Abstract
Background. Stem cell-derived conditioned medium has a promising prospect to be produced as pharmaceuticals for regenerative medicine. Objective. To investigate various methods to obtain stem cell-derived conditioned medium (CM) to get an insight into their prospect of application in various diseases. Methods. Systematic review using keywords “stem cell” and “conditioned medium” or “secretome” and “therapy.” Data concerning treated conditions/diseases, type of cell that was cultured, medium and supplements to culture the cells, culture condition, CM processing, growth factors and other secretions that were analyzed, method of application, and outcome were noted, grouped, tabulated, and analyzed. Results. Most of CM using studies showed good results. However, the various CM, even when they were derived from the same kind of cells, were produced by different condition, that is, from different passage, culture medium, and culture condition. The growth factor yields of the various types of cells were available in some studies, and the cell number that was needed to produce CM for one application could be computed. Conclusion. Various stem cell-derived conditioned media were tested on various diseases and mostly showed good results. However, standardized methods of production and validations of their use need to be conducted.
1. Introduction
Data of the use of stem cells in various diseases are accumulating. Some studies reported beneficial effects of stem cell therapy in degenerative diseases such as myocardial infarction and revealed that stem cells cause tissue repair due to their ability to secrete trophic factors that exert beneficial impact on the damaged tissue, rather than their capacity to differentiate into the needed cells [1]. Various studies on stem cell-derived secreted factors showed that the secreted factor alone without the stem cell itself may cause tissue repair in various conditions that involved tissue/organ damage. The secreted factors are referred to as secretome, microvesicles, or exosome and can be found in the medium where the stem cells are cultured; thus, the medium is called conditioned medium (CM) [2].
The use of secretome containing CM has several advantages compared to the use of stem cells, as CM can be manufactured, freeze-dried, packaged, and transported more easily. Moreover, as it is devoid of cells; there is no need to match the donor and the recipient to avoid rejection problems. Therefore, stem cell-derived conditioned medium have a promising prospect to be produced as pharmaceuticals for regenerative medicine.
To date, no clinical trial that used CM for a certain disease has been reported, except two pilot studies on the use of adipose derived mesenchymal stem cell CM for hair follicle regeneration [3] and fractional carbon dioxide resurfacing wound healing [4] in human, which showed good results. The use of CM for therapy is very appealing and may be booming in the near future, as studies on the use of CM for various diseases are accumulating [1, 3–33]. Conditioned medium contains various growth factors and tissue regenerative agents, which were secreted by the stem cells. The fact that stem cells secrete various growth factors was also shown by various proteomic studies, which revealed the presence of various growth factors and other cytokines in the CM [5, 7–9, 13, 17, 20, 22, 28, 34, 35].
However, various studies reported the use of various kinds of stem cells and various methods to get the CM to cure various kinds of degenerative diseases in various animal models. Therefore, this systematic review aimed to investigate the various methods to get the CM and the various diseases that were treated, to get an insight into the various kinds of CM and their application benefit in various diseases.
2. Materials and Methods
We performed “all text” searches without time restriction on January 23, 2014, in Pubmed/Medline using keywords “stem cell” and “conditioned medium” or “secretome” and “therapy,” “all text” searches in Cochrane library (trials) using keywords “secretome” or “conditioned medium,” and “all text” searches in ClinicalTrials.gov using keywords “stem cell” and “conditioned medium” or “secretome” and “therapy.” In addition, relevant existing articles in our library were added.
Inclusion criteria are all studies that used CM for a certain disease. Exclusion criteria are studies that did not contain complete data concerning subject condition/disease model, source of CM, and outcome of treatment with CM.
Data collection is as follows: treated conditions/diseases, type of cell that was cultured, detailed composition of medium and supplements that was used to culture the cells, culture condition (hypoxia or normoxia) to get the CM, CM processing, growth factors, and other secretions that were analyzed; method (mode) of application and outcome of CM application were noted, grouped, and tabulated.
Data synthesis is as follows: data were grouped according to treated disease and cell types that were used to produce the CM. Further, to know the growth factor yields of the various types of cells, when available, growth factor levels were tabulated and grouped according to types of cells that yielded the growth factor containing conditioned medium, in relation to the number of cells, type and duration of culture, and processing of the conditioned medium. When the data was available, the number of cells that were needed to produce the CM for one application was computed.
3. Results and Discussion
We got 39 articles that met the inclusion criteria, and 7 were excluded due to incomplete data. Various conditions/diseases were treated by various cell-derived CM and mostly showed promising results (Table 1).
Table 1.
Studies on various subjects, conditions, source of conditioned medium, and outcome.
