescence and super-resolution microscopy. Another important feature of the egg chamber as a model is its cellular simplicity—an assembly of 500–1000 cells is transformed from a spherical to an ellipsoid shape. The relatively low complexity of transition makes the egg chamber highly amenable to the automated cell tracking, biophysical approaches, and mathematical modeling that will provide deep mechanistic understanding of the cellular dynamics driving elongation.
Funding
This work was supported by the National Institutes of Health [grant number R01-GM094276].
Acknowledgments
I am grateful to Urs Schmidt-Ott, Kari Barlan, Maureen Cetera, Darcy McCoy, and the three reviewers for helpful comments on the manuscript; to Ezgi Kunttas-Tatli for enjoyable conversations about comparative aspects of insect oogenesis during the meeting; and to Nick Badovinac for illustrations.
References
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Airoldi SJ, McLean PF, Shimada Y, Cooley L. Intercellular protein movement in syncytial Drosophila follicle cells. J Cell Sci. 2011;124:4077–86. doi: 10.1242/jcs.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–27. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Brangwynne C, Huang S, Parker KK, Ingber DE, Ostuni E. Symmetry breaking in cultured mammalian cells. In Vitro Cell Dev Biol Anim. 2000;36:563–5. doi: 10.1007/BF02577523. [DOI] [PubMed] [Google Scholar]
- Broadie K, Baumgartner S, Prokop A. Extracellular matrix and its receptors in Drosophila neural development. Dev Neurobiol. 2011;71:1102–30. doi: 10.1002/dneu.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg CP. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol. 2013;15:1405–14. doi: 10.1038/ncb2869. [DOI] [PubMed] [Google Scholar]
- Deguchi S, Sato M. Biomechanical properties of actin stress fibers of non-motile cells. Biorheology. 2009;46:93–105. doi: 10.3233/BIR-2009-0528. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J Cell Sci. 2009;122:4363–74. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxzen K, Vedula SR, Leong MC, Hirata H, Gov NS, Kabla AJ, Ladoux B, Lim CT. Guidance of collective cell migration by substrate geometry. Integr Biol. 2013;5:1026–35. doi: 10.1039/c3ib40054a. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci. 2008;121:3649–63. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- Fleig R, Gutzeit HO, Engels W. Structural organization of ovarian follicle cells in the cotton bug (Dysdericus intermedius) and the honeybee (Apis mellifera) Cell Tissue Res. 1991;265:297–305. [Google Scholar]
- Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development. 2001;128:3209–20. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- Fux T, Went DF, Camenzind R. Movement pattern and ultrastructure of rotating follicles of the paedoggenetic gall midge, Heteropeza pygmea winnerz (Diptera: Cecidomyiidae) Int J Morphol Embryol. 1978;7:415–26. [Google Scholar]
- Gates J. Drosophila egg chamber elongation: insights into how tissues and organs are shaped. Fly (Austin) 2012;6:213–27. doi: 10.4161/fly.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KS. Epigenetics of the promorphology of the egg in Drosophila melanogaster. J Exp Zool. 1964;155:91–104. doi: 10.1002/jez.1401550107. [DOI] [PubMed] [Google Scholar]
- Grammont M. Adherens junction remodeling by the Notch pathway in Drosophila melanogaster oogenesis. J Cell Biol. 2007;177:139–50. doi: 10.1083/jcb.200609079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Xia B, Moshiach S, Xu C, Jiang Y, Chen Y, Sun Y, Lahti JM, Zhang XA. The microenvironmental determinants for kidney epithelial cyst morphogenesis. Eur J Cell Biol. 2008;87:251–66. doi: 10.1016/j.ejcb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit HO. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur J Cell Biol. 1990;53:349–56. [PubMed] [Google Scholar]
- Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci . 1991;100(Pt 4):781–8. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO, Haas-Assenbaum A. The somatic envelopes around the germ-line cells of polytrophic insect follicles: structural and functional aspects. Tissue Cell. 1991;23:853–65. doi: 10.1016/0040-8166(91)90035-r. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wang X, Tang HL, Montell DJ. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol. 2010;12:1133–42. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Hill J, Gerlach G, 2nd, Menegas W, Bilder D. A screen for round egg mutants in Drosophila identifies tricornered, furry, and misshapen as regulators of egg chamber elongation. G3 (Bethesda) 2012;2:371–8. doi: 10.1534/g3.111.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Brangwynne CP, Parker KK, Ingber DE. Symmetry-breaking in mammalian cell cohort migration during tissue pattern formation: role of random-walk persistence. Cell Motil Cytoskeleton. 2005;61:201–13. doi: 10.1002/cm.20077. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Petrella LN, Tanaka AJ, Cooley L. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev Biol. 2008;314:329–40. doi: 10.1016/j.ydbio.