Abstract
Fibrin is a hemostatic protein found in the clotting cascade. It is used in the operating room to stop bleeding and deliver cells and growth factors to heal wounds. However, formulations of clinically approved fibrin are optimized for hemostasis, and the extent to which biochemical and physical cues in fibrin mediate skin cell behavior is not fully understood nor utilized in the design of biomaterials. To determine if the concentration of fibrinogen and the presence of plasminogen affect cell behavior relevant to wound healing, we fabricated three-dimensional fibrin constructs made from 5, 10, or 20 mg/mL of clinical fibrin or plasminogen-depleted (PD) fibrin. We cultured dermal fibroblasts or epidermal keratinocytes in these constructs. Fibroblasts proliferated similarly in both types of fibrin, but keratinocytes proliferated more in low concentrations of clinical fibrin and less in PD fibrin. Clinical fibrin constructs with fibroblasts were less stiff and degraded faster than PD fibrin constructs with fibroblasts. Similarly, keratinocytes degraded clinical fibrin, but not PD fibrin. Fibroblast spreading varied with fibrin concentration in both types of fibrin. In conclusion, the concentration of fibrinogen and the presence of plasminogen affect fibroblast and keratinocyte proliferation, morphology, and fibrin degradation. Creating materials with heterogeneous regions of fibrin formulations and concentrations could be a novel strategy for controlling the phenotype of encapsulated fibroblasts and keratinocytes, and the subsequent biomechanical properties of the construct. However, other well-investigated aspects of wound healing remain to be utilized in the design of fibrin biomaterials, such as autocrine and paracrine signaling between fibroblasts, keratinocytes, and immune cells.
Introduction
Chronic wounds, burns, and blistering skin diseases have prompted the development of engineered skin equivalents.1–3 These include sheets of autologous or allografted skin cells, synthetic polymer scaffolds, and scaffolds derived from extracellular matrix (ECM) proteins. One such ECM protein is fibrin, which forms from fibrinogen in the final step of the coagulation cascade triggered by tissue damage.4 Because fibrin is a biocompatible and biodegradable hemostatic protein critical for wound healing, it is currently used as a surgical adhesive and skin graft matrix in the clinic.5–10 In addition to reducing blood loss, fibrin sequesters growth factors and forms a scaffold for immune cells, fibroblasts, and keratinocytes.11 A deeper understanding of cell–fibrin interactions could translate to novel regenerative therapeutics, and is thus an active area of tissue engineering research.12
Fibrin properties and subsequent cellular responses can be controlled by manipulating polymerization conditions such as fibrinogen and thrombin concentrations, solution pH, and ionic strength.13–16 Altering fibrin polymerization parameters also affects biochemical cues in fibrin, such as the concentration and distribution of ECM ligands, cytokines, and growth factors.17 These biochemical cues affect cell proliferation, differentiation, and expression of growth factors. In conjunction with biochemical stimuli, physical cues such as matrix stiffness, fibril geometry, and transduced force also affect cell behavior.18–23 Mechanical load alone causes scarring by inhibiting fibroblast apoptosis through an Akt-dependent mechanism, which results in increased collagen deposition due to higher cellularity.24 We have previously shown that fibrin concentration affects the behavior of encapsulated fibroblasts,25 keratinocytes,26 and mesenchymal stem cells.27 Recently, we also demonstrated that mesenchymal stem cell differentiation is affected by fibrinogen, collagen type I, and fibronectin.28 Unfortunately, fibrin composition and concentration varies from study to study, invalidating the direct comparison of data.
Fibrin composition varies because fibrinogen is obtained from human plasma, which contains a diverse array of proteins such as transforming growth factor beta-1, basic fibroblast growth factor, fibronectin, coagulation cascade factors, plasminogen activator inhibitor, albumin, von Willebrand factor, and immunoglobulins.29,30 Laboratory-grade fibrinogen is obtained by purifying fibrinogen through immunoprecipitation, which removes specific plasma proteins such as plasminogen—a protease zymogen that becomes plasmin, cleaves fibrin polymer, and is critical for the terminal phases of wound healing after the fibrin scaffold has served its purpose.31,32 Purifying fibrin also changes the mechanical properties of polymerized constructs. Dickneite et al. found significant differences in adhesive clot strength and hemostastic efficiency of 12 commercially available fibrin sealants,33 but cellular responses to varying fibrin formulations have not been reported. To investigate how fibrin formulation and concentration affect cell behavior relevant to wound healing, we fabricated three-dimensional (3D) fibrin constructs made from 5, 10, or 20 mg/mL of clinical fibrinogen or laboratory-grade plasminogen-depleted (PD) fibrinogen. This article reports the effects of fibrin parameters on the in vitro proliferation, morphology, and fibrinolytic activity of fibroblasts and keratinocytes.
Materials and Methods
Cell culture
Human foreskin fibroblasts (BJ HFF; American Type Culture Collection [ATCC] CRL-2522] were cultured in Dulbecco's modified Eagle's medium (DMEM) (CellGro) with 10% fetal bovine serum and 1% antibiotic solution in 5% CO2 at 37°C. Media were changed twice a week, and cells were passaged to new flasks upon reaching confluence. Passage 7–12 fibroblasts were used. Human epithelial keratinocytes (HEK001; ATCC CRL-2404) were cultured in keratinocyte serum-free media (Gibco) in 5% CO2 at 37°C. Media were changed every 3 days, and cells were passaged to new flasks upon reaching confluence. Passage 6–10 keratinocytes were used.