| Condition/disease | Subject | Source of conditioned medium | Outcome | Reference number |
|---|---|---|---|---|
| Alopecia—ID | Human | Hu-AD-MSC | Increased hair growth | [3] |
|
| ||||
| Bald—SC | C3H/HeN nude mice | Hu-AD-SC | Hair growth | [5] |
|
| ||||
| Acute hind limb ischemia—direct IM | Female athymic mouse | Hu-AD-SC | Decreased LL and F Increased BF, angiogenesis, endothelial growth, homing, and AA |
[6] |
| SCID mice | Hu-ESC—endothelial cells | Vascularization and BF: CM restored defective diabetic PB derived PAC | [7] | |
|
| ||||
| Chronic hind limb ischemia—7–10 days IM | Male nude athymic | Hu-PB-MNC-EPC Hu-UC-HUVEC |
Increased hind limb BF | [8] |
| Male NOD-SCID mouse | Hu-AF—SC—Ckit (+) | Increased arteriogenesis, capillary density, total perfusion area, and mobility, and decreased muscular deg | [9] | |
|
| ||||
| Skin wound direct—ID, [11] SC [10, 12]/topical application [4, 13] | Human | Hu-AD-SC | Enhanced wound healing Reduced adverse effects |
[4] |
| BALBc nude mice | (i) Hu-UCB-MNC ⟶ UCB-SC (endothelial + MSC) (ii) HUVEC |
Faster wound healing: UCB-SC was better than HUVEC |
[10] | |
| Diabetic immunodeficient mice | Hu-UCB-CD34-EPC | Faster wound closure Less granulation tissue area More neovascularization |
[11] | |
| Male db/db (diabetic) mice | Hu-UC-MSC | Faster wound closure Increased capillary density |
[12] | |
| BALBc-nude mouse | (i) Hu-ESC—derived EPC (ii) Hu-UCB-EPC |
Faster wound healing, granulation, and reepithelization: huESC-EPC was better than UCB-EPC | [13] | |
|
| ||||
| Skin wound—48 hour after wound—SC | Male NOD-SCID mice | Hu-BM-MSC | Faster wound healing | [14] |
|
| ||||
| MCI—direct—peri-infarct injection | Male SCID or C57BL/6 mouse | Hu-AD-SC | Improved cardiac function Reduced infarct size Effect of huAD-SC > CM |
[1] |
|
| ||||
| MCI—end of 2nd hour R—IC | Female L pig | Porcine PB-EPC | Reduced IZ-A and infarct size Increased IZ angiogenesis IZ cardiomyocyte hypertrophy Improved LV contractility and relaxation |
[15] |
|
| ||||
| MCI—4 hours—IV (jugular vein) | DL pig | Hu-ESC-MSC | Increased capillary density Reduced infarct size Preserved S-D performance |
[16] |
|
| ||||
| MCI—48 hours-IM yo | Rat nude athymic | Hu-BM-derived MPC | Improved LV function Reduced LV dilation, myocyte A, and fibrosis Increased neovascularization |
[17] |
|
| ||||
| MCI—5 min before R—IV, -at R—IC | Female DL pig | Hu-ESC derived MSC | (i) Reduced infarct size and A (ii) Improved S-D performance |
[18] |
|
| ||||
| MCI—5 min before R—IV—(tail) | Mouse | Hu-ESC derived MSC | Reduced infarct size (>1000 kD/100–220 nm) = 10–220 nm < 10–100 nm |
|
|
| ||||
| RSLT—direct—IV—(penile) | Male SD rat | Rat BM-MSC | Reduced LIB and PIC Increased survival |
[19] |
|
| ||||
| Acute hepatic failure—24 hours—intrahepatic (left liver lobe) | CCl4 injured SCID/NOD mice | 1-Hu-AF MSC 2-AF-MSC-hepatic progenitor-like cells (HPL) |
(i) AST, ALT decreased (ii) Liver phenotype improvement HPL was better than MSC-CM |
[20] |
|
| ||||
| Fulminant hepatic failure—24 hours—IV (penile) | Male SD rat | Hu-MSC | Reduced ALT, AST, TNFα, IL6, and IL1-rec-A level, and HP, ICI, and A Increased IL10 level, liver regeneration, and survival |
[21] |
| Male SD rat | Hu-BM-MSC | Reduced panlobular leucocyte infiltrate, hepatocellular death, and bile duct duplication and increased survival | [22] | |
|
| ||||
| Focal cerebral ischemia—72 hours—intranasal | Male SD rat | (i) Hu-SC-EDT (ii) BM-MSC (Lonza) |
Increased migration-diff—endogenous NPC, vasculogenesis, and motor function, and reduced infarct size (Hu SC-EDT = BM-MSC) |
[23] |
|
| ||||
| Ischemic stroke—after 8 days—lateral ventricle infusion | Male SD mice | Hu-AD-MSC | Motor function maintained, reduced infarct volume, neural cell A, and astrogliosis, and increased microvessel | [24] |
|
| ||||
| Cerebral ischemia infarction—1 day—IC/intracardiac (LV) injection | immunodeficient mice | (i) Hu-BM-MSC (ii) Hu-BM-CD133 (iii) Hu-BM-p75 (iv) Hu-fibro |
Reduced cortical infarct volume (huBM-CD133-CM < huBM-MSC-CM < hufibroCM < huBM-p75CM) |
[25] |
|
| ||||
| Fluid percussion-TBI—direct IV jugular vein | Male SD rat | Hu-BM-MSC | Reduced neuron loss, A, neuron A, infarction volume, and motor deficit Increased VEGF(+) cells |
[26] |
|
| ||||
| Fluid percussion TBI—12 hours after—IV | Male SD rat | Hu-BM-MSC | Decreased brain damage volume, brain damage incidence, and neuron A (hypoxia < normoxia) Increased motor/cognitive function and neurogenesis (hypoxia > normoxia) |
[27] |
|
| ||||
| Contusion spinal cord injury—direct | Female Wistar rat | Rat-BM-MSC | Increased motor recovery | [28] |
|
| ||||
| Chronic kidney disease—week 5—IV (tail) | Male Le rat | Hu embryonic MSC—stable—80 population doublings | Decreased systolic BP, proteinuria, and tubular + glomerular damage Increased inulin and PAH clearance, glomerular endothelium, and DNA repair |
[29] |
|
| ||||
| Nephropathy—24 hours—IV (tail) | Mouse BALBc | (i) Hu-UCB-USSC (ii) Mouse BM-MSC |
No improvement in serum urea and creatinine, HP, and physical activity score | [30] |
|
| ||||
| Normal—cancer cell line + CM xenograft | BALB mice | Hu-MSC (cell line) | Increased tumor cell proliferation (PCNA) and vascularization | [31] |
|
| ||||
| VILI—before induction—IV—(tail) | Male C57BL/6 mouse | Mouse-iPSC | Reduced tidal volume, and bronchial microstructure restored | [32] |
|
| ||||
| Intrabony periodontal defect direct—implant | Hybrid dog | Hu-MSC (Lonza) | Increased alveolar bone and cementum regeneration | [33] |
ID: intradermal, IM: intramuscular, SC: subcutaneous, MCI: myocardial infarct, R: reperfusion, IC: intracoronary artery, IV: intravenous, Imyo: intramyocardial, LV: left ventricular, RSLT: 50% reduced size liver transplantation, TBI: traumatic brain injury, VILI: ventilator induced lung injury, SCID: severe combined immunodeficient, NOD: nonobese diabetic, SD: Sprague-Dawley, DL: Dalland Landrace, L: Landrace, W: Wistar, Le: Lewis, hu: human, AD: adipose tissue derived, MSC: mesenchymal stem cells, SC: stem cell, ESC: embryonic stem cell, PB: peripheral blood, MNC: mononuclear cell, UC: umbilical cord, UCB: UC blood, BM: bone marrow, EPC: endothelial progenitor cell, HUVEC: human umbilical vein endothelial cell, AF: amniotic fluid, EDT: exfoliated deciduous tooth, MPC: mesenchymal progenitor cell, USSC: unrestricted somatic stem cell, iPSC: induced pluripotent stem cell, LL: limb lost, F: fibrosis, BF: blood flow, AA: antiapoptosis, CM: conditioned medium, PAC: proangiogenic cells, deg: degeneration, IZ: infarct zone, A: apoptosis, ALT: alanine amino transferase, AST: aspartate aminotransferase, HP: histopathology, ICI: immune cell infiltration, S-D: systolic-diastolic, LIB: liver injury biomarker, PIC: proinflammatory cytokine, Hu-SC-, IL1-rec-A: IL1 receptor antagonist, NPC: neural progenitor cell, PAH: para amino hippuric acid.
