Abstract
Study Design
The presence fibronectin fragments (FN-fs) and the cleaving enzyme, A disintegrin and metalloproteinase domain-containing protein (ADAM)-8 were examined in human intervertebral disc (IVD) tissue in vitro.
Objective
To investigate the presence and pathophysiological concentration of FN-fs and their cleaving enzyme, ADAM-8, in the human IVD tissue.
Summary of Background Data
The 29kDa FN-f has been shown to result in extracellular matrix loss in rabbit IVDs. However, the concentration of this biologically active fragment in the degenerative human IVD tissue has previously not been determined. Further, it is critical to identify the enzyme(s) responsible for FN cleavage in the IVD.
Methods
Human degenerative IVD tissues were removed during spinal surgery. A normal appearing young adult and an infant human cadaveric sample were obtained as controls. Soluble proteins were extracted, and analyzed by Western blotting utilizing antibodies specific for the human FN neoepitope VRAA271. A purified 29 kDa FN-f was used to allow estimation of the concentration of FN-fs in the tissues. ADAM-8, a FN-cleaving enzyme, was analyzed by Western blotting and immunostaining.
Results
All adult IVD tissues contain many FN-f species, but these species were absent from the infant disc tissue. Moderately degenerative discs contained the highest amount of FN-fs; the concentration was estimated to be in the nanomolar range per gram of tissue. ADAM-8, known to cleave FN resulting in the VRAA271 neoepitope, was present in the human disc. ADAM-8 primarily localized in the pericellular matrix of the nucleus pulposus (NP) tissue, as determined by immunostaining.
Conclusion
This is the first report that N-terminal FN-fs are consistently present in IVD tissues from adult subjects. The pathophysiological concentration of these fragments is estimated to be at nanomolar range per gram of IVD tissue. Further, ADAM-8, known to cleave FN, is present at the pericellular matrix of disc cells.
Keywords: intervertebral disc, fibronectin fragments, ADAM-8, human
Fibronectin (FN), a molecule with diverse biologic functions, can exist in multiple variants that arise from alternative splicing of a single pre-mRNA.3, 4 In most human tissues, the FN exists in up to 20 variants due to inclusion or exclusion of the EDA or EDB domains, and the variable (V) domain can be spliced five different ways by partial inclusion of this region. The diversity of FN forms is further increased in cartilage tissues: there is a variant with 15th type III domain and 10th type I domain together with the entire V region excluded, the [V+III15+I10]− described in canine cartilage,5 and [V+I10]−, or [V+III15]− variants, described in bovine articular cartilage. Finally, the [V+I10]− variant has been identified in the human IVD tissues and articular cartilage and meniscus (Anderson et al.6 and Zhang lab unpublished data). Thus, in the IVD tissues, there are as many as 32 splice forms. Although the functions of these diverse forms have not been thoroughly elucidated yet,7-9 their potential roles in IVD development early in life and repair in adults should not be overlooked.
The total amount of FN-fs increases with disc degeneration.10 However, neither the pathophysiological concentrations of the individual fragments nor the mechanism of FN fragmentation in the IVD were characterized previously. The N-terminal region of FN is thought to initiate matrix assembly by assisting in the formation of a fibrillar meshwork.9, 11 Free N-terminal fragments may interfere with matrix assembly in certain embryonic systems.11 In extracellular matrix networks of adult tissues, FN-fs can cause further tissue degradation as seen in osteoarthritis. The N-terminal FN-fs present in the arthritic knee 12 induce nitric oxide (NO) production by activating focal adhesion kinase (FAK) and mitogen-activated protein kinases (MAPKs) without activating α5β1 integrin.13 In the IVD tissue, data supporting a pathophysiological role of FN-fs are emerging: the 29 kDa N-terminal FN-f is capable of causing disc degeneration in the rabbit.14, 15 To assess the relevance of previous studies in human IVD disease, in the present work we aimed to determine the content of FN N-terminal fragments in human IVDs that showed different grades of degeneration. Furthermore, both enzymatic cleavage and repetitive mechanical loading could potentially result in FN fragmentation. We hypothesize that proteolytic enzymes within the IVD tissue are responsible, at least in part, for FN-fragmentation. ADAM-8 appears to be responsible for FN degradation in knee articular cartilage, resulting in the VRAA271 and 272VYQP neoepitopes.16, 17 However, the presence and activity of ADAM-8 in IVD tissues have not been previously shown. Thus, in this study, we aimed to investigate the possible presence of ADAM-8 as a FN-degrading enzyme in human disc tissue.