Fabrication of 3D fibrin constructs with encapsulated fibroblasts or keratinocytes
Three-dimensional fibrin constructs with encapsulated fibroblasts were fabricated with a protocol similar to Duong et al.16 Constructs were made from two fibrin formulations. The first formulation was clinical fibrin (Tisseel™; Baxter Healthcare Corp., BioScience). Tisseel contains fibrinogen (70–110 mg/mL), albumin (15 mg/mL), fibronectin (6 mg/mL), plasminogen (40–120 μg/mL), factor XIII (10 U/mL), and trace amounts of plasmin inhibitor, von Willebrand factor, growth factors, immunoglobulins, histidine, and niacinamide.29 The second formulation was PD fibrin (Enzyme Research Laboratories) in a buffer of 20 mM sodium citrate-HCl, with a pH of 7.4. The main difference between the two types of fibrin was the depletion of plasminogen in the PD fibrin. Fibroblasts were seeded at 200,000 cells/mL. Lyophilized fibrinogen was suspended in 40 mM tris-CaCl2. We omitted aprotinin, a serine protease inhibitor, to allow constructs to degrade. Fibroblasts were trypsinized, centrifuged, counted, diluted in media, and added to fibrinogen. Three hundred fifty microliters of fibrinogen–cell solution was pipetted into a 48-well tissue culture plate. Fifty microliters of thrombin at 40 IU/mL was added to each well and mixed to a final concentration of 5 IU/mL. Keratinocyte constructs were fabricated with an identical protocol. After polymerization, 400 μL of DMEM for fibroblasts or 400 μL of HEK media for keratinocytes was added to each well. Plates were incubated in 5% CO2 at 37°C. Final constructs were 400 μL in volume, 9 mm tall, 3 mm in diameter, with fibrin concentrations of 5, 10, and 20 mg/mL. To image cells, thinner 3D fibrin constructs were fabricated in 24-well plates. Although the dimensions of the 24-well constructs differed from those of the 48-well constructs, cells proliferated to the same extent in the 24-well versus the 48-well constructs. Cell proliferation was assessed through the alamarBlue® assay (Invitrogen Corp.) (data not shown). Constructs were fabricated in triplicate for each time point, during which cell proliferation, morphology, and 3D fibrin construct stiffness were assessed. Time points for clinical fibrin constructs were taken on days 1, 3, and 5. Time points for PD fibrin constructs were taken on days 1, 4, and 7 due to slower degradation. Because preliminary data showed that keratinocytes do not degrade PD fibrin over 1 week, time points for keratinocytes were taken on days 1, 3, and 5. We prepared gels from separate batches of the same lot (lot number 08P1498C). One batch of fibrin was used to create each triplicate set of gels.
Fibroblast and keratinocyte proliferation in 3D fibrin constructs
At each time point, the supernatant was removed, constructs were rinsed with PBS, and incubated with 10% alamarBlue in media at 37°C for 2 h. One hundred microliters of assay media was aliquoted to a 96-well plate. Fluorescent intensity was read at 535 nm excitation and 595 nm emission with a multiwell plate reader (Inifinite® F200; Tecan Group Ltd.).
Fluorescent imaging of cells in 3D fibrin constructs
Fibroblast and keratinocyte constructs in 24-well plates were stained with the Live/Dead Kit (Invitrogen). The supernatant was aspirated, and constructs were incubated for 1 h with 500 μL of DMEM with CalceinAM diluted at a 1:2000 volumetric ratio and ethidium homodimer (EthD-1) diluted 1:500 (volumetric). Labeled cells were imaged at a focal depth in the middle of the construct (Nikon). Red fluorescence was negligible, suggesting minimal cell death, so only green fluorescent images were analyzed further. Because cell spreading affects proliferation and apoptosis, we quantified fibroblast spreading morphology by calculating cell circularity with ImageJ software.34–36 Circularity is defined as 4π (area/perimeter2), with a value of 1.0 indicating a perfect circle. At least three images per construct were taken (representative images shown) and >15 cells per image were analyzed.
Measuring stiffness of 3D fibrin constructs
We measured the Young's modulus at the surface of the construct to quantify fibrin degradation. The Young's modulus E is the ratio of elastically deformable stress to strain of 3D fibrin constructs and is a measure of stiffness (see equation below). Stiff materials such as concrete, metal, and glass have high E values, while soft materials such as gelatin, fibrin, and cartilage have low E values.
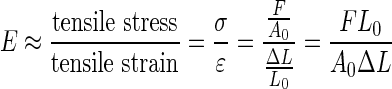 |
where E is the Young's modulus; F is the force exerted on the fibrin construct; A0 is the original cross-sectional area through which the force is applied; ΔL is the change in length of the fibrin construct; L0 is the original length of the fibrin construct.
Indentation testing was performed with an Instron 5564 Universal Material Testing Machine and a 1.5-mm-diameter cylindrical aluminum punch. One indentation per construct was performed and three constructs were tested per condition.
Statistical analysis
Data are presented as the mean±standard error, with n=3 (triplicate constructs per condition, repeated three times). To assess if cell proliferation or construct stiffness significantly differed from one time point to the next, data were assessed using Student's t-test, with p<0.05 considered significant. To determine if cell proliferation, construct stiffness, or fibroblast spreading differed among the three fibrin concentration groups at a given time point, singe-factor analysis of variance (ANOVA) without replication was performed, with p<0.05 considered significant. Excel Analysis ToolPak was used for statistical analysis, and figures were generated in MATLAB.