The various conditioned media, even when they were derived from same kind of cells, were produced by different condition, that is, from different passage, number of cells, culture medium, and culture condition (Table 2). The growth factor yields of the various types of cells can be seen in Table 3, and the cell number that is needed to produce CM for one application can be seen in Table 4.
Table 2.
Cell type, medium, culture condition, cell number, duration, passage, and processing of conditioned medium.
| Reference number | Cell type | Medium/vessel | Culture condition | Cell number | Duration | Passage | CM processing |
|---|---|---|---|---|---|---|---|
| [7] | Hu-ESC-EC HUVEC |
EBM2 | NA | NA | 5 days | NA | NA |
| [13] | (i) Hu-ESC-CD133/KDR-EPC (ii) Hu-UCB-EPC |
EGM-2 (Lonza)—15 mL 150 mm culture dish |
NA | 80% | 48 hours | P5–8 | Conc. 50x 10 kD |
| [16] [29] |
Hu-ESC-MSC Hu-ESC-MSC |
DMEM—insulin, transferrin, selenoprotein, FGF2, PDGF-AB, glutamine, and β-ME | NA NA |
NA NA |
3 days 3 days |
≥80 PD 80 PD |
Conc. 25x 10 kD—220 nm → 0.5 mg/mL protein |
| [18] | Hu-ESC-MSC | Chemically defined medium | NA | NA | 3 days | 80 PD | Conc. 25x 10 kDa |
| [9] | Hu-AF SC—Ckit (+) |
αMEM Six-well plate |
5% CO2 | 150.000 70% |
16 hours | NA | from 500.000 cells—1 mL—Conc. → 80 µL |
| [20] | 1-Hu-AF-MSC 2-hu-AF-MSC-HPL |
DMEM 0.5% FBS 25 cm2 TC flask |
5% CO2 | 1.5 × 106
80% |
24 hours | P5–13 | Conc. 25x 3 kD |
| [8] | (i) Hu-PB-MNC-EPC (ii) HUVEC |
EBM2 (Lonza) 1% FBS | 1.5% O2, 5% CO |
NA | 72 hours | NA | NA |
| [15] | Porcine PB-MNC-EPC | Ex vivo 15 (Lonza)—VEGF 1 ng/mL—FC plate | 5% CO2 | From 30–40 mL PB | 48 hours | P0 | Centr. 600 g 5 min, 0.2 μm—ice |
| [12] | Hu-UC-MSC | M199 | 5% CO2 | NA | 24 hours | P3 | Conc.- 0.2 μm |
| [10] | (i) Hu-UCB-MNC-SC (endothelial + MSC) (ii) HUVEC |
EGM-2–15 mL 150 mm culture dish |
5% CO2 | 80% | 48 hours | P5–8 | Conc. 50x—10 kD |
| [11] | Hu-UCB-CD34-EPC | M199 basal medium | 5% CO2 | 1 × 106 | 24 hours | NA | Conc. |
| [30] | (i) Hu-UCB-USSC (ii) mBM-MSC |
Ultra CULTURE medium 7.5% BABLc serum | 5% CO2 | 60% | 48 hours | NA | NA |
| [14] | Hu-BM-MSC | αMEM 10% FBS | 5% CO2 | 2 × 107/flask 60–70% | Till 60–70% | P5 | Conc. 50x—5 kD 5 flask → 100 μL |
| [22] | Hu-BM-MSC | NA-0.05% BSA | NA | 2 × 106 | 24 hours | P3–7 | Conc. 25x—3 kD |
| [26] | Hu-BM-MSC | DMEM-0.05% BSA | normoxia | 2 × 106 | 24 hours | P3–7 | Conc. 25x—3 kD |
| [27] | Hu-BM-MSC | DMEM-0.05% BSA | 5% CO2
0.5% O2 |
2 × 106 → split 1 : 2 → confl |
24 hours | P3–7 | Conc. 25x 3 kD |
| [17] | Hu-BM-MNC-stro-3-MPC | αMEM | NA | 1 × 106 MPC | NA | P5 | Conc. |
| [25] | (i) Hu-BM-MSC (ii) Hu-BM-CD133 (iii) Hu-BM-p75 (iv) Hu-fibro |
αMEM | 5% CO2
(i) 1% O2 (ii) 21% O2 |
1 × 106
90% |
48 hours | P4-5 | 0.2 µm—80°C conc. 40x—5 kD |
| [28] | Rat-BM-MSC | DMEM T75 flask |
NA | 90% | 48 hours | P2–4 | Conc. 40x—10 kD: 10mL → 250 µL 0.22 µm—80°C |
| [19] | Rat BM-MSC | DMEM-0.05% BSA 10 mL |
NA | 80–90% | 12 hours | P3-4 | Conc. 25x 3 kD |
| [23] | (i) Hu-SC-EDT (ii) BM-MSC (Lonza) |
DMEM | 5% CO2 | 4 × 105 | 48 hours | P3–5 | 3000 rpm—3 min → supernatant |
| [3] | Hu-AD-MSC | NA | Hypoxia | NA | NA | NA | Conc.-freeze dried |
| [24] | Hu-AD-MSC | αMEM 70 mL | Spheroid 1% O2—5% CO2 | 4.2 × 107 | 2 days | NA | Centr. |
| [6] | Hu-AD-SC | CRM/αMEM CRM—hu allo 10% αMEM—FBS 10% |
1% O2—5% CO2 | 2.5–3 × 105/mL—24 mL—150 cm dish | 2 days | Up to P5 | Centr. |
| CRM/αMEM CRM—hu allo 10% αMEM FBS 10% |
Spheroid 1% O2—5% CO2 |
6–12 × 105/mL—30 μL → 70 mL flask | |||||
| [1] | Hu-AD-SC | NA | NA Reference [13, 14] |
1 × 105 →80% | 24 hours | NA | Centr. 300 g 5 min—220 nm |
| [5] | Hu-AD-SC | DMEM/F12 | 2% O2
5% CO2 |
4 × 105 | 72 hours | P4-5 | Centr. 300 g 5' 0.22 µm, 3 kD |
| [23] | Hu-MSC | DMEM-0.05% BSA 15 mL—175 cm2 flask | NA | 1 × 106
70–80% |
24 hours | NA | Conc. 25x 3 kD |
| [31] | Hu-MSC (cell line) | DMEM-10% FBS 5 mL |
5% CO2 | 70% | 48 hours | NA | 100,000 g—1 hour → supernatant 0.22 µm |
| [33] | Hu-MSC (Lonza) | DMEM | 5% CO2 | 70% | 48 hours | P3–9 | 4°C or −80°C |
| [32] | Mouse-iPSC | NA | NA | NA | NA | NA | NA |
Hu: human, ESC: embryonic stem cell, EC: endothelial cell, HUVEC: human umbilical vein endothelial cell, EPC: endothelial progenitor cell, AF: amniotic fluid, SC: stem cell, MSC: mesenchymal SC, HPL: hepatic progenitor-like cell, AD: adipose tissue derived, PB: peripheral blood, MNC: mononuclear cell, UC: umbilical cord, UCB: UC blood, m: mouse, BM: bone marrow, MPC: mesenchymal progenitor cell, fibro: fibroblast, EDT: exfoliated deciduous tooth, iPSC: induced pluripotent SC, TC: tissue culture, NA: not available, FBS: fetal bovine serum, allo: allogenic serum, BSA: bovine serum albumin. ∗Filtered 220 nm → 10 nm → 100 nm yielded 10–220 nm versus 10–100 nm (<1000 kD) versus 100–220 nm (>1000 kD). FC: fibronectin coated, P: passage, PD: population doubling, CM: conditioned medium, conc.: concentrated, centr.: centrifugation.
