Abstract
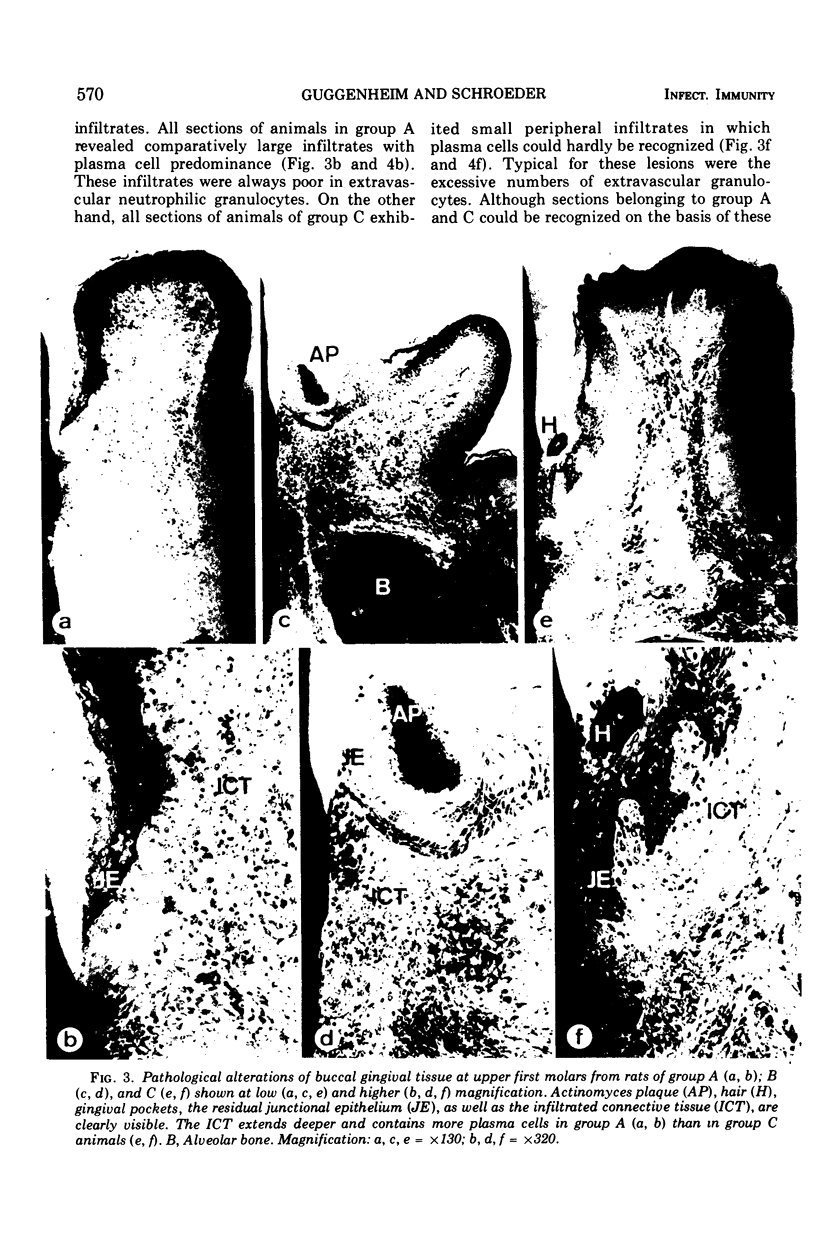
The purpose of our experiment was to evaluate the destructive potential of a strain of Actinomyces viscosus on the periodontium of sensitized rodents, describing the induced lesions on the basis of quantitative cytology. The experimental design comprised in principle the following procedures. Young germfree rats were immunized either by intravenous or intradermal injections with heat-killed cells of A. viscosus Ny 1 or sham immunized intradermally with physiological saline. After a period suitable for activation of the humoral and cell-mediated immune systems, Ny 1 was monoassociated in all animals by oral implantation. During the ensuing 42 days the animals were allowed to react against the continuous peripheral oral antigen challenge. Since A. viscosus is known as a heavy dental plaque-forming organism, the animals could be expected to develop local immunopathological lesions in the periodontal tissues. These lesions were then studied by quantitative cytology after sampling procedures allowing optimal tissue preservation. The degree of bone loss observed in all treatments was unrelated to the destructive capacity of the infiltrates, suggesting the presence of distinct mechanisms responsible for the activation of osteoclasts and factors interfering with fibroblast activity. Although the cellular composition of the infiltrated tissues was analyzed at the end of the experiment only, distinct stages of the lesions in the different treatments allowed reconstruction of the sequence of events. After an acute inflammatory phase, a classic delayed hypersensitivity reaction developed which was transformed after some time by a large superimposed plasma cell accumulation. The particular but undefined immune status was the significant factor determining the final type of peripheral infiltrative reaction.
