In this analysis of 85 patients with metastatic renal cell carcinoma (RCC) and nonclear or sarcomatoid histologies, treatment benefit from mTOR inhibitors was limited for most patients. While a few patients achieved prolonged responses, no histologic variant was found to be predictive. These findings argue in favor of investigating predictive biomarkers to identify patients most likely to respond.
Keywords: mTOR inhibitors, nonclear-cell rCC, renal cell carcinoma, sarcomatoid
Abstract
Background
The clinical trials that reported benefit of the rapalogs temsirolimus and everolimus in advanced renal cell carcinoma (RCC) were primarily conducted in patients with clear-cell histology (ccRCC). We assessed outcome with these mammalian target of rapamicin (mTOR) inhibitors in two subsets of kidney cancer: sarcomatoid variant ccRCC and nonclear-cell RCC.
Patients and methods
Baseline clinical features, information on prior treatment, and histologic subtypes were collected for patients previously treated with rapalogs for metastatic RCC of either nonclear phenotype or ccRCC with sarcomatoid features. Outcome was assessed centrally by a dedicated research radiologist for determination of tumor response, progression-free survival (PFS), and overall survival (OS).
Results
Eighty-five patients received temsirolimus (n = 59) or everolimus (n = 26). Nonclear-cell phenotypes included papillary (n = 14), chromophobe (n = 9), collecting duct (n = 4), translocation-associated (n = 3), and unclassified (n = 32) RCC. Twenty-three patients had clear-cell histology with sarcomatoid features. The response rate in assessable patients (n = 82) was 7% (all partial responses); 49% of patients achieved stable disease, and 44% had progressive disease as their best response. Tumor shrinkage was observed in 26 patients (32%). Median PFS and OS were 2.9 and 8.7 months, respectively. Nine patients (11%) were treated for ≥1 year, including cases of papillary (n = 3), chromophobe (n = 2), unclassified (n = 3) RCC, and ccRCC with sarcomatoid features (n = 1). No tumor shrinkages were observed for patients with collecting duct or translocation-associated RCC.
Conclusions
A subset of patients with nonclear-cell and sarcomatoid variant ccRCC subtypes benefit from mTOR inhibitors, but most have poor outcome. Histologic subtype does not appear to be helpful in selecting patients for rapalog therapy. Future efforts should include the identification of predictive tissue biomarkers.
introduction
Kidney cancer is not an uncommon malignancy, with an anticipated incidence of 65 150 new cases and 13 680 deaths in the United States in 2013 [1]. Based on the Heidelberg classification of kidney cancers, there are several variants of renal cell carcinoma (RCC) [2]. The most common histologic subtype is conventional or clear-cell RCC (ccRCC), which makes up ∼60%–80% of cases. The remaining variants comprise a heterogeneous group of diseases and are often summarized as nonclear-cell RCC (ncRCC). Specific subtypes differ in their incidence and include papillary (7%–14%), chromophobe (6%–11%), and collecting duct RCC with its variant medullary type RCC (<1%) [2, 3]. More recently, translocation-associated RCC, typically with translocations involving the transcription factor E3 (TFE3) gene located on Xp11.2, have been recognized in the WHO classification as yet another rare, clinically distinct entity [4]. RCC phenotypes that do not meet sufficient histopathologic criteria for any of these subtypes are generally grouped as unclassified tumors and makeup about 3%–5% of cases.
The presence of sarcomatoid differentiation is not considered to constitute a separate RCC subtype; rather, it can be identified as a morphologic feature across all RCC histologies, including ccRCC and ncRCC. As such, its incidence has been reported in up to 29% of collecting duct carcinomas and about 8%–10% of clear-cell, chromophobe, and unclassified RCC, and is less frequently associated with papillary histology (3%) [5]. Regardless of the underlying histology, sarcomatoid features are considered a hallmark of more aggressive disease biology, thus adversely affecting clinical course and prognosis, with an estimated overall survival (OS) of <10 months in most series [6–8].