Methods
Patient selection and sample collection
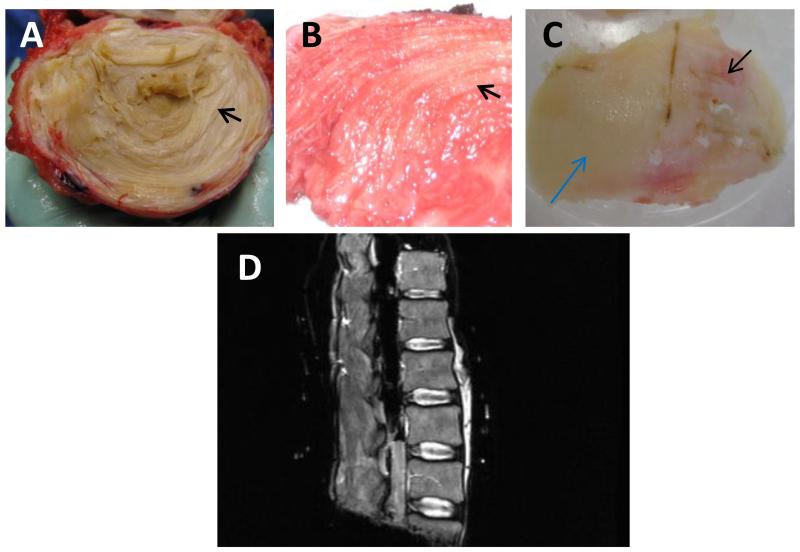
IVD tissues were collected from patients undergoing spinal surgery (approved by the Institutional Review Board) and from deceased organ donors through our relationship with Gift of Hope Organ & Tissue Donor Network. Figure 1, panel A, shows a cadaveric donor intervertebral disc to illustrate the distinction between nucleus pulposus (NP) and annulus fibrosus (AF) tissues within the IVD. The lumbar spines (L1-L5) from cadaveric donors have been harvested en-block, the T2 weighted MR images of the isolated lumbar spine were obtained at 1.5 Tesla resolution (Panel D).
Figure 1. Gross morphology of human cadaveric intervertebral disc (IVD) tissue (A), surgical IVD tissue (B) and femoral articular cartilage (C), and MR image of a cadaveric spine (D).
Panels A and B, arrows point to the innermost annular ring; panel C, blue arrow points to minimally degenerative appearing articular cartilage, and black arrow points to severely degenerative cartilage tissue.
Figure 1, panel B, shows disc tissue from a patient who had undergone discectomy for back pain. Tissues with annular rings were identified as AF. Because of degeneration and damage during surgery, only a small number of surgical tissues contained clearly distinguishable NP tissues. The IVD tissues excised during surgery were rinsed with phosphate buffered saline (PBS) with protease inhibitors, and were transported to the laboratory immediately. All patients underwent routine T2-weighted magnetic resonance (MR) imaging at 1.5 Tesla resolution as part of routine examination before surgery. The severity of disc degeneration for patients and cadaveric donors was graded on T2-weighted images by the principal investigator and two other physicians, using criteria described by Pfirrmann et al.18 Specifically, when all 3 physicians agree, the grade is as agreed. When 2 physicians agree but the remaining physician’s grading is 1 grade different, the first 2 physicians’ grading was used. When any of the 3 physicians’ grades is 2 or more grades different from the others’, all 3 physicians re-grade the spine. As controls, human articular cartilage tissues were obtained from patients undergoing knee replacement surgery. Figure 1, panel C, shows a femoral condyle from a patient undergoing total knee arthroplasty. Articular cartilage (AC) tissues from relatively intact areas (black arrow) and degenerative areas (blue arrow) were isolated. The disc and AC tissues were rinsed in 1xPBS containing protease inhibitors (Roche), and stored at −70 °C.
Quantitative Western blot analysis
An infrared imager (LI-COR Odyssey) was utilized to allow identification of two antigen epitopes simultaneously. A known FN (Sigma) N-terminal fragment was used to quantify the FN-fs. Specifically, for protein extraction, frozen tissues were crushed into a fine powder using a pre-cooled Bio-Pulverizer (Biospec Products). Approximately 100 mg (wet weight) of the powdered tissue was extracted with lysis buffer (Cell Signaling) and additional protease inhibitors (Roche) at 4°C for 24 hours. Protein concentration was determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL).