Full text
PDF



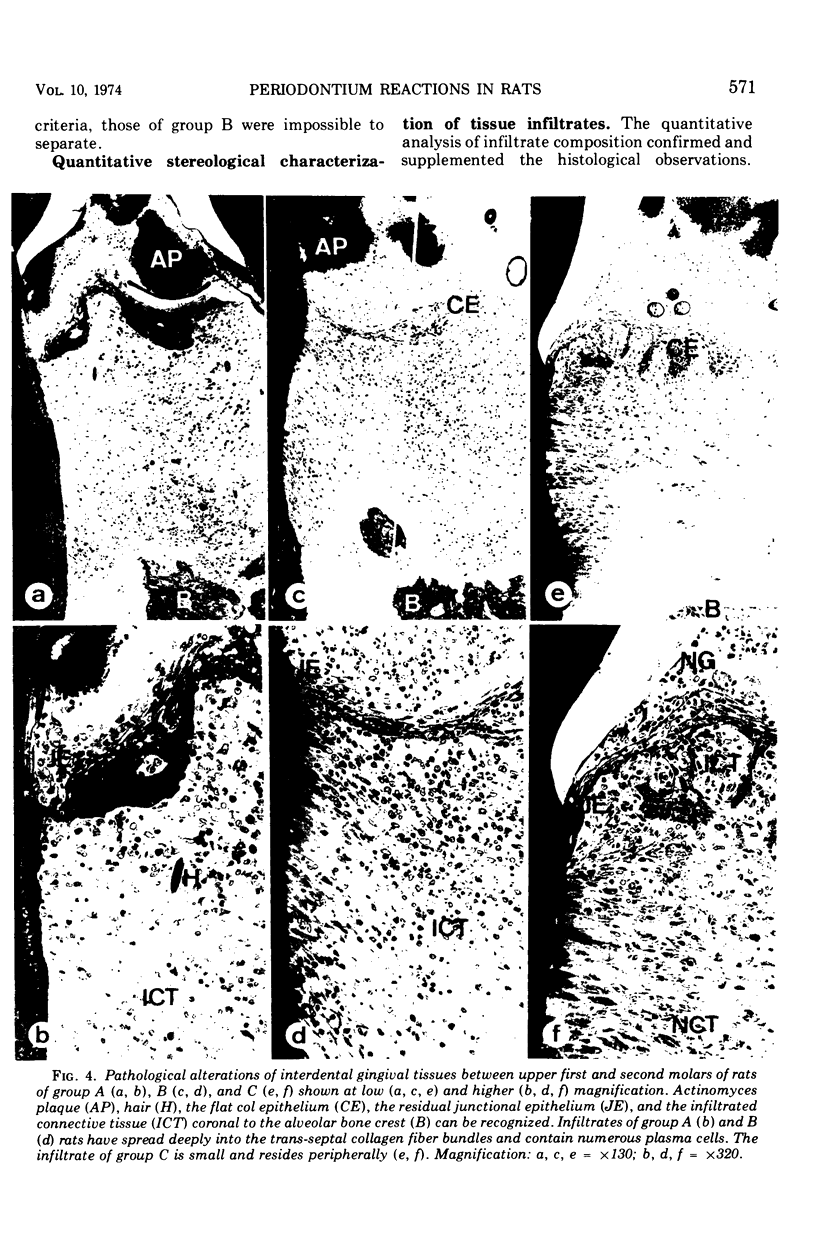
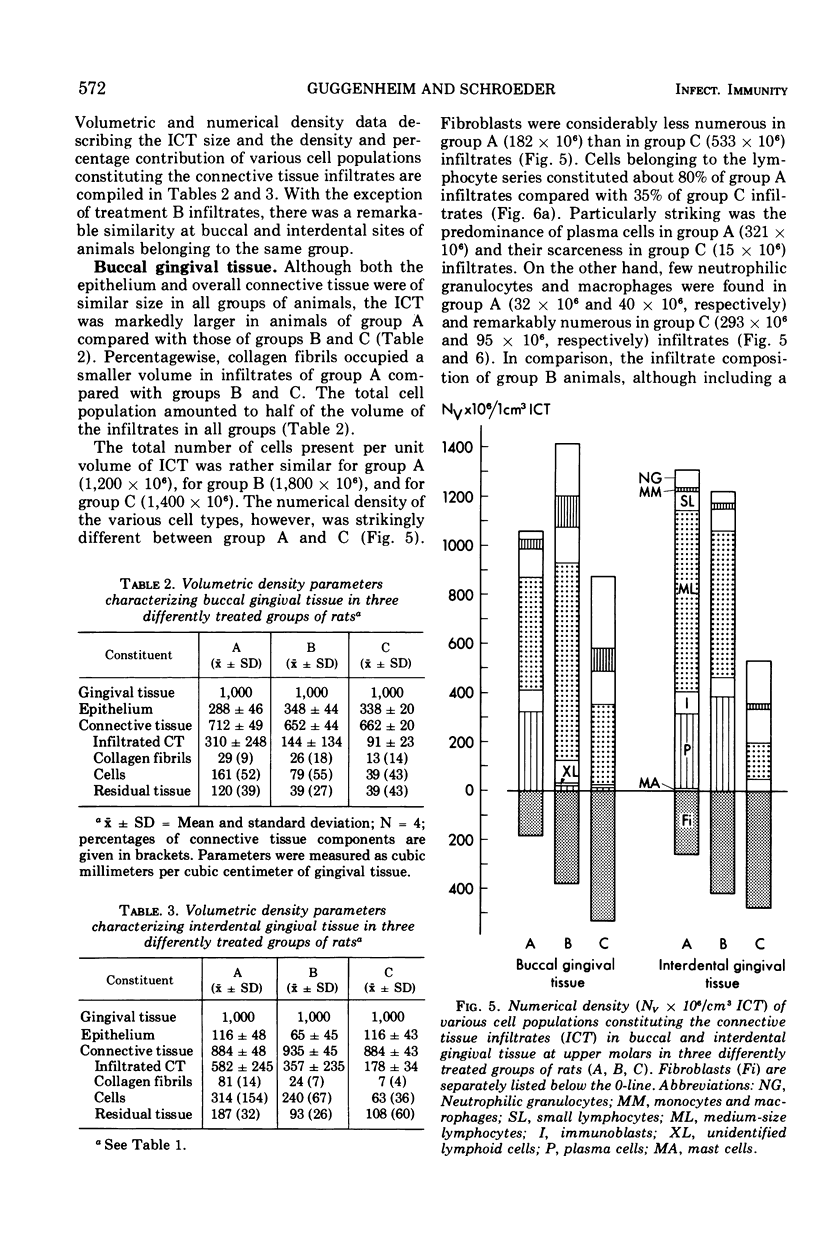
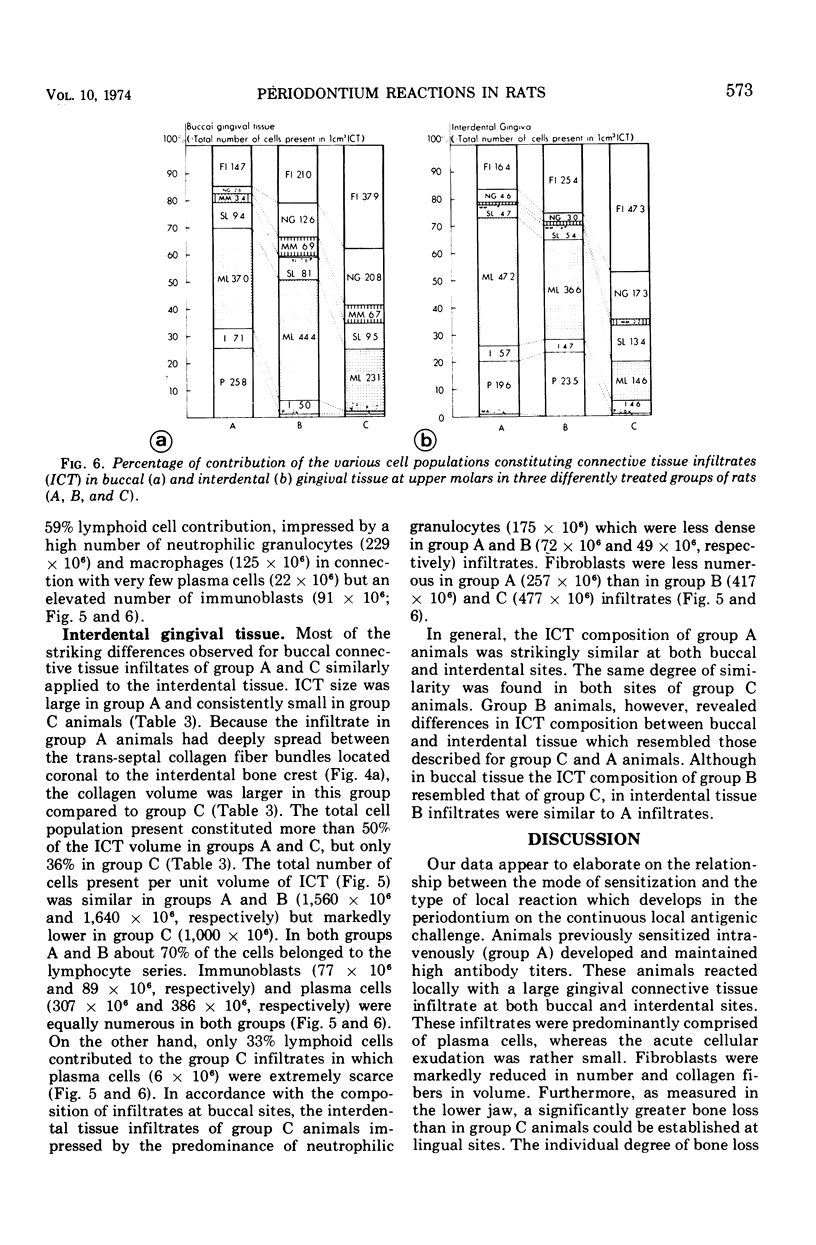




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax M. H., Elliott J. H., Daly J. J., Willms-Kretschmer K., McCarthy J. S., Leskowitz S. Local plasmacytopoiesis in delayed hypersensitivity reactions. J Immunol. 1969 May;102(5):1214–1219. [PubMed] [Google Scholar]
- Greenberg A. H., Shen L. A class of specific cytotoxic cells demonstrated in vitro by arming with antigen-antibody complexes. Nat New Biol. 1973 Oct 31;245(148):282–285. doi: 10.1038/newbio245282a0. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H. Studies on the Bacteriology of Hamster Periodontal Disease. Am J Pathol. 1965 May;46(5):843–857. [PMC free article] [PubMed] [Google Scholar]
- KEYES P. H., GOLD H. S. Periodontal lesions in the Syrian hamster. I. A method of evaluating alveolar bone resorption. Oral Surg Oral Med Oral Pathol. 1955 May;8(5):492–499. doi: 10.1016/0030-4220(55)90080-3. [DOI] [PubMed] [Google Scholar]