Results
Fibroblasts proliferate similarly in clinical and PD fibrin
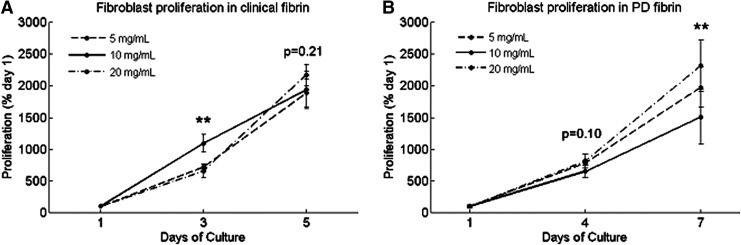
Fibroblasts in clinical fibrin proliferated 20-fold over 5 days (Fig. 1A). Day 3 proliferation differed among clinical fibrin concentrations (ANOVA, p<0.01) with the most proliferation in 10 mg/mL clinical fibrin. Day 5 proliferation did not differ among clinical fibrin concentrations (ANOVA, p=0.21). For each concentration group of clinical fibrin, proliferation increased from each time point to the next (Student's t-test, p<0.05). Fibroblasts proliferated 15- to 20-fold in PD fibrin over 7 days (Fig. 1B). For each concentration of PD fibrin constructs, proliferation increased from each time point to the next (Student's t-test, p<0.05). Day 4 proliferation did not differ among PD fibrin concentrations (ANOVA, p=0.10). Day 7 proliferation differed among PD fibrin concentrations (ANOVA, p<0.01) with the greatest proliferation in 20 mg/mL. Fibroblast proliferation differed in varying concentrations of fibrin on day 3 for clinical fibrin and on day 7 for PD fibrin, although the overall growth of fibroblasts in both types of fibrin was similar. Depletion of plasminogen did not affect fibroblast proliferation in the tested concentration range.
FIG. 1.
Proliferation of human foreskin fibroblasts cultured in three-dimensional (3D) fibrin constructs at (A) days 1, 3, and 5 in clinical fibrin and (B) days 1, 4, and 7 in plasminogen-depleted (PD) fibrin, measured by alamarBlue®. Cell growth is presented as a percentage of day 1. Data are mean±SEM, n=3. At each time point after day 1, a single-factor analysis of variance (ANOVA) without replication was performed to compare fibroblast proliferation in different fibrin concentrations. “*” Indicates significance at p<0.05, and “**” indicates significance at p<0.01. p-Values are reported on the figure for nonsignificant results. From each time point to the next, fibroblast proliferation significantly increased in each concentration group (p<0.05, * not shown).
Keratinocytes proliferate better in low concentrations of clinical fibrin and do not proliferate well in PD fibrin
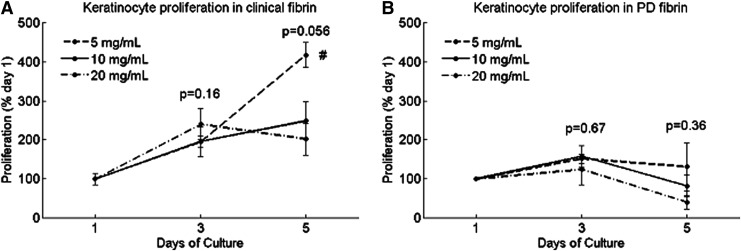
Keratinocytes in clinical fibrin proliferated two- to fourfold over 5 days (Fig. 2A), with the highest proliferation in the lowest concentration of 5 mg/mL. Keratinocytes in PD fibrin proliferated slightly at day 3, but on day 5, proliferated only between 50% and 150% of day 1.
FIG. 2.
Proliferation of human epidermal keratinocytes cultured in 3D fibrin constructs at (A) days 1, 3, and 5 in clinical fibrin and (B) days 1, 3, and 5 in PD fibrin, measured by alamarBlue. Cell growth is presented as a percentage of day 1. Data are mean±SEM, n=3. At each time point after day 1, a single-factor ANOVA without replication was performed to compare keratinocyte proliferation in different fibrin concentrations. “*” indicates significance at p<0.05. From each time point to the next, fibroblast proliferation significantly increased in each concentration group (p<0.05, * not shown). “#” indicates a significant difference on day 5 between 5 and 10 mg/mL clinical fibrin (p=0.046), as well as between 5 and 20 mg/mL clinical fibrin (p=0.015).
Clinical fibrin constructs with fibroblasts are less stiff and degrade faster than PD fibrin constructs with fibroblasts
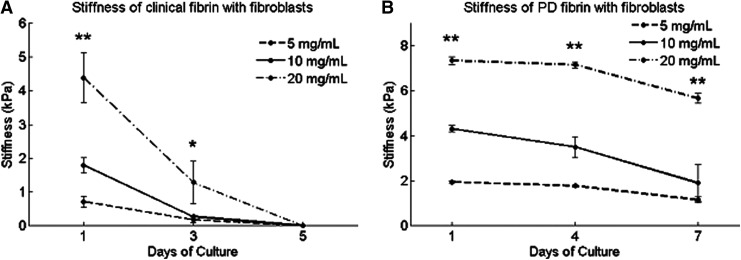
Day 1 clinical fibrin construct stiffness varied with fibrinogen concentrations, ranging from 0.3 kPa for 5 mg/mL to 4.5 kPa for 20 mg/mL (Fig. 3A). For each concentration group of clinical fibrin, stiffness decreased from day 1 to 3 and from day 3 to 5 (Table 1). Fibroblasts completely degraded clinical fibrin constructs between days 3 and 5. Acellular control constructs made from clinical fibrin did not degrade over 5 days, suggesting that stiffness decrease was due to fibroblast-mediated fibrinolysis (data not shown).
FIG. 3.