For Western blots, 10 μg of protein extracts from disc tissues were treated with chondroitinase ABC (Sigma) 0.1 U/ml in 50 mM Tris-acetate EDTA buffer at 37°C for 1 hour and resolved on NuPAGE 4-12% Bis-Tris Gels (Invitrogen). Proteins were transferred onto Immobilon® Membrane, PVDF type (Millipore).
To identify FN-fs containing the N-terminus and the neoepitope VRAA271, the following primary antibodies were used: mouse monoclonal antibody (mAB) to the 29kDa FN N-terminal fragment (mAB1936, Chemicon/Millipore Clone 616) and rabbit polyclonal neoepitope antibody VRAA271 (kindly supplied by Pfizer, Inc.). Membranes were incubated with infrared (IR) Dye 680-conjugated goat anti-mouse IgG and IRDye 800-conjugated goat anti-rabbit IgG. The experiment was repeated 4 times, each with tissues collected from different people (n = 17).
For ADAM-8 Western blotting, goat anti-human ADAM-8 antibody at 0.2μg/ml (AF1031, R&D Systems) was incubated overnight at 4°C. Recombinant human ADAM-8 (R&D Systems) was used as positive control. After washing with PBS, IRDye 800-conjugated donkey anti-goat secondary antibody (diluted 1:20,000) was incubated for 1 hr at room temperature. The experiment was repeated 3 times, with 3 NP and 12 AF tissues from 12 different patients. ADAM-8 Western blotting has also been performed on 10 cadaveric tissues (5 NP and 5 AF, from 2 different people; data not shown).
Membranes were analyzed using an Odyssey Infrared Imaging System (LI-COR Biosciences). Where relevant, signal intensities were determined using LI-COR imaging software and exported to Microsoft Excel. Comparison of signal intensity among different degrees of degeneration and in various age groups was performed with the Kruskal-Wallis test followed by Dunn’s multiple comparison test.
Immunostaining for ADAM-8
Human NP and AF tissues were embedded in O.C.T. Compound (Tissue Tek) and cryosectioned to 5 μm thickness with a cryostat (Lyca). Sections were fixed in 4% paraformaldehyde, washed in PBS, incubated with 0.1M sodium periodate to inhibit endogenous peroxidase and washed as before. A one hour blocking step was done with 3% donkey serum and 1% BSA in PBS. Goat anti-Human ADAM-8 at 5μg/ml (R & D Systems) was applied overnight at 4°C, followed by washing with PBS. Donkey anti-goat secondary antibody conjugated to horseradish peroxidase (diluted 1:3000) was applied for 1 hr at room temperature. After washing, Vector ABC solution was applied for 75 minutes, followed by washing. Tissues were then incubated with Diaminobenzidine (DAB, Sigma) reaction solution until color was detected, followed by counterstaining with hematoxylin.
Results
The fibronectin (FN) antibody (to the neoepitope VRAA271) and the N-terminal antibody (mAB 1936) specifically recognize full-length FN and its fragments (FN-fs)
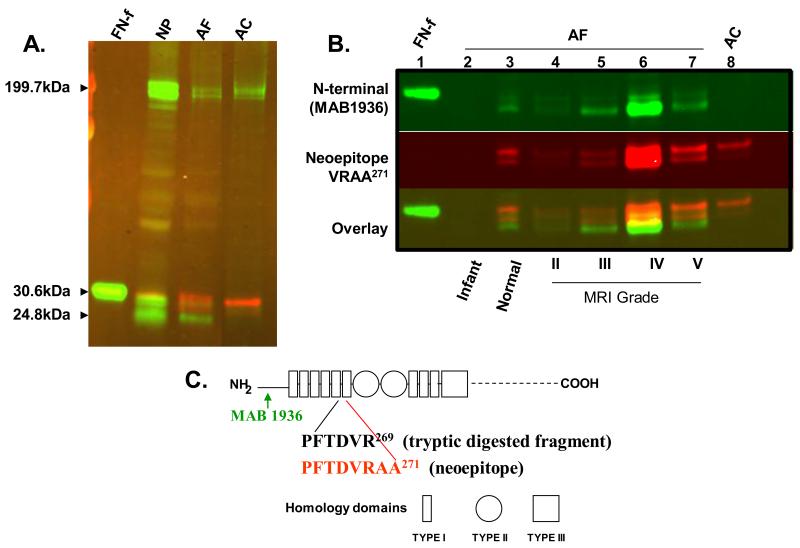