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Mechanisms of elongation in embryogenesis. Development. 2006;133:2291–302. doi: 10.1242/dev.02406. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahi KS, White PF, Shreter DM, Classen AK, Bilder D, Mofrad MR. Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: new insights based on morphometric analysis and mechanical modeling. Dev Biol. 2009;331:129–39. doi: 10.1016/j.ydbio.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Andreeva A, Sipe CW, Liu L, Cheng A, Lu X. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr Biol. 2012;22:956–66. doi: 10.1016/j.cub.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S. A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell. 2013;24:159–68. doi: 10.1016/j.devcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellyn L, Cetera M, Horne-Badovinac S. Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. J Cell Biol. 2013;200:721–9. doi: 10.1083/jcb.201209129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Spradling AC. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev Biol. 1993;159:140–52. doi: 10.1006/dbio.1993.1228. [DOI] [PubMed] [Google Scholar]
- Marmaras A, Berge U, Ferrari A, Kurtcuoglu V, Poulikakos D, Kroschewski R. A mathematical method for the 3D analysis of rotating deformable systems applied on lumen-forming MDCK cell aggregates. Cytoskeleton (Hoboken) 2010;67:224–40. doi: 10.1002/cm.20438. [DOI] [PubMed] [Google Scholar]
- McLean PF, Cooley L. Protein equilibration through somatic ring canals in Drosophila. Science. 2013;340:1445–7. doi: 10.1126/science.1234887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ, Keshishian H, Spradling AC. Laser ablation studies of the role of the Drosophila oocyte nucleus in pattern formation. Science. 1991;254:290–3. doi: 10.1126/science.254.5029.290. [DOI] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231:446–54. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Olguin P, Glavic A, Mlodzik M. Intertissue mechanical stress affects Frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr Biol. 2011;21:236–42. doi: 10.1016/j.cub.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008;182:801–15. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–9. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Rorth P. Fellow travellers: emergent properties of collective cell migration. EMBO Rep. 2012;13:984–91. doi: 10.1038/embor.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–15. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Nystul T, Lighthouse D, Morris L, Fox D, Cox R, Tootle T, Frederick R, Skora A. Stem cells and their niches: integrated units that maintain Drosophila tissues. Cold Spring Harb Symp Quant Biol. 2008;73:49–57. doi: 10.1101/sqb.2008.73.023. [DOI] [PubMed] [Google Scholar]
- Srdic Z, Jacobs-Lorena M. Drosophila egg chambers develop to mature eggs when cultured in vivo. Science. 1978;202:641–3. doi: 10.1126/science.100884. [DOI] [PubMed] [Google Scholar]
- Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci USA. 2012;109:1973–8. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JB, Meats M. Microtubules and control of insect egg shape. J Cell Biol. 1976;71:207–17. doi: 10.1083/jcb.71.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallenius T. Actin stress fibre subtypes in mesenchymal-migrating cells. Open Biol. 2013;3:130001. doi: 10.1098/rsob.130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinova I, Dahmann C. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr Biol. 2013;23:1472–7. doi: 10.1016/j.cub.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–32. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Vlachos S, Harden N. Genetic evidence for antagonism between Pak protein kinase and Rho1 small GTPase signaling in regulation of the actin cytoskeleton during Drosophila oogenesis. Genetics. 2011;187:501–12. doi: 10.1534/genetics.110.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc Natl Acad Sci USA. 2013;110:163–8. doi: 10.1073/pnas.1201141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–55. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Waring GL. Morphogenesis of the eggshell in Drosophila. Int Rev Cytol. 2000;198:67–108. doi: 10.1016/s0074-7696(00)98003-3. [DOI] [PubMed] [Google Scholar]
- Went DF. Pulsating oocytes and rotating follicles in an insect ovary. Dev Biol. 1977;55:392–6. doi: 10.1016/0012-1606(77)90182-8. [DOI] [PubMed] [Google Scholar]
- Willard SS, Ozdowski EF, Jones NA, Cronmiller C. Stall-mediated extrinsic control of ovarian follicle formation in Drosophila. Genetics. 2004;168:191–8. doi: 10.1534/genetics.104.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. pii: a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D, Ferrari A, Ulmer J, Veligodskiy A, Fischer P, Spatz J, Ventikos Y, Poulikakos D, Kroschewski R. Three-dimensional modeling of mechanical forces in the extracellular matrix during epithelial lumen formation. Biophys J. 2006;90:4380–91. doi: 10.1529/biophysj.105.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]