Table 3.
Growth factor level from various cell sources, culture duration, cell number and processing of conditioned medium.
| Reference number | Cell source | Culture/duration | Cell number/processing | Growth factor level | |
|---|---|---|---|---|---|
| [7] | Hu-ESC-EC | Monolayer —5 days | NA |
Angiogenic cytokine: VEGF, SDF-1, PlGF, leptin, EGF, bFGF, and HGF: CM DM-PAC < CM cPAC Angiogenic cytokine: VEGF, PDGF, ICAM-1, EGF, and bFGF: CM ESC-EC > CM c-PAC = CM D-PAC |
|
|
| |||||
| [13] | (i) Hu-ESC-CD133/KDR-EPC (ii) Hu-UCB-EPC |
Monolayer—48 hours | 80% in 150 mm culture dish/Conc. 50x | EGF | ESC-EPC versus CB-EPC versus EGM-2 12584 versus 12654 versus 9 pg/mL |
| FGF-2 | 383 versus 652 versus 61 | ||||
| Fractalkine | 1605 versus 150 versus 133 | ||||
| GM-CSF | 755 versus 323 versus 313 | ||||
| IL-6 | 4332 versus 1961 versus 2463 | ||||
| IL-8 | 239030 versus 13629 versus 7 | ||||
| IL-9 | 345 versus 42 versus 9 | ||||
| IP-10 | 458 versus 513 versus 511 | ||||
| MCP-1 | 63 versus 3201 versus 1902 | ||||
| PDGF-AA | 6667 versus 5568 versus 41 | ||||
| PDGF-AB/BB | 17 versus 1884 versus 75 | ||||
| VEGF | 4265 versus 538 versus 42 | ||||
|
| |||||
| [9] | Hu-AF SC—Ckit (+) | Monolayer—16 hours | 15 × 104—70%/from 5 × 105 cells Conc. 12.5x | VEGF, IL-8, SDF-1 | 1 ng/170.000 cell (1 μg DNA) |
| IL-6, MCP-1 | 0.5 ng/170.000 cell | ||||
| TGF-b | 0.2 ng/170.000 cell | ||||
| IFNg | — | ||||
| IP-10 /CXCL10 | — | ||||
| IL-1a | — | ||||
|
| |||||
| [20] | (i) Hu-AF-MSC (ii) Hu-AF-MSC-HPL |
Monolayer—24 hours | 1.5 × 106
80%/ Conc. 25x |
Proteome analysis Hu-AF-MSC-HPL-CM: Anti-inflammatory cytokine: IL-10, IL-27, IL-17E, IL-13, IL-12p70, and IL-1ra Liver protection: MCP- 1, IL-1b Hu-AF-MSC-HPL-CM, AF-MSC-CM: Anti-inflammatory cytokine: TGFβ1 Tissue repair: serpin E1, SDF-1 Angiogenesis: VEGF, PDEGF, and endostatin/collagen XVIII Liver regeneration: UPA, thrombospondin 1 and 2, HEGF, FGF-7, EGF, and HGF Anti-apoptotic markers: TIMP-1, IGFBP |
|
|
| |||||
| [8] | (i) Hu-PB-MNC- EPC (ii) HUVEC |
Monolayer—72 hours | NA | IL-8/CXCL8 Hypox versus norm |
29090.7 ± 12279.4 pg/mL versus 2282.1 ± 406.3 pg/mL |
| SDF-1/CXCL12 | 6059.9 ± 654.6 pg/mL versus 3179.9 ± 488.0 pg/mL |
||||
| HGF | 539.5 ± 141.7 pg/mL versus 343.4 ± 74.8 pg/mL |
||||
| Angiogenin | 144.6 ± 68.2 pg/mL versus 72.5 ± 15.8 pg/mL |
||||
| PDGF-BB | 111.6 ± 27.02 pg/mL versus 19.9 ± 2.2 pg/mL |
||||
| VEGF-A | 25.5 ± 4.8 pg/mL versus 11.4 ± 5.2 pg/mL |
||||
|
| |||||
| [10] | Hu-UCB SC (endothelial + MSC) | Monolayer—48 hours | 80%/Conc. 50x | EGF | 3,286 ± 419 pg/mL |
| VEGF | 2,463 ± 151 pg/mL | ||||
| G-CSF | 3,615 ± 173 pg/mL | ||||
| GM-CSF | 3,623 ± 345 pg/mL | ||||
| TGF-β1, PDGF, bFGF, and KGF |
=HUVEC | ||||
| HUVEC | Monolayer—48 hours | 80%/Conc. 50x | EGF | UCB-SC-4.8X | |
| VEGF | UCB-SC-42x | ||||
| G-CSF | UCB-SC-3.7x | ||||
| GM-CSF | UCB-SC-2.4x | ||||
|
| |||||
| [22] | Hu-BM-MSC | Monolayer—24 hours | 2 × 106/Conc. 25x |
69 from 174 prot. tested (+) (concentration NA) |
|
|
| |||||
| [27] | Hu-BM-MSC | Monolayer—24 hours | 4 × 106/Conc. 25x | VEGF HGF |
Normoxia: 230 pg/mL Hypoxia: 450 pg/mL Normoxia: 600 pg/mL Hypoxia: 750 pg/mL |
|
| |||||
| [17] | Hu-BM-MNC-stro-3-MPC | NA | 1 × 106/Conc. | IL-6 = 2x C | 118.04 ± 0.27 pg/mL |
| MCP-1 = 2x C | 521.89 ± 1.48 pg/mL | ||||
| VEGF = 2x C | 33.95 ± 2.98 pg/mL | ||||
|
| |||||
| [25] | (i) Hu-BM-MSC (ii) Hu-BM-CD133 (iii) Hu-BM-p75 |
Monolayer—48 hours | 1 × 106