Figure 2, panel A is a Western blot using two sets of antibodies: 1) a mouse monoclonal antibody specific for the N-terminus of human FN (mAB1936, Chemicon/Millipore), with goat anti-mouse secondary antibody conjugated to infrared dye (IRDye) of 680nM in wavelength (green bands); 2) a rabbit polyclonal antibody recognizing the FN-f neoepitope VRAA271, with a goat anti-rabbit secondary antibody conjugated to IRDye of 800nM in wavelength (red bands). Protein extracts from human NP and AF tissues from a surgical patient with a Grade IV degenerative disc were loaded in separate lanes as indicated. In both NP and AF tissues, there are two full length FN polypeptides with an apparent molecular weight (MW) of about 200 kDa, and multiple FN-fs with apparent MW of around 25-29 kDa. Some FN-fs are recognized by the N-terminus mAb (green), while others are recognized by the VRAA271 neoepitope antibody (red). As expected, arthritic human ankle articular cartilage (AC) tissue contained fragments recognized by the neoepitope VRAA271 antibody (red). There were two products with MW of 25-29 kDa, recognized by the VRAA271 neoepitope antibody (red), as well as 2 full length FN polypeptide species recognized by the N-terminus mAb. The neoepitope antibody did not recognize the 29-kDa tryptic fragment (lane 1, FN-f) which is only 2 amino acids shorter than the fragment containing the neoepitope VRAA271 (asillustrated in figure 2C).17, 19 This finding confirms that the neoepitope antibody is highly specific.
Figure 2. The fibronectin (FN) antibody to neoepitope VRAA271 is specific for the fibronectin fragment (FN-f), while the N-terminal antibody (mAB 1936) binds to both full length fibronectin and its fragments.
Panel A: Western blot using the neoepitope VRAA271 antibody (shown in red) and the N-terminal antibody (mAB 1936, shown in green). Lanes, from left to right: 29-kDa fibronectin fragment (FN-f); degenerative nucleus pulposus (NP) tissue; degenerative human annulus fibrosus (AF) tissue; arthritic articular cartilage (AC, as positive control). Panel B: FN and FN-f species in human AF tissues by western blot. Lane 1: human plasma derived 29-kDa FN-f; lane 2: infant AF tissue; lane 3: normal young adult AF tissue; lane 4-7: adult AF surgical samples with increasing degree of degeneration; Lane 8: arthritic AC. Panel C: Schematic diagram of the human FN protein structure. Arrows below the diagram illustrate the location of the antibody epitopes; Diagram modified from Hynes (1990);3 numbering of amino acids according to Zack et al. (2009).16
The content of fibronectin N-terminal fragments appears to increase as disc degeneration progresses
Figure 2, panel B shows one Western blot of human AF protein extracts from patients with different grades of disc degeneration detected by the two FN monoclonal antibodies. Bands detected by the N-terminus mAb are depicted in green, by the neoepitope antibody in red, and by both antibodies in yellow. AF tissue lysates were loaded in lanes 4-7, representing surgical samples from patients with varying degrees of degeneration (MRI grades II-V). In this set of samples, the intensity of the positive bands corresponding to fragments recognized by both antibodies was strongest in the sample from the patient with Grade IV degeneration (lane 6). As previously reported, infant AF tissue contained full length FN, but not FN-f (lane 2, as negative control). Protein extract from normal-appearing adult AF tissue from a 25-year old cadaveric donor (Lane 3) contained multiple FN-fs with estimated MW of 25-29 kDa, detected by both antibodies. This suggests that disc degeneration may have started, although such degeneration is not yet visible to the naked eye in this individual.