Stiffness of fibroblast-encapsulating 3D fibrin constructs fabricated from clinical or PD fibrin. Stiffness was measured by means of mechanical indentation testing. (A) Clinical fibrin construct stiffness varies with fibrinogen concentration, ranging from 0.7 kPa for 5 mg/mL to 4.4 kPa for 20 mg/mL clinical fibrin. Clinical fibrin constructs with fibroblasts degrade over 5 days of culture. (B) PD fibrin construct stiffness also varies with fibrinogen concentration, ranging from 2.0 kPa for 5 mg/mL to 7.2 kPa for 20 mg/mL PD fibrin. Fibroblasts degrade PD fibrin constructs less than clinical fibrin constructs. Data are mean±SEM, n=3. At each time point after day 1, a single-factor ANOVA without replication was performed to compare if construct stiffness differed among different concentrations. “*” indicates significance at p<0.05, and “**” indicates significance at p<0.01.
Table 1.
p-Values from Student's t-Test Comparing Stiffness of Clinical and Plasminogen-Depleted Fibrin Constructs with Fibroblasts
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
|---|---|---|---|
| Clinical fibrin | |||
| Day 1 vs. 3 | <0.01 | <0.01 | <0.01 |
| Day 3 vs. 5 | <0.01 | 0.02 | 0.03 |
| PD fibrin | |||
| Day 1 vs. 4 | <0.01 | 0.07 | 0.32 |
| Day 3 vs. 7 | <0.01 | 0.06 | <0.01 |
PD, plasminogen depleted.
PD fibrin constructs were stiffer than those made of equivalent concentrations of clinical fibrin (Fig. 3B). Initial stiffness varied with fibrinogen concentration, ranging from 2.0 kPa for 5 mg/mL to 7.2 kPa for 20 mg/mL PD fibrin. On day 7, the stiffness of PD fibrin constructs decreased to 50–80% of initial values, but the constructs did not completely degrade. Stiffness decreased from each time point to the next for 5 mg/mL PD fibrin (Table 1). However, from day 1 to 4, the 10 and 20 mg/mL PD fibrin constructs did not degrade. Additionally, from day 3 to 7, the 10 mg/mL PD fibrin constructs did not degrade. Acellular control constructs made from PD fibrin did not degrade over 7 days, suggesting that stiffness decrease was due to fibroblast-mediated fibrinolysis (data not shown). PD fibrin constructs lacking plasminogen and other plasma proteins were stiffer and more resistant to fibroblast-mediated degradation than clinical fibrin constructs.
Keratinocytes degrade clinical fibrin constructs, but do not degrade PD fibrin constructs
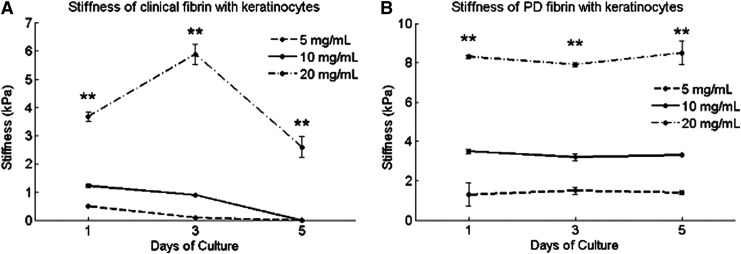
On day 1, the stiffness of clinical fibrin constructs with keratinocytes varied with fibrinogen concentrations, ranging from 0.3 kPa for 5 mg/mL to 3.8 kPa for 20 mg/mL (Fig. 4A). These values were similar to stiffness values of the same constructs with fibroblasts. For each concentration group of clinical fibrin, stiffness decreased from day 1 to 3 and from day 3 to 5, suggesting keratinocyte-mediated degradation (Table 2). One exception was 20 mg/mL clinical fibrin, which interestingly increased in stiffness to 6 kPa on day 3, but fell to 2.5 kPa on day 5, enduring 5 days of culture. Lower concentration clinical fibrin constructs (5 and 10 mg/mL) completely degraded between days 3 and 5. Acellular control constructs made from clinical fibrin did not degrade over 5 days, suggesting that the decrease in stiffness was due to keratinocyte-mediated fibrinolysis and not due to hydrolysis or proteolytic factors in culture media (data not shown). Each line in both Figure 4A and B represents one fibrinogen concentration.
FIG. 4.
Stiffness of keratinocyte-encapsulating fibrin constructs fabricated from clinical or PD fibrin. Constructs contained 200,000 keratinocytes/mL. Stiffness was measured by means of mechanical indentation testing. (A) Keratinocytes degrade clinical fibrin constructs over 5 days. (B) Keratinocytes do not degrade PD fibrin. Data are mean±SEM, n=3. At each time point after day 1, a single-factor ANOVA without replication was performed to compare if construct stiffness differed among different concentrations. “**” indicates significance at p<0.01. Each line represents one fibrinogen concentration.
Table 2.
p-Values from Student's t-Test Comparing Stiffness of Clinical and Plasminogen-Depleted Fibrin Constructs With Keratinocytes
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
|---|---|---|---|
| Clinical fibrin | |||
| Day 1 vs. 3 | <0.01 | 0.012 | <0.01 |
| Day 3 vs. 5 | <0.01 | <0.01 | <0.01 |
| PD fibrin | |||
| Day 1 vs. 3 | 0.67 | 0.21 | 0.12 |
| Day 3 vs. 5 | 0.46 | 0.50 | 0.45 |
PD fibrin constructs were stiffer than those made of equivalent concentrations of clinical fibrin (Fig. 4B). At each time point, the stiffness of PD fibrin constructs with keratinocytes varied with fibrin concentrations (ANOVA, p<0.01). These values were similar to stiffness values reported for the same constructs with fibroblasts. PD fibrin constructs with keratinocytes did not degrade over 7 days of culture. Keratinocyte-mediated fibrinolysis did not require plasminogen or other plasma proteins absent in PD fibrin.