90%/ Conc. 40x |
Secretion/cell | P75 versus CD133 versus BMMSC |
| IL6—norm | 3.8 versus 0.8 versus 0.6 fg | ||||
| IL6—hypox | 0.25 = 0.25 versus 0.1 fg | ||||
| PlGF—norm | 0.045 versus 0.01 versus 0 fg | ||||
| PlGF—hypox | 0.043 versus 0.025 versus 0.15 fg | ||||
| ADM—norm | 0.1 versus 0.05 versus 0.2 fg | ||||
| ADM—hypox | 5.8 versus 5.4 versus 11.5 fg | ||||
| VEGF—norm | 1.5 versus 1.0 versus 1.35 fg | ||||
| VEGF—hypox | 0.7 versus 0.9 versus 0.95 fg | ||||
| SDF-1—norm | 1.35 versus 0.75 versus 0.15 fg | ||||
| SDF-1—hypox | 0.4 versus 0.7 versus 1.0 fg | ||||
| HGF—norm | 0.84 versus 0.7 versus 0.25 fg | ||||
| HGF—hypox | 0.01 versus 0.25 versus 0.01 fg | ||||
| DKK-I—norm | 4 versus 4 versus 4.5 fg | ||||
| DKK-1—hypox | 6.8 versus 6.5 versus 10.5 fg | ||||
|
| |||||
| [28] | Rat-BM-MSC | Monolayer—48 hours | 90% T75/Conc. 40x |
23 from 90 prot. tested (+) | |
| NGF | 356 ± 117 pg/mL | ||||
| BDNF | 208 ± 57 pg/mL | ||||
| IL-6 | 427± 168 pg/mL | ||||
|
| |||||
| [24] | Hu-AD-MSC | Spheroid—2 days | 4.2 × 107/Centr. | CM versus αMEM:hTGF-b1 | 14.33 ± 6.71 versus 2.49 ± 2.39 pg/mL |
| hVEGF | 1,015.17 ± 170.97 pg/mL versus ND | ||||
| hPDGF-AA | Both ND | ||||
|
| |||||
| [6] | Hu-AD-SC In αMEM—FBS |
Spheroid—2 days | 105/Centr. | VEGF | 14.4 ± 0.4 ng/mL |
| FGF2 | 13.2 ± 2.2 ng/mL | ||||
| HGF | 13.3 ± 2.3 ng/mL | ||||
| CXCL12 | 16.6 ± 2.9 ng/mL | ||||
| In CRM-hu allo | No diff >< αMEM-FBS | ||||
| In CRM-serum (−) | GF < | ||||
| In αMEM—FBS | Monolayer—2 days | 105/Centr. | GF<<< | ||
| In CRM-hu allo | GF<<< | ||||
|
| |||||
| [5] | Hu-AD-SC | Monolayer— 72 hours |
4 × 105/Conc. | Spot density array hypox versus nomoxia | |
| GCSF | 14.07 ± 3.84 versus 10.13 ± 4.21 | ||||
| GM-CSF | 13.53 ± 1.26 versus 10.21 ± 1.44 | ||||
| IGFBP-1 | 9.48 ± 0.44 versus 5.56 ± 0.44 | ||||
| IGFBP-2 | 8.91 ± 0.02 versus 6.73 ± 0.31 | ||||
| IGF-II | 10.62 ± 0.85 versus 4.61 ± 0.93 | ||||
| M-CSF | 14.06 ± 0.13 versus 7.46 ± 1.69 | ||||
| M-CSF R | 9.09 ± 0.20 versus 3.31 ± 1.75 | ||||
| PDGF Rβ | 17.67 ± 1.32 versus 11.47 ± 1.40 | ||||
| PDGF-AA | 16.63 ± 1.33 versus 12.14 ± 2.12 | ||||
| VEGF | 13.47 ± 1.26 versus 5.59 ± 1.22 | ||||
| EGF | 11.06 ± 2.45 versus 34.14 ± 6.75 | ||||
|
| |||||
| [36] | (i) AD-SC (ii) Hu dermal fibroblast |
NA | NA | AD-SC | |
| VEGF | 810.65 ± 56.92 pg/μg DNA | ||||
| IGF-I | 328.33 ± 22.7 pg/μg DNA | ||||
| Hu dermal fibroblast | |||||
| VEGF | 28.4 ± 2.25 pg/μg DNA | ||||
| IGFI | Undetectable | ||||
|
| |||||
| [33] | Hu-MSC (Lonza) | Monolayer—48 hours | 70%/(—) | IGF-1 | 1515.6 ± 211.8 pg/mL |
| VEGF | 465.8 ± 108.8 pg/mL | ||||
| TGF-b1 | 339.8 ± 14.4 pg/mL | ||||
| HGF | 20.3 ± 7.9 pg/mL, | ||||
| FGF-2, PDGFBB, BMP-2, and SDF-1 (—) | |||||
CRM: clinically relevant med, hu allo: human allogenic serum, MP: microparticle, ND: not detected, SDF-1: stromal derived factor-1, PlGF: placental GF, bFGF: basic FGF, HGF: hepatocyte GF, PAC: peripheral blood angiogenic cells (from PB MN cells-floating), cPAC: healthy control PAC, ESC-EC: ESC derived endothelial cell, MCP-1: monocyte chemotactic protein-1, PDEGF: platelet derived endothelial cell GF, UPA: urokinase plasminogen activator, HEGF: heparin binding epidermal GF, TIMP-1: tissue inhibitor of metalloproteinase-1, IGFBP: insulin-like GF binding protein, IP-10: interferon inducible protein-1, ADM: adrenomedullin, DKK-1: Dickkopf-1, norm: normoxic, hypox = hypoxic, fg = fentogram.