FN-fs and association with IVD degeneration and patient age
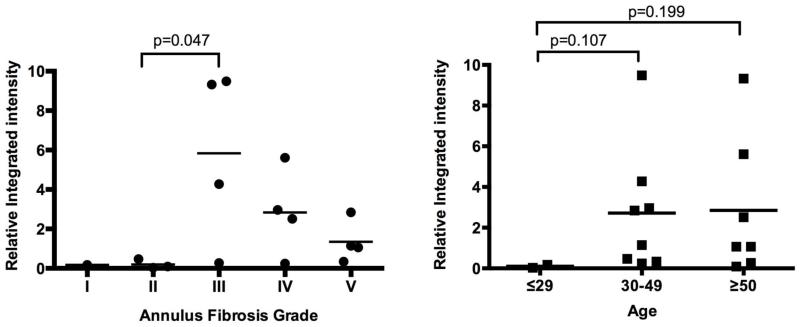
In order to further investigate the correlation of FN-fs and IVD degeneration, Western blots were performed 4 times (n=17), with AF tissues from patients with various degree of disc degeneration (infant, n = 1; grade I, n = 1; grade II, n = 3; grade III, n = 4; grade IV, n = 4; grade V, n = 4). Signal intensity of individual bands was quantified using LI-COR imaging software. Figure 3, left panel shows the ratio of integrated intensity of the FN N-terminal fragments to 50ng of known FN-f (Sigma), recognized by the mAB antibody clone 1936. Grade III IVDs contain more FN-fs than Grade II IVDs (p=0.047, n=3 or 4, respectively). Because 50 ng of the purified 29 kDa FN-f (Sigma) was loaded in lane 1 of figure 2B and each subsequent blot, we estimate that the concentrations of the naturally occurring 25-29 kDa FN-fs were in the nanomolar range per gram of tissue.
Figure 3. Integrated intensity ratio of N-terminal fragments (FN-fs) recognized by mAB1936 in human annulus fibrosus (AF) tissues to positive control (50ng of 29 kDa fragment by Sigma).
Left panel: correlation of FN-f-content in AF tissues to intervertebral disc degeneration. Right panel: correlation of FN-f-content in AF tissues to patient age. The concentration of small endogenous FN-fs is at nanomolar range per gram of AF tissue.
Figure 3, right panel shows that there is a tendency for AF tissues from patients age 30 and above (30-49, n=8; ≥50, n=7) to contain a higher concentration of FN-fs compared with such tissues from patients 29 years or younger (n=2, P=0.107 or 0.199, respectively).
ADAM-8 is present in the both the human adult NP and AF tissues, as shown by Western blotting and immunostaining
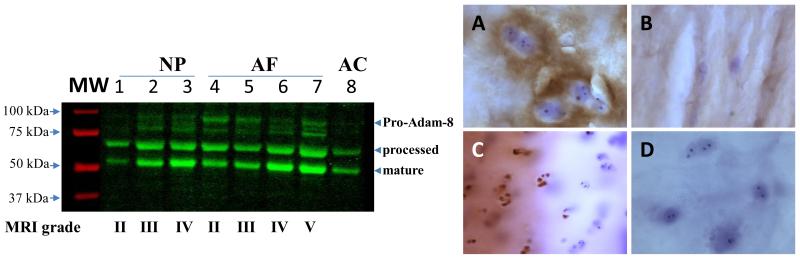
Figure 4, left panel, shows that ADAM-8 is detected in human IVD tissues from 12 different patients (3 NPs and 12 AFs, with Pfirrmann grades II-V). The largest molecule identified with this antibody has an apparent MW of 95 kDa. Two major bands with molecular weights ranging from 50 kDa to 75 kDa were also detected in both NP and AF tissues. There are several necessary processing steps for activation of ADAM-8, resulting in a few different possible active species in vitro.20 Based on these observations and the predicted M.W. of the proteins, we believe that the band with an apparent MW of 95 kDa represents pro-ADAM-8. There are two major bands with molecular weights in the range 50-70 kDa, which may represent partially processed ADAM-8. These designations are indicated in Figure 4. In addition, the 50-70 kDa products are more pronounced in cadaveric tissues (n=10, data not shown), which may represent autolysis.
Figure 4. A Disintegrin and metalloproteinase domain-containing protein (ADAM)-8 is present in the degenerative intervertebral disc.
Left panel: Adam-8 Western blot using tissues from human nucleus pulposus (NP) and annulus fibrosus (AF) tissues, and rhAdam-8 (as control); Molecular weight (MW) markers are shown in red. Right panel: ADAM-8 immunostaining; A: NP tissue; B: AF tissue; C: articular cartilage (AC, as positive control); D: NP tissue, no primary antibody control.