Fibroblasts spread in both clinical and PD fibrin, although cell spreading is decreased in high concentrations of PD fibrin
In two-dimensional (2D) and 3D culture, fibroblasts adhere to the substrate and spread out. We performed fluorescent microscopy of CalceinAM-stained fibroblasts cultured in clinical fibrin and observed spread fibroblast morphology by day 3 in lower fibrinogen concentrations of 5 and 10 mg/mL (Fig. 5A). Qualitatively, fibroblasts appeared less elongated and more circular in 20 mg/mL. By day 5, clinical fibrin constructs were mostly degraded and fibroblasts spread in a confluent monolayer over the entire area of the well. Fibroblasts in the remaining clinical fibrin networked together while spreading. Imaging was also performed on fibroblasts cultured in PD fibrin constructs, in which fibroblasts elongated and spread by day 4 in 5 and 10 mg/mL, but maintained ball-like morphology with low spreading in 20 mg/mL (Fig. 5B). This trend continued to day 7, where fibroblasts in 20 mg/mL PD fibrin constructs spread less than in 5 and 10 mg/mL.
FIG. 5.
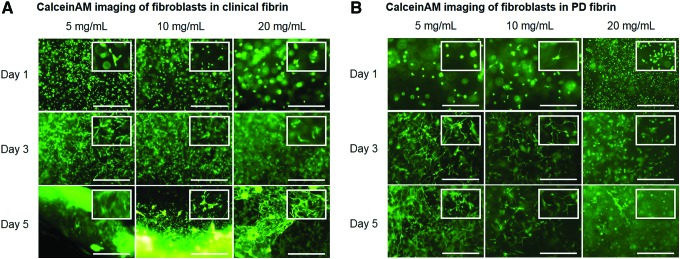
Fluorescent imaging and spreading quantification of CalceinAM-stained fibroblasts (scale bar=250 μm). (A). Fibroblasts in clinical fibrin constructs spread by day 3 in all concentrations and degraded most of the constructs by day 5. (B) Fibroblasts in PD fibrin constructs spread more in 5 and 10 mg/mL and less in 20 mg/mL. Insets feature a magnified region of the image to highlight cellular morphology. Color images available online at www.liebertpub.com/tea
We quantified fibroblast cell spreading by calculating 1−circularity, where a higher value indicated more spreading. At each time point after day 1, fibroblast spreading in clinical fibrin constructs differed among concentration groups (Table 3). On day 5, the spreading ranged from 0.5 to 0.7, with fibroblasts in the lowest concentration of clinical fibrin spreading the most. A more noticeable difference was observed in PD fibrin constructs. Similar to the fibroblasts in clinical fibrin, spreading in PD fibrin differed among concentration groups (Table 3). However, on day 7, fibroblasts cultured in the lower PD fibrin concentrations of 5 and 10 mg/mL both had a spread of 0.9, while fibroblasts cultured in the highest PD fibrin concentration of 20 mg/mL had a lower spread of 0.25. Plasminogen and other plasma proteins absent in PD fibrin, and/or the stiffer mechanical environment may have opposed cell spreading in lower concentrations of fibrin.
Table 3.
p-Values from Student's t-Test Comparing Fibroblast Spreading in Clinical and Plasminogen-Depleted Fibrin Constructs
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
|---|---|---|---|
| Clinical fibrin | |||
| Day 1 vs. 3 | <0.01 | <0.01 | 0.46 |
| Day 3 vs. 5 | <0.01 | 0.65 | <0.01 |
| PD fibrin | |||
| Day 1 vs. 4 | <0.01 | <0.01 | <0.01 |
| Day 3 vs. 7 | <0.01 | <0.01 | <0.01 |
Keratinocyte morphology is qualitatively identical in clinical and PD fibrin
Keratinocytes assume a cobblestone morphology in vitro and do not extend and spread like fibroblasts. We performed fluorescent microscopy of CalceinAM-stained keratinocytes cultured in clinical fibrin and observed normal cobblestone morphology (Fig. 6A). On day 5, keratinocytes clustered together more in 10 and 20 mg/mL clinical fibrin than in 5 mg/mL, or in any PD fibrin construct. Imaging was also performed on keratinocytes cultured in PD fibrin constructs, in which identical morphologies were qualitatively observed, although with less keratinocyte clustering on day 5 (Fig. 6B).
FIG. 6.
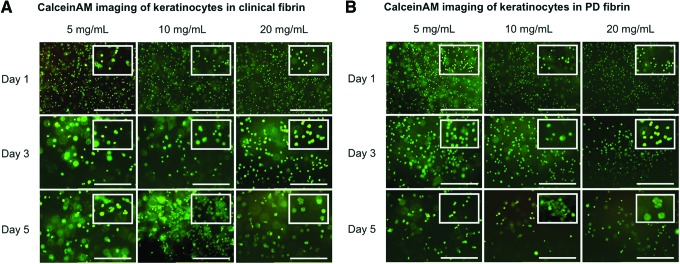
Fluorescent imaging of CalceinAM-stained keratinocytes in (A) clinical fibrin and (B) PD fibrin (scale bar=250 μm). On day 5, keratinocytes clustered together more in 10 and 20 mg/mL clinical fibrin than in 5 mg/mL clinical fibrin, or in any PD fibrin construct. Insets feature a magnified region of the image to highlight cellular morphology. Color images available online at www.liebertpub.com/tea
Discussion
In this study, we investigated if (1) the presence of plasminogen and other plasma proteins and (2) fibrinogen concentrations affect the behavior of skin cells. We fabricated cellularized 3D fibrin constructs made from 5, 10, or 20 mg/mL of clinical or PD fibrin, and examined the proliferation and morphology of fibroblasts and keratinocytes. We also assessed cell-mediated fibrinolysis by measuring construct stiffness.