Table 4.
Cell number to produce CM per application, volume, and mode of delivery of various cell sources for various conditions and the outcome.
| Reference number | Condition/disease | Species | Cell source of CM | Culture medium/culture type—condition | Cell number/application | Volume and mode of delivery | Outcome |
|---|---|---|---|---|---|---|---|
| [6] | Hind limb ischemia—direct | Female athymic mice—20–25 gr | Hu-AD-SC | αMEM—FBS 10%/monolayer—hypox 1% | 12.000 | 40 μL—IM—7x | Good result |
| CRM—Hu allo10%/spheroid—hypox 1% | 48.000 | Better result | |||||
| αMEM—FBS 10%/spheroid—hypox 1% | Better result | ||||||
|
| |||||||
| [9] | Hind limb ischemia—10 days | Male NOD-SCID mice—10–12 weeks | Hu-AF-SC–Ckit (+) | αMEM—(−)/monolayer—normoxia | 500.000 | 80 μL—IM—4x | Good result |
|
| |||||||
| [11] | Full thickness wound—5 mm direct | Diabetic-immunodef. mice—17–23 g | Hu-UCB-CD34-EPC | M199 basal medium (−)/monolayer—normoxia | 1 × 106 | 100 μL—intradermal injection | Good result |
|
| |||||||
| [14] | Wound 30–50 mm2; 120–140 mm2—48 hours |
Male NOD-SCID mice—4-5weeks | Hu-BM-MSC | αMEM—10% FBS/monolayer—normoxia | 1 × 108 | 100 μL—SC—periphery wound | Good result |
|
| |||||||
| [17] | MCI 48 hours | Nude-athymic rat—6–8 weeks | Hu-BM-MNC-stro-3-MPC | αMEM—(−)/monolayer—normoxia | 1 × 106 | 250 μL Intramyocardial | Good result |
|
| |||||||
| [20] | CCl4 injured acute hepatic failure—24 hours | SCID-NOD mice—6–8 weeks | Hu-AF-MSC | DMEM—0.5% FBS/monolayer—normoxia | 1.5 × 106 | 200 μL—intrahepatic (left liver lobe) | Good result |
| Hu-AF-MSC- HPL | Better result | ||||||
|
| |||||||
| [21] | Fulminant hepatic failure—24 hours | Male SD rat—250–300 g | Hu-MSC | DMEM—0.05% bovine serum albumin/monolayer—normoxia | 1.5 × 106 | 900 μL penile vein | Good result Increased survival |
| [22] | Male SD rat—280–370 g | Hu-BM-MSC | NA—0.05% BSA/monolayer—normoxia | 2 × 106 | 900 μL CM Penile vein |
Good result Increased survival |
|
|
| |||||||
| [23] | Focal cerebral ischemia—72 hours | Male SD rat—350–400 g | Hu-EDT-SC | DMEM (−)/monolayer—normoxia | 400.000 | 10x10 µL—intranasal (left-right) Every day D3-D15 |
Good result |
| BM-MSC (Lonza) | Good result | ||||||
|
| |||||||
| [24] | Ischemic Stroke—8 days |
Male SD mouse—8 weeks | Hu-AD-MSC | αMEM—(−)/spheroid—hypoxia 1% | 50.400 | Infusion 0.5 µL/hour-7 days—lateral ventricle | Good result |
SCID: severe combined immunodeficiency, NOD: nonobese diabetic, SD: Sprague-Dawley, Hu: human, AD: adipose tissue, SC: stem cell, AF: amniotic fluid, UCB: umbilical cord blood, EPC: endothelial progenitor cell, BM: bone marrow, MSC: mesenchymal SC, MNC: mononuclear cell, MPC: mesenchymal progenitor cell, HPL: hepatic progenitor-like cell, and EDT: exfoliated deciduous tooth.
Various studies showed that conditioned medium have been tested in various kinds of diseases/conditions (Table 1) [1, 3–33], that is, alopecia [3, 5], acute and chronic hind limb ischemia [6–9], acute and chronic wound healing [4, 10–14], myocardial infarct [1, 15–18], acute liver injury/failure [19–22], cerebral injury/ischemia/stroke [23–27], spinal cord injury [28], lung injury [32], and bone defect [33], and showed improvement of the conditions. Moreover, chronic kidney disease that was treated using human embryonic stem cell-derived mesenchymal stem cell (huESC-MSC) CM showed decreased systolic blood pressure and proteinuria and improvement in tubular and glomerular damage, renal blood flow, and glomerular filtration rate [29]. However, nephropathy that was treated using CM from human umbilical cord blood unrestricted somatic stem cell (huUCB-USSC) or mouse bone marrow mesenchymal stem cell (mBM-MSC) CM did not show improvement in serum urea and creatinine level, histopathological damage, and physical activity score [30]. Moreover, prevention of cancer using human mesenchymal stem cell line CM showed increased tumor cell proliferation and vascularization [31].
In the two cases of kidney disease, it can be concluded that CM from hu-ESC-MSC can improve the condition, and the needed growth factor level is presumably enough as CM processing includes a 25-time concentration step [29]. However, for hu-UCB-USSC or mBM-MSC-CM, lack of data concerning CM processing and growth factor level of the CM [30] prevent further analysis to conclude whether the failure to improve the condition is due to the lack of certain growth factor or due to the level of growth factors that was too low to give an effect.
The conditioned medium can be harvested from various kinds of cells (Table 2). Moreover, there are various methods to get the conditioned medium, which may interfere with the growth factor types and levels that were harvested by the methods. Only some of the various studies using CM checked the growth factor levels (Table 3) [5–10, 13, 17, 20, 22, 24, 25, 27, 28, 33, 36] and the same type of cells yielded different growth factor levels, when cell number, culture medium and condition, and CM processing were different [6, 24]. Moreover, growth factor measures also differed, that is, pg/mL or ng/mL [6, 8, 10, 13, 17, 24, 27, 28, 33], pg/μg DNA [9, 36], fg/cell [25], spot density [5], and positive/negative [20] (Table 3). The measure of pg/μg DNA and fg/cell can be computed into pg or ng/mL provided the DNA content/cell and cell number is known. However, in some studies, the exact cell number that was used to produce the CM was not mentioned [7, 8, 10, 13, 28, 33, 36]. In addition, most studies measured different sets of growth factors and other cytokines/factors (Table 3).