Despite recent advances in the treatment of metastatic ccRCC, the optimal therapy for patients with advanced RCC with less common histologies has not been established. Most pivotal trials of VEGF-targeted agents or rapamycin-like mammalian target of rapamicin (mTOR) inhibitors (rapalogs) exclusively enrolled patients with clear-cell histology, with the exception of the multicenter Advanced Renal Cell Carcinoma (ARCC) trial, a randomized trial that demonstrated superiority of temsirolimus over interferon-α [9]. An unplanned subgroup analysis of patients with ncRCC suggested that superior efficacy of temsirolimus over interferon likely applies in this population [10]. Although the report has led to the frequent use of mTOR inhibitors in ncRCC, it did not provide insight into the distinct ncRCC subtypes. Similarly, the effect of sarcomatoid differentiation on the therapeutic benefit with rapalogs remains to be elucidated.
In this study, we carried out a retrospective analysis of patients with metastatic RCC of sarcomatoid clear-cell and nonclear-cell subtypes previously treated with mTOR inhibitors at Memorial Sloan–Kettering Cancer Center (MSKCC). The aim was to explore the efficacy of these agents across the various RCC variants.
patients and methods
Cases were identified from an institutional database of 298 patients with RCC previously treated at our center with rapalog therapy between April 2007 and April 2013. Histopathologic diagnosis, including RCC subtype, was established via review of tumor tissue at MSKCC. We included the following variants: papillary RCC, chromophobe RCC, collecting duct carcinoma, medullary RCC, translocation-type, and unclassified RCC, as well as ccRCC with any element of sarcomatoid differentiation. Patients who had received rapalogs in combination with other agents were excluded from the analysis. This study was reviewed and approved by the MSKCC Institutional Review Board.
Individual charts were retrospectively reviewed to determine patient demographics, details of rapalog administration, clinical features at treatment start, extent of prior treatment, as well as follow-up and survival status. We defined the first day of rapalog administration as the entry point for our analysis. For inclusion in the response assessment analysis, patients had to have received ≥4 weeks of rapalog therapy with baseline and subsequent tumor reassessment carried out at MSKCC. Patients with clinical progression before 4 weeks of mTOR inhibitor therapy were included in the response evaluation as having had progressive disease. Generally, cross-sectional imaging was obtained every 6–8 weeks, according to our clinical standard. All scans carried out during rapalog therapy were reviewed by the same research radiologist for formal tumor response assessment using Response Evaluation Criteria in Solid Tumors (RECIST) criteria v1.1 [11]. Progression-free survival (PFS) and OS were determined using the Kaplan–Meier method.
results
patient characteristics
A total of 85 rapalog-treated patients were identified for this analysis. Baseline patient characteristics are summarized in the Table 1. RCC subtypes included 23 clear-cell sarcomatoid variants and 62 patients with ncRCC. The latter group comprised 14 patients with papillary (16%), 9 with chromophobe (10%), 4 with collecting duct (5%), 3 with TFE translocation-type (4%), and 32 with unclassified RCC (37%). Among the ncRCC patients, 11 (13%) had sarcomatoid features. Disease was widely disseminated in most patients, with more than half of the patients demonstrating ≥3 metastatic sites of disease. The majority of patients (82%) had undergone nephrectomy before starting rapalog treatment. Sixty-five percent were pretreated with other targeted agents (median of one prior agent), with all having received VEGF-directed therapy.
Table 1.
Patient characteristics (N = 85)
| Characteristics | No. of patients | % |
|---|---|---|
| Sex | ||
| Male | 54 | 64 |
| Female | 31 | 36 |
| Median age (range), years | 60 (15–86) | |
| Karnofsky performance status | ||
| 90% | 20 | 24 |
| 80% | 42 | 49 |
| ≤70% | 23 | 27 |
| Prior nephrectomy | ||
| Yes | 70 | 82 |
| No | 15 | 18 |
| Histology | ||
| Sarcomatoid clear cell | 23 | 27 |
| Papillary | 14 | 16 |
| Chromophobe | 9 | 10 |
| Unclassified | 32 | 38 |
| Collecting duct | 4 | 5 |
| Translocation type | 3 | 4 |
| MSKCC Risk Group [1]a | ||
| Favorable | 13 | 15 |
| Intermediate | 57 | 68 |
| Poor | 14 | 17 |
| No. of metastatic sites | ||
| 1 | 13 | 15 |
| 2 | 24 | 28 |
| ≥3 | 48 | 57 |
| No. of prior systemic therapies | ||
| 0 | 30 | 35 |
| 1 | 41 | 48 |
| ≥2 | 14 | 17 |
| mTOR inhibitor | ||
| Everolimus | 26 | 30 |
| Temsirolimus | 59 | 70 |
aLDH was unavailable for one patient; thus, MSKCC risk group could not be assigned.