Fibroblasts proliferated at least 15-fold regardless of fibrin composition and concentration (Fig. 1). This may be due to competing biochemical and physical cues that vary with fibrinogen concentration—when one increases, the other decreases. The ECM structure mediates cell behavior by presenting ligands to bind to integrin receptors on the cell surface.37 Fibrinogen and fibronectin, two such ligands, bind fibroblasts through the αvβ3 integrin, which stimulates cell adhesion and proliferation.38 A higher concentration of fibrinogen provides a higher ligand density for integrin binding, which regulates actin filament organization, cell adhesion, and motility.39 The concentration of fibrinogen also determines the fibril geometry and size, factors also mediating cell behavior. Fibroblasts cultured on collagen grooves demonstrate different gene expression patterns than when cultured on flat surfaces,40 potentially due to physical interactions between the microenvironment, cytoskeleton, and nuclear matrix.36 However, too high concentrations of fibrin prevent fibroblast spreading and mechanically inhibit proliferation in 3D culture.26,41 On the other hand, fibrin at a low concentration features a porous microstructure that encourages fibroblast spreading and proliferation, but presents fewer ECM ligands and growth factors. Although PD fibrin is stiffer than clinical fibrin at identical concentrations (Figs. 3 and 4), fibroblasts may receive more biochemical input from clinical fibrin and more biophysical input from PD fibrin, resulting in similar proliferation profiles.
Fibrin, fibronectin, and plasminogen represent some components of the natural wound milieu, which support keratinocyte growth. Keratinocytes actually do not express integrin αvβ3—the receptor for fibrin and fibrinogen. Fibrin indirectly mediates keratinocyte behavior by presenting fibronectin and releasing plasminogen.42,43 In response to wounding, keratinocytes upregulate integrin subunit α5, the receptor for fibronectin.44,45 This subunit promotes keratinocyte migration and survival in a Bcl-2 pathway-dependent fashion.46–48 Keratinocytes proliferating in clinical fibrin therefore adhered to fibronectin present in the construct. However, keratinocytes did not proliferate in PD fibrin (Fig. 2). Plasminogen activates matrix metalloproteinases crucial for epithelial cell migration,49 converts latent transforming growth factor beta and hepatocyte growth factor into active forms,50,51 and induces keratinocyte migration, while reducing proliferation.52 Our data suggest that plasminogen may be a vital component of a fibrin construct containing keratinocytes.
Whereas fibroblast proliferation was nearly identical in clinical fibrin compared with PD fibrin, the stiffness and degradation of fibrin constructs varied with composition and concentration (Table 4). Fibroblasts rapidly degraded clinical fibrin within 3–5 days, with higher concentrations of fibrin corresponding to stiffer constructs, which degraded more slowly than constructs fabricated from lower concentrations (Fig. 3A). In agreement with our results, several studies have shown that a lower fibrinogen concentration results in thicker fibers, which Gabriel et al. demonstrated to be more susceptible to plasmin digestion than thin fibers found in higher fibrin concentrations.53
Table 4.
Summary of Fibroblast and Keratinocyte Proliferation, and Fibrin Construct Stiffness
| Final cell proliferation (% of day 1) | 5 mg/mL | 10 mg/mL | 20 mg/mL |
|---|---|---|---|
| Fibroblasts | |||
| Clinical fibrin | 1890±220 | 1940±300 | 2170±160 |
| PD fibrin | 1980±310 | 1510±430 | 2320±410 |
| Keratinocytes | |||
| Clinical fibrin | 420±30 | 250±50 | 200±40 |
| PD fibrin | 130±60 | 82±30 | 40±20 |
| Final construct stiffness (kPa) | 5 mg/mL | 10 mg/mL | 20 mg/mL |
|---|---|---|---|
| Fibroblasts | |||
| Clinical fibrin | 0 | 0 | 0 |
| PD fibrin | 1.2±0.1 | 1.9±0.8 | 5.7±0.2 |
| Keratinocytes | |||
| Clinical fibrin | 0 | 0 | 2.6±0.4 |
| PD fibrin | 1.4±0.1 | 3.3±0.1 | 8.5±0.6 |
Mean values±standard error.
PD fibrin constructs with fibroblasts were twice as stiff and degraded slower than clinical fibrin constructs (Fig. 3B), likely due to the lack of plasminogen. Compared with clinical fibrin, PD fibrin may contain different amounts of structurally important proteins such as collagen, factor XIIIa, fibronectin, and albumin. Even without plasminogen, fibroblasts degraded PD fibrin over 1 week (Fig. 3B), suggesting activity of other proteases, such as matrix metalloproteinases.54 We omitted aprotinin to allow fibroblasts to degrade fibrin and observed 15- to 20-fold proliferation in both types of fibrin. Fibroblasts have been shown to proliferate significantly less (only threefold) in fibrin constructs fabricated with aprotonin.25 Aprotonin prevents the degradation of the ECM, which may prevent subsequent liberation of degradation products and growth factors, thus reducing the survival, proliferation, and differentiation of both encapsulated and endogenous cells. The initial composition of fibrin may play a large role in mediating the dynamic reciprocity of the wound microenvironment.55
Keratinocytes slightly degraded clinical fibrin over 5 days (Fig. 4A). The stiffness of 20 mg/mL clinical fibrin constructs with keratinocytes rose at day 3 but fell at day 5. Batch variation of clinical fibrin may explain the increased day 3 stiffness. However, the overall trend of keratinocyte-mediated fibrin degradation in clinical fibrin was similar to that from fibroblast constructs. As expected, higher concentrations of fibrin resulted in stiffer constructs, both for clinical and PD fibrin. Unlike fibroblasts, keratinocytes did not degrade PD fibrin (Fig. 4B). Keratinocytes may express plasminogen activators ineffective without plasminogen or may not express sufficient amounts of nonplasmin proteases to degrade PD fibrin.