3.1. Culture Medium and Supplement
Some studies used fetal bovine serum or other supplement containing complete medium, while other studies used serum-free media. Moreover, the basal media used were variable, for example, αMEM, DMEM, DMEM/F12, M199, EBM2, EGM-2, in vivo 15, or chemically defined medium, and the same type of cell might be cultured in different kind of basal medium (Table 2). Culture medium in in vitro culture represents microenvironment in in vivo condition and may determine cell fate and thus cell secretion [37]. Therefore, the same type of cells may secrete different level of growth factors when they were cultured in different medium, as can be seen in Table 3 [25, 27].
3.2. Culture Duration
Production of CM varies in culture duration from sixteen hours to five days (Table 3). In case complete medium was used, short culture duration might leave certain serum derived growth factors that was not consumed by the cells and might add to the growth factor level, or, on the contrary, suppress growth factor secretion by the cells. Possibility of the presence of residual growth factor from the medium can be seen in a study, which showed that medium without cell contained a TGF-b1 level of 2.49 ± 2.39 pg/mL (Table 3) [24].
3.3. Culture Condition
Some studies produce CM from cell culture in normoxia (O2 level 20-21%) and variable oxygen deprived (hypoxia O2 level 0.5%, 1%, 1.5%, and 2%) condition (Table 2). Some studies on various stem cells showed that most growth factors were upregulated in hypoxia condition, for example, vascular endothelial derived growth factor (VEGF) [5, 8, 27], hepatocyte growth factor (HGF) [8, 27], platelet derived growth factor (PDGF) [5, 8], placenta growth factor (PlGF) [25], and insulin-like growth factor II (IGF-II) [5], except epidermal growth factor (EGF) that was downregulated [5]. However, another study showed the contrary, that is, down regulation of VEGF and HGF in hypoxia condition [25].
Most studies produced CM in monolayer culture, but several studies used spheroid cultures (Table 3). Spheroid cultures need a special handling and equipment (spinner flask) but yield more cells compared to conventional monolayer cultures, and thus more secreted factors [6, 24] (Table 4). In addition, cells located at the center of the spheroid may be in relative hypoxic condition compared to cells on the surface, thus further increasing certain growth factor yield.
3.4. Secreted Factor's Role in Improvement of Diseases
Various cytokines were secreted by stem cells into the CM, and they played a role in the improvement of various diseases/conditions. Those cytokines can be grouped into growth factors, proinflammatory and anti-inflammatory cytokines, and other cytokines. Various studies used various methods to assess various cytokines in the conditioned CM, from the conventional ELISA assays [6, 10, 24, 25, 27, 33, 36] to proteomic profiling methods [5, 7–9, 13, 17, 20, 22, 28, 34, 35].
3.4.1. Growth Factors
Growth factors that are secreted by various kinds of stem cells are vascular endothelial derived growth factor (VEGF) [5, 6, 8, 10, 13, 17, 24, 25, 27, 33, 36], platelet derived growth factor (PDGF) [5, 8, 10, 13, 24], epidermal growth factor (EGF) [5, 10, 13, 20], insulin-like growth factor I (IGF-I) [33, 36], insulin-like growth factor II (IGF-II) [5], hepatocyte growth factor (HGF) [6, 8, 20, 25, 27, 33], fibroblast growth factor 2/basic fibroblast growth factor (FGF-2/bFGF) [6, 7, 10, 13], keratinocyte growth factor/fibroblast growth factor 7 (KGF/FGF-7) [10, 20], platelet derived endothelial cell growth factor (PDEGF) [20], heparin binding epidermal growth factor (HEGF) [20], placenta growth factor (PlGF) [25], neural growth factor (NGF) [28], and brain derived neurotrophic factor (BDNF) [28].
Further, studies that analyzed various growth factors reported the presence of the various growth factors, which were secreted by various stem cells into their conditioned medium (Table 3), except for human MSC (Lonza) that did not secrete FGF-2, PDGFBB, BMP-2, and SDF-1 but secreted IGF-1, VEGF, TGF β1, and HGF [33]. Moreover, different culture condition and medium may yield different level of growth factor secretions [6].
3.4.2. Pro- and Anti-Inflammatory Cytokines
Anti-inflammatory cytokines that are secreted by stem cells are TGFβ1 [9, 10, 20, 24, 33] and some interleukins (IL), that is, IL-6 [9, 13, 17, 25, 28], IL-10, IL-27, IL-17E, IL-13, IL-12p70, and IL-1 receptor antagonist (IL-1ra) [20], while the secreted proinflammatory cytokines are IL-8/CXCL-8 [8, 9, 13], IL-9 [13, 35], and IL-1b [20].
3.4.3. Other Cytokines
Other secreted factors are leptin [7], angiogenin [8], granulocyte colony stimulating factor (GCSF) [5, 10], granulocyte macrophage CSF (GM-CSF) [5, 10, 13], macrophage CSF (MCSF) [5], fractalkine [13], monocyte chemotactic protein (MCP-1) [9, 13, 17, 20], serpin E-1 [20], endostatin/collegen XVIII [20], UPA, thrombospondins 1 and 2 [20], tissue inhibitor of metalloproteinase-1 (TIMP-1) [20], IGF binding protein (IGFBP) [5, 20], stem cell-derived factor 1 (SDF-1)/CXCL-12 [6–9, 20, 25], adrenomedullin (ADM) [25], Dickkopf-1 (DKK-1) [25], and receptors, that is, MCSF receptor (MCSFR) [5] and PDGF receptor (PDGFR) [5].
3.5. Translation of Conditioned Medium Usage in Patients
In conditioned medium, various factors may be present as a cocktail and act in concert to promote regeneration. Therefore, it is important to analyze a complete set of growth factor and cytokine levels for every kind of stem cell-derived conditioned medium and to know the culture condition, conditioned medium processing, and diseases/conditions that are responsive to a certain conditioned medium treatment. When the content of the various cytokines in a certain conditioned medium is known, the result of the conditioned medium on a certain disease/condition can be determined, and the way to translation into patients is open.
From studies that analyzed VEGF level we can conclude that most stem cells secrete VEGF. As VEGF plays a role on angiogenesis [36] that is important in regeneration of injured/damaged tissues/organs, various stem cell-derived conditioned media are able to cure various diseases and will have more impact on diseases with ischemia. In addition, VEGF may prevent apoptosis in hypoxic condition, thus preventing further damage [6].
