Abstract
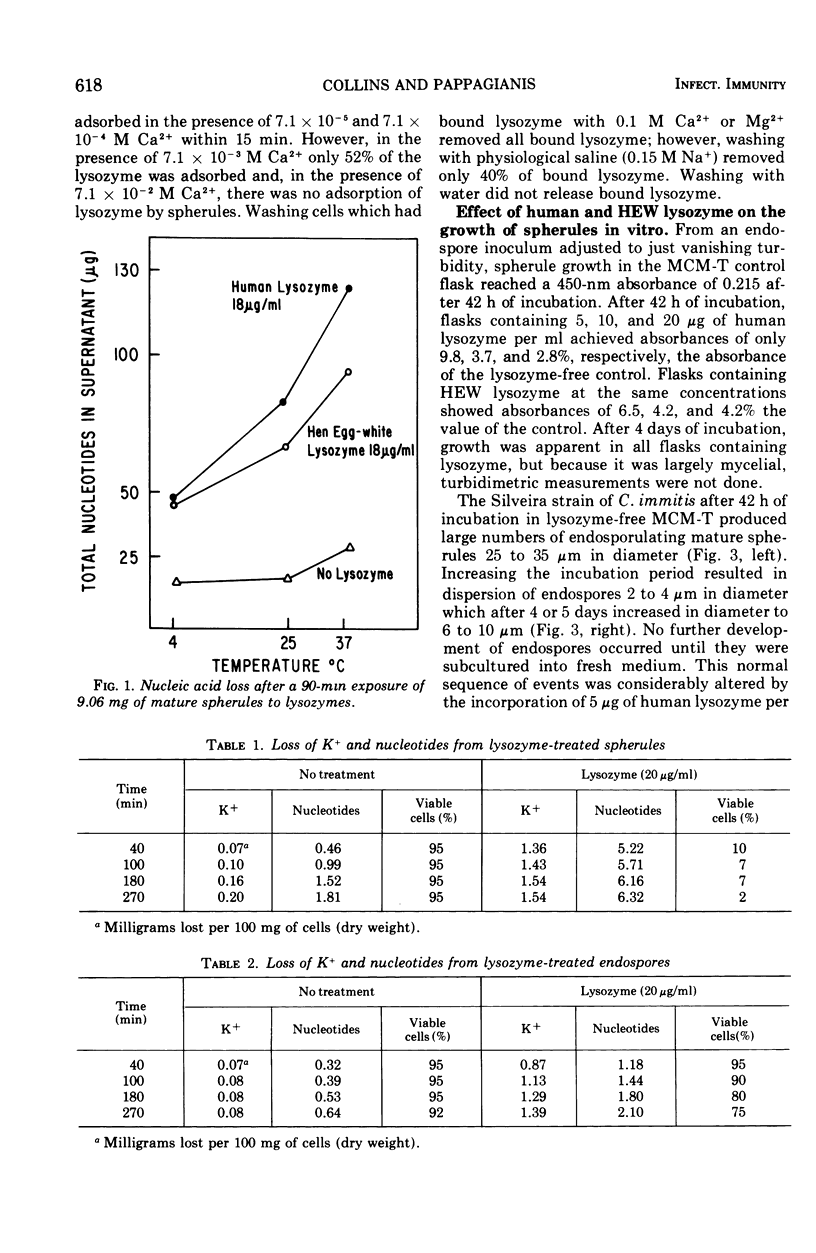
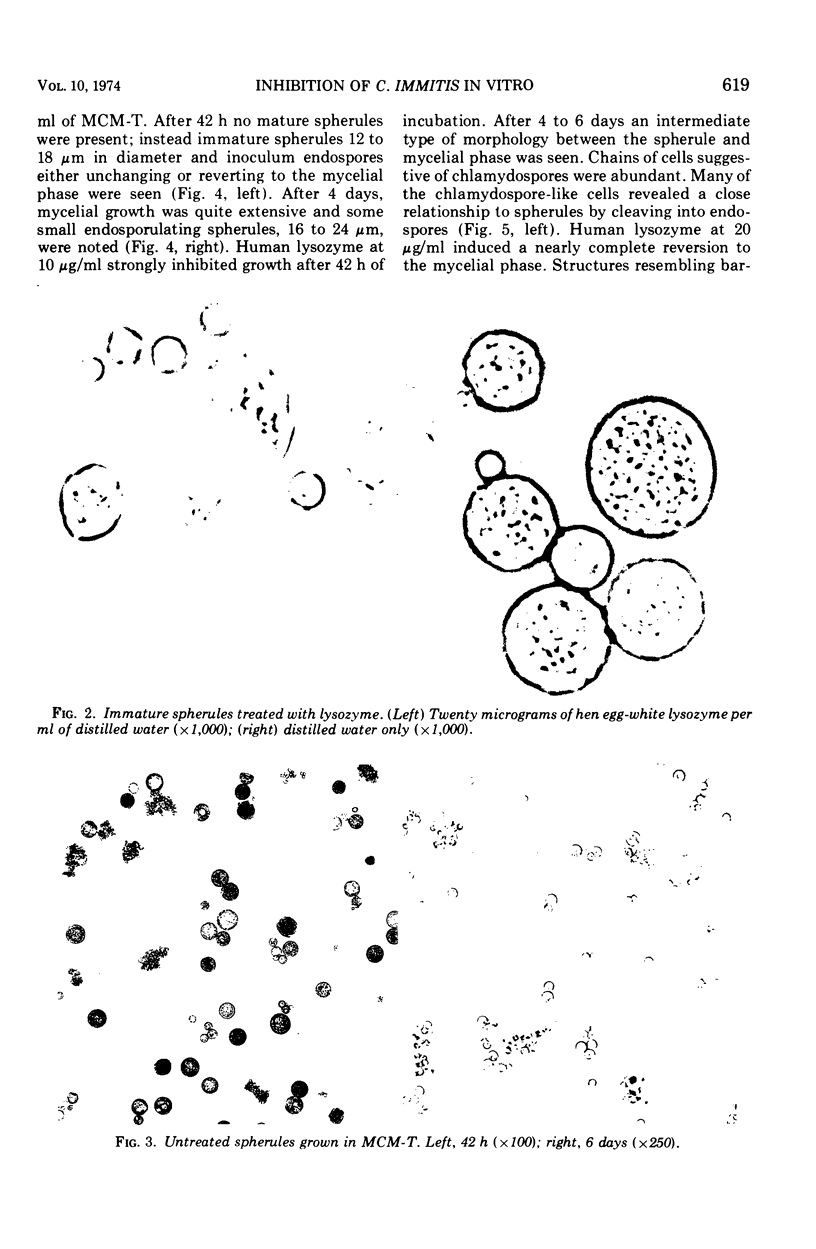
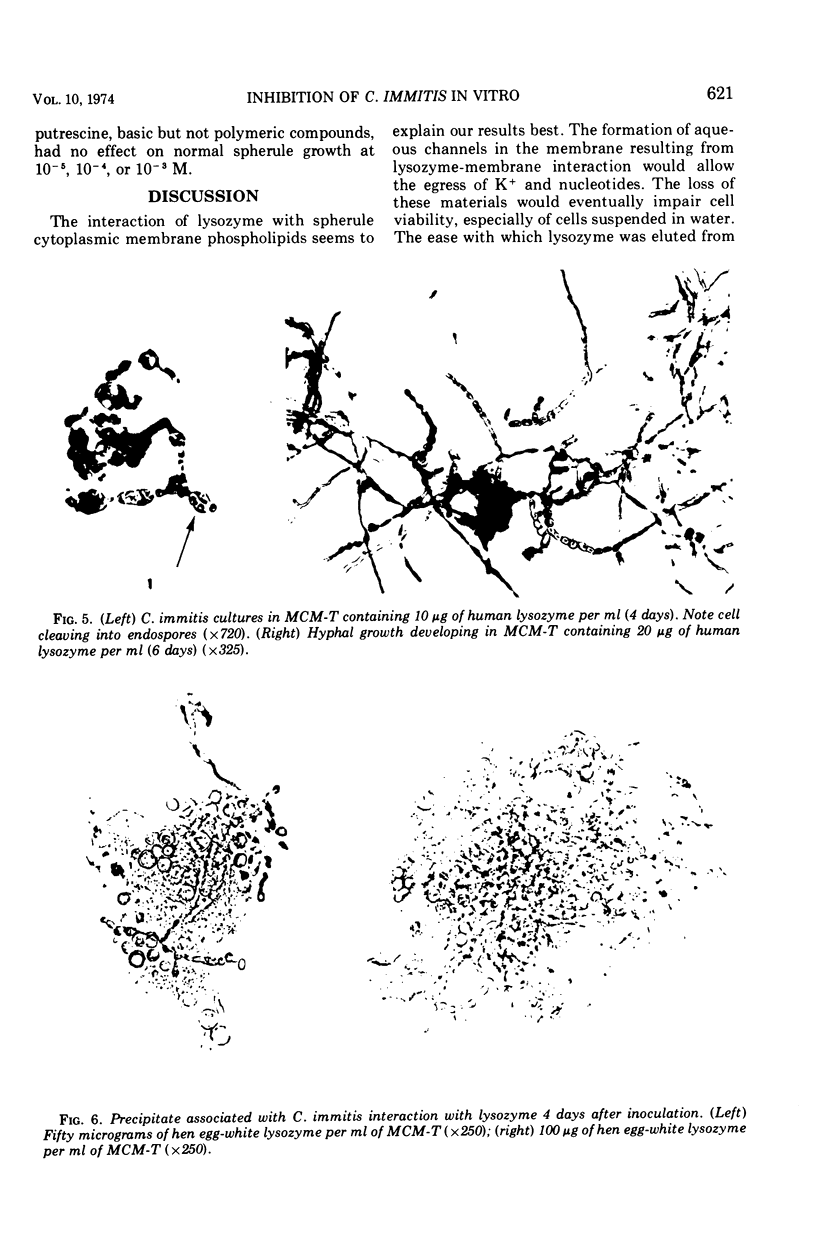
Development of mature endosporulating spherules from an endospore inoculum was markedly inhibited by human or hen egg-white (HEW) lysozyme at 5 μg/ml. Mature spherules formed in medium containing 5 μg per lysozyme per ml (3.3 × 10−7 M) were approximately 50% smaller than control spherules. In addition, lysozyme induced a large portion of the endospore inoculum to revert to the mycelial growth phase. Increasing lysozyme concentrations to 10 or 20 μg/ml prompted a nearly complete reversion of the inoculum to the mycelial phase. Mature endosporulating spherules removed from growth medium and resuspended in a solution of human or HEW lysozyme at 18 μg/ml in distilled water prompted leakage of four to five times as much of materials absorbing maximally at 260 nm into the supernatant as untreated control spherules during 90 min of incubation. This four- to fivefold increase in nucleotide loss was evident at 4, 25, and 37 C. The permeability of 1-day-old immature spherules and 8-day-old endospores was considerably altered by lysozyme treatment of cells suspended in distilled water. Large amounts of potassium and nucleotides were rapidly lost by each type of cell when treated with 20 μg of lysozyme per ml. After 270 min of exposure to lysozyme, 98% of the immature spherules and 25% of the endospores were nonviable. Lysozyme adsorption by formalin-killed spherules in the presence of varying concentrations of calcium ion and the rapid alteration of permeability seen after lysozyme treatment suggested that the cell membrane was damaged