- Kosunen T. U., Flax M. H. Experimental allergic thyroiditis in the guinea pig. IV. Autoradiographic studies of the evolution of the cellular infiltrate. Lab Invest. 1966 Mar;15(3):606–616. [PubMed] [Google Scholar]
- Lehner T. Cell-mediated immune responses in oral disease: a review. J Oral Pathol. 1972;1(1):39–58. [PubMed] [Google Scholar]
- Leskowitz S. Is delayed sensitivity a preparation for antibody synthesis? J Immunol. 1968 Sep;101(3):528–533. [PubMed] [Google Scholar]
- Lindhe J., Helldén L. Neutrophil chemotactic activity elaborated by dental plaque. J Periodontal Res. 1972;7(4):297–303. doi: 10.1111/j.1600-0765.1972.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Nisengard R., Beutner E. H., Hazen S. P. Immunologic studies of periodontal diseases. IV. Bacterial hypersensitivity and periodontal disease. J Periodontol. 1968 Nov;39(6):329–332. doi: 10.1902/jop.1968.39.6.329. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney R. R., Zander H. A. Allergic periodontal disease in sensitized squirrel monkeys. J Periodontol. 1970 Jan;41(1):12–21. doi: 10.1902/jop.1970.41.1.12. [DOI] [PubMed] [Google Scholar]
- Rizzo A. A., Mitchell C. T. Chronic allergic inflammation induced by repeated deposition of antigen in rabbit gingival pockets. Periodontics. 1966 Jan-Feb;4(1):5–10. [PubMed] [Google Scholar]
- Schroeder H. E., Lindhe J., Hugoson A., Münzel-Pedrazzoli S. Structural constituents of clinically normal and slightly inflamed dog gingiva. A morphometric study. Helv Odontol Acta. 1973 Oct;17(2):70–83. [PubMed] [Google Scholar]
- Schroeder H. E., Münzel-Pedrazzoli S. Correlated morphometric and biochemical analysis of gingival tissue. Morphometric model, tissue sampling and test of stereologic procedures. J Microsc. 1973 Dec;99(3):301–329. doi: 10.1111/j.1365-2818.1973.tb04629.x. [DOI] [PubMed] [Google Scholar]
- Schroeder H. E. Transmigration and infiltration of leucocytes in human junctional epithelium. Helv Odontol Acta. 1973 Apr;17(1):6–18. [PubMed] [Google Scholar]
- Schroeder H. E. Ultrastructure of the junctional epithelium of the human gingiva. Helv Odontol Acta. 1969 Oct;13(2):65–83. [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- TAKATSY G. The use of spiral loops in serological and virological micro-methods. Acta Microbiol Acad Sci Hung. 1955;3(1-2):191–202. [PubMed] [Google Scholar]
- TERNER C. ARTHUS REACTION IN THE ORAL CAVITY OF LABORATORY ANIMALS. Periodontics. 1965 Jan-Feb;3:18–22. [PubMed] [Google Scholar]
- Weibel E. R. An automatic sampling stage microscope for stereology. J Microsc. 1970 Feb;91(1):1–18. doi: 10.1111/j.1365-2818.1970.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Zachrisson B. U., Schultz-Haudt S. D. A comparative histological study of clinically normal and chronically inflamed gingivae from the same individuals. Odontol Tidskr. 1968 Mar 29;76(2):179–192. [PubMed] [Google Scholar]