response and clinical outcomes
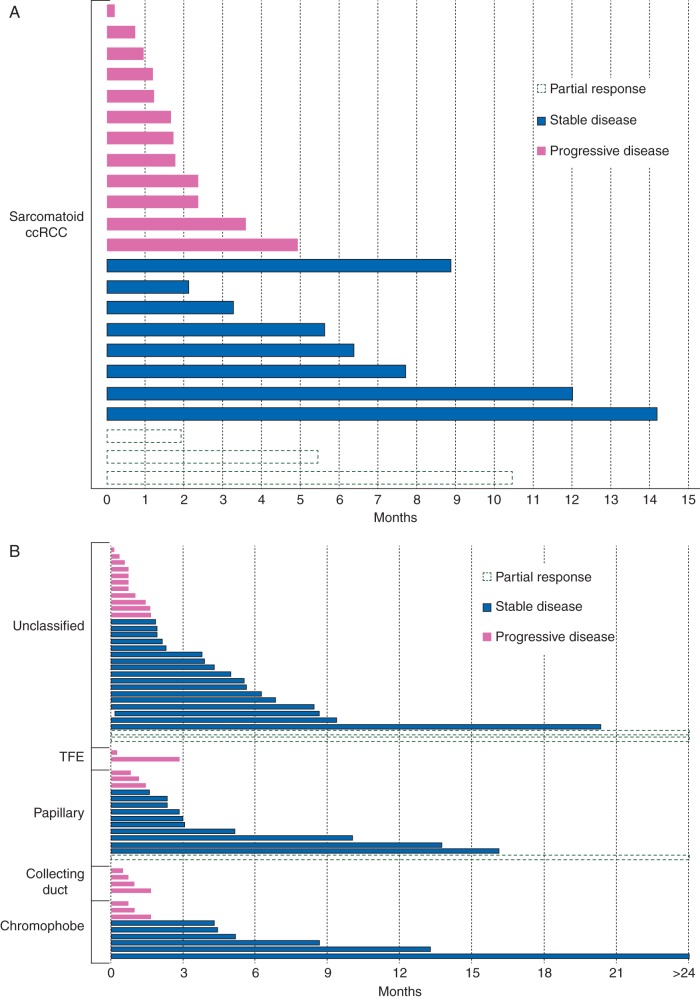
Twenty-seven patients (30%) received everolimus, and 59 (70%) were treated with temsirolimus. Three patients were not included in the response assessment analysis because they received <4 weeks of mTOR inhibitor therapy due to toxicity. Of 82 patients assessable for treatment effect, best response was partial response, stable disease, and progressive disease in 7%, 49%, and 44%, respectively (Table 2). Seventeen patients experienced clinical progression before 4 weeks of therapy. Among all assessable subjects, tumor shrinkage was observed in 32% of patients but was short-lived for most cases (Figure 1). Antitumor effect was seen across multiple RCC subtypes without any particular variant standing out as most responsive to therapy. None of the four patients with collecting duct and none of the three patients with translocation-associated RCC showed any tumor shrinkage.
Table 2.
Response rate by RECIST of assessable patients
| Objective response (RECIST 1.1) | Assessable patients | Sarcomatoid ccRCC | Nonclear cell |
|---|---|---|---|
| No. of patients (%) | 82 (100) | 23 (100) | 59 (100) |
| PR, n (%) | 6 (7) | 3 (13) | 3 (5) |
| SD, n (%) | 40 (49) | 7 (30) | 33 (56) |
| PR or SD ≥ 6 months, n (%) | 20 (24) | 4 (17) | 14 (24) |
| PD, n (%) | 36 (44) | 13 (57) | 23 (39) |
PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Duration of mammalian target of rapamicin inhibitor treatment of patients with sarcomatoid clear-cell RCC (A) and nonclear-cell RCC (B) according to histologic subtypes. Each bar represents an individual patient. Line pattern/color distinguishes best response by RECIST.