as a result of binding lysozyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. R., WEISER R. S. The beta-glucosaminidase activity of egg-white lysozyme. Biochim Biophys Acta. 1957 Dec;26(3):517–521. doi: 10.1016/0006-3002(57)90098-7. [DOI] [PubMed] [Google Scholar]
- Collins M., Pappagianis D. Effects of lysozyme and chitinase on the spherules of Coccidioides immitis in vitro. Infect Immun. 1973 May;7(5):817–822. doi: 10.1128/iai.7.5.817-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966 Dec;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- Jollès P. Relationship between chemical structure and biological activity of hen egg-white lysozyme and lysozymes of different species. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):350–364. doi: 10.1098/rspb.1967.0033. [DOI] [PubMed] [Google Scholar]
- Joos R. W., Carr C. W. The binding of calcium to phospholipid-protein complexes. Proc Soc Exp Biol Med. 1969 Dec;132(3):865–870. doi: 10.3181/00379727-132-34325. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Interactions of basic proteins with phospholipid membranes. Binding and changes in the sodium permeability of phosphatidylserine vesicles. J Biol Chem. 1971 Feb 25;246(4):1142–1148. [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Phospholipid-protein interactions: membrane permeability correlated with monolayer "penetration". Biochim Biophys Acta. 1971 Jun 1;233(3):805–809. doi: 10.1016/0005-2736(71)90181-7. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Dreyfus J., 3rd, Canfield R. E. Effect of human lysozyme on gram-positive and gram-negative bacteria. Antimicrob Agents Chemother (Bethesda) 1968;8:442–444. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Saito S. G. The diagnostic value of lysozyme (muramidase) estimation in biological fluids. Am J Med Sci. 1969 Dec;258(6):405–415. doi: 10.1097/00000441-196912000-00005. [DOI] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. The inhibition of lysozyme by acidic polymers from pathogenic bacteria. J Bacteriol. 1955 Jul;70(1):110–112. doi: 10.1128/jb.70.1.110-112.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Madin S. H. Cellular responses in lungs of immunized mice to intranasal infection with Coccidioides immitis. Sabouraudia. 1968 Feb;6(2):94–102. doi: 10.1080/00362176885190181. [DOI] [PubMed] [Google Scholar]
- Senn H. J., Chu B., O'Malley J., Holland J. F. Experiemental and clinical studies on muramidase (lysozyme). I. Muramidase activity of normal human blood cells and inflammatory exudates. Acta Haematol. 1970;44(2):65–77. doi: 10.1159/000208664. [DOI] [PubMed] [Google Scholar]
- TARBET J. E., BRESLAU A. M. Histochemical investigation of the spherule of Coccidioides immitis in relation to host reaction. J Infect Dis. 1953 Mar-Apr;92(2):183–190. doi: 10.1093/infdis/92.2.183. [DOI] [PubMed] [Google Scholar]
- TARBET J. E., WRIGHT E. T., NEWCOMER V. D. Experimental coccidioidal granuloma; developmental stages of sporangia in mice. Am J Pathol. 1952 Sep-Oct;28(5):901–917. [PMC free article] [PubMed] [Google Scholar]
- Welsh I. R., Spitznagel J. K. Distribution of lysosomal enzymes, cationic proteins, and bactericidal substances in subcellular fractions of human polymorphonuclear leukocytes. Infect Immun. 1971 Aug;4(2):97–102. doi: 10.1128/iai.4.2.97-102.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]