Figure 4, right panels show immunohistochemical staining of ADAM-8 in human cadaveric NP (A) and AF (B) tissues. In NP tissue (A), ADAM-8 is detected in areas closely surrounding NP cells. In the AF tissue, ADAM-8 is detected diffusely in the extracellular matrix (B). Human articular cartilage tissue from the femoral condyle of a patient undergoing total knee replacement was used as a positive control (C). In this tissue, only the superficial layers of degenerative articular cartilage stained positive for ADAM-8 (C). Normal appearing areas were negative for ADAM-8 (data not shown). Negative control (AC) with no primary antibody added did not show any non-specific staining (D).
Discussion
In this study, FN-fs were identified in IVD tissues derived both from cadaveric donors and surgical samples. We estimated that the concentrations of the naturally occurring 25-29 kDa FN-fs were in the nanomolar range per gram of surgical tissue. This information should prove helpful for researchers examining pathophysiological functions of FN-fs in the IVD.
This is the first report demonstrating that a fragment containing the VRAA271 neoepitope, previously described in degenerative articular cartilage, is present in both the NP and AF tissues. The neoepitope is also identifiable in normal appearing IVD tissue, suggesting that FN fragmentation may occur before visible degenerative changes can be detected. Thus, it may be useful as a molecular marker for early disc degeneration. Also detected in the IVD were FN-fs that do not contain the VRAA271 neoepitope, suggesting further proteolysis from the C-terminus of the fragment. Tiaden et al. have shown that high temperature requirement serine protease A1 (HTRA1) cleaves FN in the IVDs, but the precise cleavage site(s) have not been characterized.21 This suggests that HTRA1 may be, at least in part, responsible for, generating the FN N-terminal fragments.
We have identified ADAM-8 in degenerative human NP and AF tissues. The presence of ADAM-8 has not been previously described in human IVD tissues, although it has been shown to generate the VRAA271 neoepitope in articular cartilage tissues by Zack et al.16 These findings support the hypothesis that ADAM-8 plays a role in FN fragmentation associated with disc tissue degeneration.
FN-fs have been shown to downregulate proteoglycan accumulation and accelerated IVD degeneration in the rabbit.15 Our preliminary observation shows that there is a trend for increased expression of genes encoding type II collagen, aggrecan and MMP-9 and MMP-13 in cultured bovine IVD cells in response to FN-f stimulation (data not shown). Although the effects are small over the short term, disturbance of extracellular matrix metabolism by the FN-f might be mechanistically significant in a slowly evolving disease such as disc degeneration.
One limitation of the current study is that cadaveric tissues were utilized as negative control in comparison of NP and AF from surgically-obtained discs that showed various degrees of degeneration. Use of cadaveric tissues reflected limited access to less degenerated (Grade I and II) IVD tissues from surgical patients. Patients with normal appearing discs, or with very mild disc degeneration are not typically subjected to surgical intervention. Cadaveric tissues were collected within a 24-hour time frame (from time of death to tissue collection) to ensure that the cadaver tissues remained fresh. To further validate this comparison, we compared the FN-f content of grade IV tissues from cadaveric sources and from living subjects, because Grade IV tissues were available from both types of donors. The FN fragment pattern is similar in both patient-derived and cadaver-derived tissues (data not shown). It is also worth noting that FN and its fragments shown in Figures 2 & 3 represent the soluble protein fraction only; cross-linked proteins were not extracted by the method used in these studies.
Most of the Grade I & II tissues were from deceased donors and patients in their 20s, while most of the grade III-V tissues were from surgical patients 40-60 years old. Although there is a trend for an increase in FN-fs in patients aged 30 years and older, any effect of age on FN-f content cannot be reliably determined from the present study in view of the small number of samples analyzed. A possible additional future goal is to determine whether there is a correlation between the concentration of FN-fs in IVDs and degree of back pain.
ADAM-8 enzymatic activity is regulated at the protein level, by autoactivation, resulting in multiple partially processed but enzymatically active species in vitro.16 Consistent with the report by Zack et al., we identified multiple bands reactive with the anti-ADAM8 antibody in IVD tissues.16 Future investigations of ADAM-8 inhibitors such as batimastat22 might lead to interventions that would prevent FN fragmentation thus delay IVD degeneration.
In summary, we have identified a FN VRAA271 neoepitope in IVD tissue, and estimated the pathophysiological concentration of FN N-terminal fragments to be in the nanomolar range per gram of AF tissue. We have further shown the presence of ADAM-8, likely responsible for FN cleavage. Both FN-f and ADAM-8 are present in the early stages of disc degeneration, before visible changes to the disc tissue occur, suggesting that FN-fs may play an important role in the initiation and progression of disc degeneration.