Fibroblasts exhibited spreading morphology in both clinical fibrin (Fig. 5A) and PD fibrin (Fig. 5B), except in the highest concentration of PD fibrin. Surprisingly, the proliferation in 20 mg/mL PD fibrin constructs was similar to the proliferation in lower concentration constructs. Several studies have shown that substrate stiffness plays a critical role on cell morphology, survival, and differentiation. Yeung et al. found that the stiffness of a 2D substrate must be at least 2–3 kPa for fibroblast spreading, a range similar to that of our data.56 At each time point, fibroblast spreading differed significantly depending on the fibrin concentration (Fig. 5C, D). Interestingly, fibroblasts in 10 mg/mL clinical fibrin appear to spread the least, although significant differences were observed between all three concentration groups. Fibroblasts in 5 and 10 mg/mL PD fibrin spread similarly, while fibroblasts in 20 mg/mL PD fibrin did not, suggesting a threshold of mechanical resistance beyond which fibroblasts cannot spread. Our data agree with previous work showing that mechanical stiffness regulates cell spreading. Plasminogen may also mediate cell morphology and spreading in 3D culture, although we are yet to discern the relative contributions of mechanical stiffness and biochemical signaling toward cell spreading. If ECM ligands such as fibronectin and fibrin enable cell spreading and mechanical resistance opposes cell spreading, fibroblast spreading should be greater in clinical fibrin than in PD fibrin. However, clinical fibrin constructs may have degraded before fibroblasts could maximally spread, while stiffer and more degradation-resistant PD fibrin constructs could allow more time for fibroblasts to spread.
We also performed fluorescent microscopy on CalceinAM-stained keratinocytes in clinical and PD fibrin. Keratinocytes demonstrated normal cobblestone morphology (Fig. 6A) and were distributed homogenously throughout the construct. On day 5, keratinocytes clustered more in high-stiffness clinical fibrin. Keratinocytes are known to form focal adhesions to the ECM in a high-stiffness environment.57,58 However, keratinocytes did not cluster in PD fibrin constructs that were stiffer than clinical fibrin constructs of the same fibrinogen concentration (Fig. 6B). These data, consistent with other studies previously discussed, demonstrate that plasminogen and other plasma proteins, which may be absent in PD fibrin, mediate keratinocyte adhesion.
In summary, our data show that fibrin stiffness and the presence of plasminogen affect the behavior of fibroblasts and keratinocytes. These results could be translated into a novel strategy of delivering cells to a dermal wound site using a heterogeneous fibrin construct, containing subregions of biomaterial optimized for controlling specific cell phenotypes. However, the interplay between various cells and the matrix is complex, and different wound pathologies present unique spatiotemporal profiles of inflammatory molecules, ECM ligands, and proteases.
Fibroblasts and keratinocytes signal to each other through autocrine and paracrine mechanisms to regulate tissue homeostasis, as well as wound healing. Further complicating matters, cells bind to the ECM that changes cell behavior, which in turn leads to changes in the ECM—a phenomenon coined “dynamic reciprocity.”55 To better understand the complexity of the wound-healing milieu, we are currently investigating constructs with multiple cell types and ECM proteins. Adding more components to our in vitro models will improve our understanding of (1) the relative contribution of fibrinogen, fibronectin, plasminogen, and type I collagen toward the mechanical and biological properties of the microenvironment and (2) how communication between dermal, epidermal, immune, and mesenchymal stem cells regulates cell proliferation, migration, differentiation, and fibrinolysis.59–64 Once we identify in vitro fibrin construct formulations and cell profiles that optimally form skin-like phenotypes, we will test the ability of these constructs to heal wounds in animal models. Studying simplified in vitro models of wound healing using individual cell populations, specific growth factors, or biomechanical forces is important, but findings must be scaled to more complex in vivo models that translate into novel therapeutic strategies.
Acknowledgments
This study was funded by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (AR053250:01A). The authors thank Haison Duong, Abigail Corrin, and Chase Linsley for technical assistance and conceptual input.
Disclosure Statement
No competing financial interests exist.