Median PFS for the entire cohort, for patients with ncRCC, and for those with sarcomatoid ccRCC was 2.9 months [95% confidence interval (CI) 1.8–4.3], 2.8 months (95% CI 1.8–4.2), and 3.5 months (95% CI 1.6–5.4), respectively (Table 3; Figure 2). For treatment-naïve ncRCC patients (n = 27), the median PFS with rapalogs was 3.8 months (95% CI −2.2–6.8), whereas pretreated patients showed a median PFS of 2.7 months (95% CI 1.6–5.1). Nine patients (10%) received rapalogs for ≥1 year (range 13.3–37.8 months), including cases of papillary (n = 3), chromophobe (n = 2), unclassified (n = 3), and sarcomatoid ccRCC (n = 1). With a median follow-up of 33 months, the median OS for the entire cohort, for patients with ncRCC, and for those with sarcomatoid ccRCC was 8.7 months (95% CI 6.5–12.0), 9.1 months (95% CI 6.5–12.6), and 8.2 months (95% CI 4.8–14.3), respectively (Table 3; Figure 2). Across the entire cohort, the median survival differed significantly when stratifying patients by MSKCC risk group [12] (9.3, 9.0, and 6.4 months for patients with favorable, intermediate, and poor risk, respectively; P = 0.04; see Figure 3).
Table 3.
Progression-free survival and overall survival according to histology
| All patients | Sarcomatoid ccRCC | Nonclear cell | |
|---|---|---|---|
| No. of patients (%) | 85 (100) | 23 (27) | 62 (73) |
| Median progression-free survival (95% CI), months | 2.9 (1.8–4.3) | 3.5 (1.6–5.4) | 2.8 (1.8–4.2) |
| Median overall survival (95% CI), months | 8.7 (6.5–12.0) | 8.2 (4.8–14.3) | 9.1 (6.5–12.6) |
CI, confidence interval.
Figure 2.
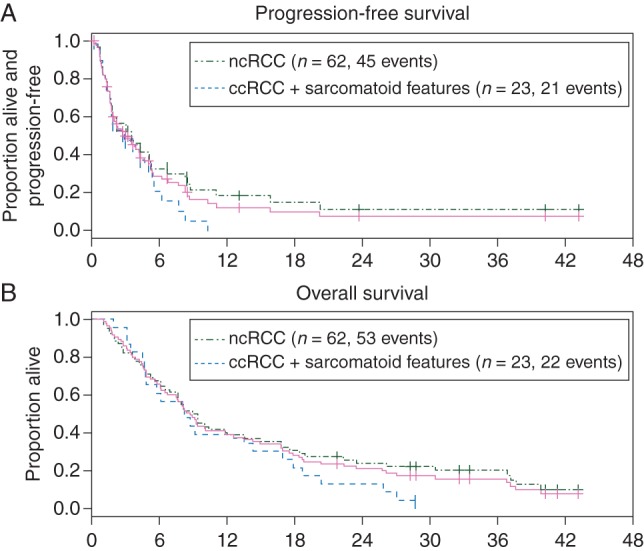
Kaplan–Meier curves for progression-free survival (A) and overall survival (B) for the entire cohort. Patients who discontinued treatment due to toxicity were censored at the time of last dose.
Figure 3.
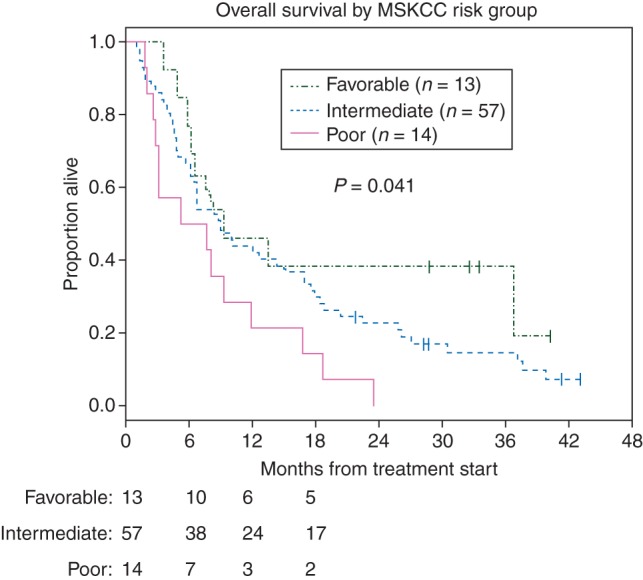
Kaplan–Meier curves for overall survival according to MSKCC risk category for the entire cohort.