Acknowledgments
National Institute of Child Health and Human Development (NICHD, 1K08 HD049598-01) funds were received to support this work.
Relevant financial activities outside the submitted work: consultancy, grants/grants pending, royalties, and Medical Advisory Board.
Footnotes
The Manuscript submitted does not contain information about medical device(s)/drug(s).
References
- 1.United States bone and joint decade: The burden of musculoskeletal diseases in the united states. 2008. [Google Scholar]
- 2.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: Four-year results for the spine patient outcomes research trial (SPORT) Spine (Phila Pa 1976) 2008;33:2789–2800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes R, editor. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- 4.Gehris AL, Oberlender SA, Shepley KJ, Tuan RS, Bennett VD. Fibronectin mRNA alternative splicing is temporally and spatially regulated during chondrogenesis in vivo and in vitro. Dev Dyn. 1996;206:219–230. doi: 10.1002/(SICI)1097-0177(199606)206:2<219::AID-AJA11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Burton-Wurster N, Borden C, Lust G, Macleod JN. Expression of the (V+C)-fibronectin isoform is tightly linked to the presence of a cartilaginous matrix. Matrix Biol. 1998;17:193–203. doi: 10.1016/s0945-053x(98)90058-0. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DG, Markova D, Adams SL, Pacifici M, An HS, Zhang Y. Fibronectin splicing variants in human intervertebral disc and association with disc degeneration. Spine (Phila Pa 1976) 2010;35:1581–1588. doi: 10.1097/BRS.0b013e3181c6ef1a. [DOI] [PubMed] [Google Scholar]
- 7.White DG, Hall JW, Brandli DW, Gehris AL, Bennett VD. Chick cartilage fibronectin differs in structure from the fibronectin in limb mesenchyme. Exp Cell Res. 1996;224:391–402. doi: 10.1006/excr.1996.0149. [DOI] [PubMed] [Google Scholar]
- 8.White DG, Hershey HP, Moss JJ, Daniels H, Tuan RS, Bennett VD. Functional analysis of fibronectin isoforms in chondrogenesis: Full-length recombinant mesenchymal fibronectin reduces spreading and promotes condensation and chondrogenesis of limb mesenchymal cells. Differentiation. 2003;71:251–261. doi: 10.1046/j.1432-0436.2003.7104502.x. [DOI] [PubMed] [Google Scholar]
- 9.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 10.Oegema TR, Jr, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Darribere T, Schwarzbauer JE. Fibronectin matrix composition and organization can regulate cell migration during amphibian development. Mech Dev. 2000;92:239–250. doi: 10.1016/s0925-4773(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 12.Peters JH, Carsons S, Yoshida M, et al. Electrophoretic characterization of species of fibronectin bearing sequences from the N-terminal heparin-binding domain in synovial fluid samples from patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2003;5:R329–39. doi: 10.1186/ar1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gemba T, Valbracht J, Alsalameh S, Lotz M. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem. 2002;277:907–911. doi: 10.1074/jbc.M109690200. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- 15.Greg Anderson D, Li X, Tannoury T, Beck G, Balian G. A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine (Phila Pa 1976) 2003;28:2338–2345. doi: 10.1097/01.BRS.0000096943.27853.BC. [DOI] [PubMed] [Google Scholar]
- 16.Zack MD, Malfait AM, Skepner AP, et al. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at ala(271) Arthritis Rheum. 2009;60:2704–2713. doi: 10.1002/art.24753. [DOI] [PubMed] [Google Scholar]
- 17.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 18.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Pardo A, Pearlstein E, Frangione B. Primary structure of human plasma fibronectin. the 29,000-dalton NH2-terminal domain. J Biol Chem. 1983;258:12670–12674. [PubMed] [Google Scholar]
- 20.Hall T, Leone JW, Wiese JF, et al. Autoactivation of human ADAM8: A novel pre-processing step is required for catalytic activity. Biosci Rep. 2009;29:217–228. doi: 10.1042/BSR20080145. [DOI] [PubMed] [Google Scholar]
- 21.Tiaden AN, Klawitter M, Lux V, et al. Detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287:21335–21345. doi: 10.1074/jbc.M112.341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall T, Shieh HS, Day JE, et al. Structure of human ADAM-8 catalytic domain complexed with batimastat. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:616–621. doi: 10.1107/S1744309112015618. [DOI] [PMC free article] [PubMed] [Google Scholar]