References
- 1.Metcalfe A.D., and Ferguson M.W.J.Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4,413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böttcher-Haberzeth S., Biedermann T., and Reichmann E.Tissue engineering of skin. Burns 36,450, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Greaves N.S., Iqbal S.A., Baguneid M., and Bayat A.The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 21,194, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Singer A.J., and Clark R.A.F.Cutaneous wound healing. N Engl J Med 341,738, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed T.A.E., Dare E.V., and Hincke M.Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng B Rev 14,199, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Eyrich D., Göpferich A., and Blunk T.Fibrin in tissue engineering. Tissue Eng 6,379, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Janmey P.A., Winer J.P., and Weisel J.W.Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 6,1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser H.W., et al. Cultured autologous keratinocytes in fibrin glue suspension, exclusively and combined with STS-allograft (preliminary clinical and histological report of a new technique). Burns 20,23, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Mazlyzam A.L., et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 33,355, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Radosevich M., Goubran H.I., and Burnouf T.Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang 72,133, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Mosesson M.W., Siebenlist K.R., and Meh D.A.The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci 936,11, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Daley W.P., Peters S.B., and Larsen M.Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121,255, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Shen L., Mcdonagh P., McDonagh J., and Hermans J., Jr.Fibrin gel structure: influence of calcium and covalent cross-linking on the elasticity. Biochem Biophys Res Commun 56,793, 1974 [DOI] [PubMed] [Google Scholar]
- 14.Carr M., Jr., Carr S., et al. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagul Fibrinolysis 6,79, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Rowe S.L., Lee S., and Stegemann J.P.Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater 3,59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duong H., Tawil B., and Wu B.M.Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen–thrombin compositions and by extrinsic cellular activity. Tissue Eng Part A 15,1865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner S., and Grose R.Regulation of wound healing by growth factors and cytokines. Physiol Rev 83,835, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Markowski M.C., Brown A.C., and Barker T.H.Directing epithelial to mesenchymal transition through engineered microenvironments displaying orthogonal adhesive and mechanical cues. J Biomed Mater Res Part A 100,2119, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Brown A.C., Fiore V.F., Sulchek T.A., and Barker T.H.Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol 229,25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker B.M., and Chen C.S.Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125,3015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cukierman E., Pankov R., and Yamada K.M.Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 14,633, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Chen C.S., and Fu J.Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys 41,519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong V.W., Akaishi S., Longaker M.T., and Gurtner G.C.Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 131,2186, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Aarabi S., et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 21,3250, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Cox S., Cole M., and Tawil B.Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng 10,942, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Sese N., Cole M., and Tawil B.Proliferation of human keratinocytes and cocultured human keratinocytes and fibroblasts in three-dimensional fibrin constructs. Tissue Eng Part A 17,429, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Ho W., Tawil B., Dunn J.C.Y., and Wu B.M.The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng 12,1587, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Linsley C., Wu B., and Tawil B.The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng Part A 19,1416, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Buchta C., Hedrich H.C., Macher M., Höcker P., and Redl H.Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials 26,6233, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Jackson M.R.Fibrin sealants in surgical practice: an overview. Am J Surg 182,1S, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Rømer J., et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 2,287, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Li W., and Chong S.Plasminogen activator/plasmin system: a major player in wound healing? Wound Repair Regen 11,239, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Dickneite G., Metzner H., Pfeifer T., Kroez M., and Witzke G.A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thromb Res 112,73, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Chen C.S., Mrksich M., Huang S., Whitesides G.M., and Ingber D.E.Geometric control of cell life and death. Science 276,1425, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Singhvi R., et al. Engineering cell shape and function. Science 264,696, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Thomas C.H., Collier J.H., Sfeir C.S., and Healy K.E.Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A 99,1972, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hynes R.O.The extracellular matrix: not just pretty fibrils. Science 326,1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juhasz I., Murphy G.F., Yan H.C., Herlyn M., and Albelda S.M.Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol 143,1458, 1993 [PMC free article] [PubMed] [Google Scholar]
- 39.Maheshwari G., Brown G., Lauffenburger D.A., Wells A., and Griffith L.G.Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci 113 (Pt 1),1677, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Dalby M.J., Riehle M.O., Yarwood S.J., Wilkinson C.D., and Curtis A.S.Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp Cell Res 284,272, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Bott K., et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31,8454, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Geer D.J., and Andreadis S.T.A novel role of fibrin in epidermal healing: plasminogen-mediated migration and selective detachment of differentiated keratinocytes. J Invest Dermatol 121,1210, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kubo M., et al. Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol 117,1369, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Larjava H., Salo T., Haapasalmi K., Kramer R.H., and Heino J.Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest 92,1425, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larjava H., Haapasalmi K., Salo T., Wiebe C., and Uitto V.J.Keratinocyte integrins in wound healing and chronic inflammation of the human periodontium. Oral Dis 2,77, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Varner J.A., Emerson D.A., and Juliano R.L.Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell 6,725, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z., Vuori K., Reed J.C., and Ruoslahti E.The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A 92,6161, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geer D.J., Swartz D.D., and Andreadis S.T.Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng 8,787, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Mirastschijski U., and Impola U.Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol 1,55, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Naldini L., et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J 11,4825, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odekon L.E., Blasi F., and Rifkin D.B.Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J Cell Physiol 158,398, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Szabo I., Simon M., and Hunyadi J.Plasmin promotes keratinocyte migration and phagocytic-killing accompanied by suppression of cell proliferation which may facilitate re-epithelialization of wound beds. Clin Dev Immunol 11,233, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabriel D.A., Muga K., and Boothroyd E.M.The effect of fibrin structure on fibrinolysis. J Biol Chem 267,24259, 1992 [PubMed] [Google Scholar]
- 54.Santoro M.M., and Gaudino G.Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 304,274, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., and Herman I.M.Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 19,134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung T., et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60,24, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Zhu A.J., Haase I., and Watt F.M.Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci U S A 96,6728, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trappmann B., et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 11,642, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Werner S., and Smola H.Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol 11,143, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Witte R.P., and Kao W.J.Keratinocyte-fibroblast paracrine interaction: the effects of substrate and culture condition. Biomaterials 26,3673, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Maas-Szabowski N., Shimotoyodome A., and Fusenig N.E.Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci 112,1843, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Eckes B., et al. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol 19,325, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Werner S., Krieg T., and Smola H.Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127,998, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Barker J.N., Mitra R.S., Griffiths C.E., Dixit V.M., and Nickoloff B.J.Keratinocytes as initiators of inflammation. Lancet 337,211, 1991 [DOI] [PubMed] [Google Scholar]