discussion
This study set out to assess therapeutic effect of rapalog-type mTOR inhibitors in patients with advanced RCC of nonclear-cell and sarcomatoid variant clear-cell histologic subtypes. Our findings suggest that outcome with this class of agents is poor for the majority of patients. In interpreting our data, it is important to note that even in conventional RCC, objective responses are infrequently seen with rapalogs, and other authors have suggested that RECIST criteria may not optimally reflect treatment benefit [13]. That said, although we noted tumor shrinkage in 32% of our cohort, antitumor effect was short-lived in most cases, as reflected by the median PFS of only 2.9 months observed for this cohort. Furthermore, 17 patients (21%) experienced clinical progression before 4 weeks of mTOR inhibitor therapy. However, a small subset of patients derived noteworthy benefit from rapalogs, with treatment duration beyond 2 years for isolated cases. There was no correlation of such benefit with any particular RCC type.
Despite a paucity of prospective data, rapalogs are considered agents of choice for ncRCC by many oncologists. The National Comprehensive Cancer Network (NCCN) guidelines recommend temsirolimus as category 1 for patients with poor-risk metastatic ncRCC and category 2A for patients belonging to other prognostic risk groups [14]. This recommendation is based on a subset analysis of patients with ncRCC treated in the global ARCC trial of temsirolimus versus interferon in patients with poor risk features. Of note, this unplanned analysis included only 37 patients with ncRCC treated with temsirolimus and 36 patients treated with interferon. The authors reported median PFS, median OS, and objective response rate (ORR) of 7 months, 11.6 months, and 5.4%, respectively, with temsirolimus. The efficacy of temsirolimus appeared superior to interferon, which is not surprising given the previously reported lack of efficacy for interferon for nonclear-cell histologies [10]. Details on histologic subtype were limited in this report, and ncRCC cases were summarized jointly for the efficacy analysis. Chromophobe and collecting duct cases were included in the analysis, but the only dedicated outcome data provided for one particular subtype was in papillary RCC (n = 10) with a median OS of 13.2 months [9, 10]. A phase II trial of everolimus in advanced ncRCC enrolled 49 patients of various ncRCC subtypes, the majority of which were pretreated, and reported a median OS and PFS of 14 and 5.2 months, respectively [15]. Although the authors noted a trend for a longer PFS in chromophobe RCC, the small number of patients (n = 8) and lack of statistical significance (P = 0.084) do not allow definitive conclusions. The recently reported RCC expanded access trial for everolimus, included 75 patients with metastatic ncRCC and reported an overall response rate of 1.3% and a median treatment duration of 12 weeks for this group [16].
In our study, we report outcome data with mTOR inhibitors in patients with advanced sarcomatoid ccRCC, again with varying degrees of therapeutic benefit (Figure 1). A defined standard of care for this group of patients has not been established. None of the pivotal trials leading to the approval of targeted therapeutics in RCC assessed presence of sarcomatoid differentiation by central pathology. Previously published retrospective series suggest that VEGF inhibitors can be effective for some sarcomatoid patients [7, 17]. Case reports have previously suggested that treatment benefit from rapalogs in sarcomatoid ccRCC varies [18]. We found similar heterogeneity across our cohort, which is, to our knowledge, the largest group of patients with sarcomatoid ccRCC treated with rapalog therapy reported to date. Overall, patients responded poorly, with >50% suffering progressive disease as their best response to treatment. Similar to the ncRCC cohort, however, isolated patients achieved disease control for ≥12 months.
No specific RCC subtype, including chromophobe RCC, was predictive of higher response rates or prolonged PFS. None of the few patient with translocation-associated or collecting duct RCC included in this series derived benefit from rapalog therapy, but definite conclusions are limited by the small sample size. Interestingly, ∼10% (n = 9) of all patients derived prolonged clinical benefit and remained on treatment of more than 1 year with four of these patients continuing rapalog therapy for more than 2 years without progression. We previously reported on our efforts to determine the oncogenomic basis for such unusual responses. Tumor and healthy DNA from three of these outliers were analyzed on a targeted next-generation sequencing platform in search for candidate biomarkers of therapeutic response [19, 20]. For two of three patients, plausible genomic determinants of prolonged disease control were identified with alterations in key components of the PI3K/mTOR pathway. For three patients with ncRCC and rapid progression of disease on rapalog therapy, no such changes were noted. These important early findings offer proof-of-concept and provide plausible explanation for the variability in response to treatment that we observed. In this heterogeneous group of diseases, it is likely that differences in underlying tumor genetics, rather than the histopathologic phenotype alone, determines response to targeted therapies.
In summary, patients with metastatic ncRCC and sarcomatoid ccRCC can benefit from mTOR-targeted therapy, but the majority of patients respond poorly with these agents. Therapeutic effect varies greatly between individual patients, even within the same subgroups of disease. Importantly, objective responses or prolonged disease stabilization can be seen for a subset of patients across several of these rare cancers without clear association with any particular histologic phenotype. Future efforts should include the identification and prospective study of genomic tissue biomarkers with the goal of developing predictive tools that can help to rationally select those patients who are most likely to derive benefit from rapalog therapy.
funding
This work was supported by the Young Investigator Award, Conquer Cancer Foundation.
disclosure
MHV has received honoraria from Novartis and research funding from Pfizer; AMM has received research funding and consultancy from Novartis; RJM has received research funding from Novartis, Pfizer, and GlaxoSmithKline and consultancy from Pfizer and Aveo Oncology. All remaining authors have declared no conflicts of interest.
acknowledgements
The authors thank Sheila Higgins and Michael J. McGregor for their contribution to this article.
references
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Reuter VE, Presti JC., Jr Contemporary approach to the classification of renal epithelial tumors. Semin Oncol. 2000;27:124–137. [PubMed] [Google Scholar]
- 4.Lopez-Beltran A, Scarpelli M, Montironi R, et al. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 5.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol. 2002;167:65–70. [PubMed] [Google Scholar]
- 7.Molina AM, Tickoo SK, Ishill N, et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am J Clin Oncol. 2011;34:454–459. doi: 10.1097/COC.0b013e3181f47aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuch B, Bratslavsky G, Shih J, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU Int. 2012;109:1600–1606. doi: 10.1111/j.1464-410X.2011.10785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 10.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 13.Oudard S, Thiam R, Fournier LS, et al. Optimisation of the tumour response threshold in patients treated with everolimus for metastatic renal cell carcinoma: analysis of response and progression-free survival in the RECORD-1 study. Eur J Cancer. 2012;48:1512–1518. doi: 10.1016/j.ejca.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (NCCN) Kidney Cancer (version 1. 2013). http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. (23 August 2013, date last accessed) [PubMed] [Google Scholar]
- 15.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol. 2013;24:1026–1031. doi: 10.1093/annonc/mds582. [DOI] [PubMed] [Google Scholar]
- 16.Blank CU, Bono P, Larkin JMG, et al. Safety and efficacy of everolimus in patients with non-clear cell renal cell carcinoma refractory to VEGF-targeted therapy: subgroup analysis of REACT. J Clin Oncol. 2012;30 (suppl 5; abstr 402) [Google Scholar]
- 17.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235–241. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 18.Areses MC, Herranz UA, Ferran BB, et al. Temsirolimus in renal cell carcinoma with sarcomatoid differentiation: a report of three cases. Med Oncol. 2012;29:795–798. doi: 10.1007/s12032-011-9976-y. [DOI] [PubMed] [Google Scholar]
- 19.Voss MH, Hakimi AA, Scott SN, et al. Genetic determinants of long-term response to rapalog therapy in advanced renal cell carcinoma (RCC) J Clin Oncol. 2012;30 (suppl; abstr 4604) [Google Scholar]
- 20.Voss MH, Bastos DA, Karlo C, et al. Treatment outcome with mTOR inhibitors for 65 cases of non-clear cell renal cell carcinoma: association of long-term responses and oncogenomic events. J Clin Oncol. 2013;31 (suppl 6; abstr 391) [Google Scholar]