Concerning angiogenesis, other than VEGF, other growth factors that may play a role in angiogenesis are FGF2 [7, 38], EGF [7], HGF [7, 8], PlGF [7], SDF-1 [7], PDGF [7, 38], TGFβ1 [38], and PDEGF [39]. In addition, various cytokines, that is, interleukin [38], IL-8 [8, 9, 13], chemokines [38], monocyte chemotactic protein (MCP-1) [9, 13, 17, 20], leptin [7], angiogenin [8], and endostatin/collagen XVIII [20], also play a role in angiogenesis.
Moreover, FGF2 is a more potent angiogenic factor compared to VEGF, with additional effect on proliferation of fibroblasts, preadipocytes, and endothelial, epithelial, and neural stem cells, on migration of neural crest derived glial and myogenic cells and on differentiation of neuroepithelial cells into mature neurons and glial cells [38].
Other growth factors contribute in the regeneration of injured/damaged tissue organs, with special emphasis on proliferation, that is, PDGF for connective tissue, glial, and other cells, EGF for mesenchymal, glial, and epithelial cells, and IGF-I and IGF-II for various kinds of cells [40]. In addition, PlGF that is a member of VEGF family increases the activity of VEGF in vitro and in vivo [41], KGF inhibits oxidative stress induced epithelial cell death [42], NGF promotes neurite outgrowth and neural cell survival [40], BDNF is neuroprotective, promotes cell survival, and reduces astroglial scar formation [28], and some growth factors, including HEGF, FGF-7, EGF, and HGF promote liver regeneration [20].
Proinflammatory cytokines that play a role in regeneration are IL-1b due to its liver protective role [20], IL-8 due to its angiogenic activity [8, 9, 13], and IL-9 due to wound healing promotion activity [13, 43]. In addition, anti-inflammatory cytokines prevent inflammation and promote liver regeneration [20].
Other cytokines, that is, UPA and thrombospondins 1 and 2, promote liver regeneration [20], serpin E-1 [20] and SDF-1 [6–9, 20, 25] promote tissue repair [20], TIMP-1 and IGFBP [5, 20] prevent apoptosis [20], ADM causes vasodilatation and reduces cellular oxidative stress and apoptosis [25], DKK-1 initiates bone marrow stem cell proliferation [25], and fractalkine prevents apoptosis [13, 44].
Various colony stimulating factors, that is, granulocyte colony stimulating factor (GCSF) [5, 10], granulocyte macrophage CSF (GM-CSF) [5, 10, 13], and macrophage CSF (MCSF) [5], may recruit various resident stem cells/progenitor cells including endothelial progenitors to site of injury/damage and promote wound healing process [10, 13] or hair growth [5].
MCSF receptor (MCSFR) [5] promotes myeloid progenitor, mononuclear phagocyte, and placental trophoblast growth and development [45], and PDGFR [5] may interact with various signaling molecules or integrin to cause cell proliferation, motility, differentiation, or survival by apoptosis inhibition [46].
Moreover, one factor may contribute to more than one mode of regenerative action, such as MCP-1 that is involved in angiogenesis [9, 13, 17, 20] and liver protection activity [20]. Further, for production of CM to be applied in various human diseases, data from animal studies that showed promising outcome are very valuable.
3.5.1. Production of CM for Translation into Various Human Diseases
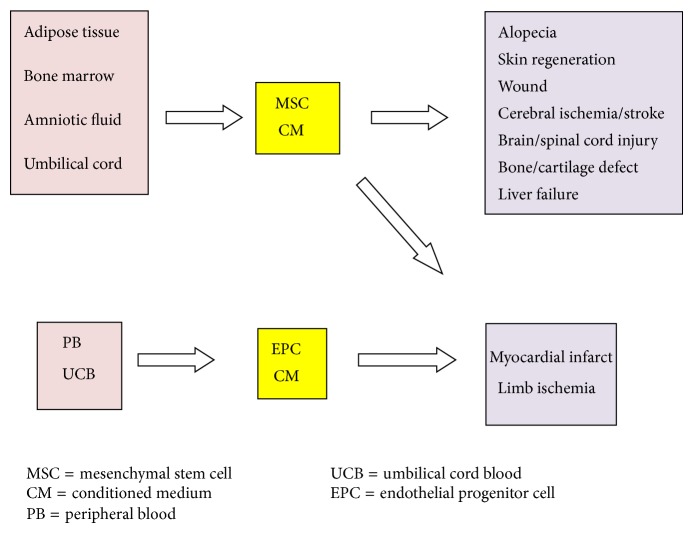
To use CM for various human diseases, production method of the CM needs to be standardized in terms of the type and number of cells that were needed to produce the CM, culture medium and condition, and conditioned medium processing. In addition, the volume and mode of delivery are also important. As various studies used various numbers and type of cells and various doses of CM, it is important to know the number of cells that yielded the CM for one application, which may be interpolated for human studies. Therefore, in Table 4 we summarized all data that may be needed for interpolation into human studies, that is, diseases that were treated, species and age or body weight of the animal, type of cell, culture medium and condition, number of cells to produce CM for one application, volume, and mode of application. Moreover, various possible applications of CM for various conditions are summarized in Figure 1.
Figure 1.
Various possible applications of CM for various conditions.
In addition, for translation into patients, it is very important to analyze and to note the various cytokine contents of the various conditioned media. Further, for every conditioned medium with known cytokine content, validation of its use on various diseases needs to be conducted. Finally, the possibility of promotion of existing cancer should be tested for every CM, and caution should be taken before CM therapy to ensure that the recipient is free from cancer.
Advantages of production of various CM for patients lie in the possibility of mass production by pharmaceutical companies, when production methods have been standardized. Conditioned media are not like stem cells that need a good manufacturing practice (GMP) facility to be applied to patients [47]. When CM has been packaged properly, it can be transported easily as drugs and does not need cryopreservation, such as that the stem cells need. However, compared to stem cells that may survive for a rather long period, CM needs to be given more frequently, as cytokines' and growth factors' half-lives are mostly shorter [48, 49], which is a disadvantage for the patients but will give more profit to pharmaceutical companies.
4. Conclusion
Various stem cell-derived conditioned media were produced by various methods and processing and tested on various diseases and mostly showed good results. However, standardized methods for various conditioned media production and validations of their use on various diseases need to be conducted.
Acknowledgment
This study was funded by the research grant from Indonesian Ministry of Education and Culture (Pusnas 2014), Contract no. 2218/H2.R12/HKP.05.00/2014.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Yang D., Wang W., Li L., Peng Y., Chen P., Huang H., Guo Y., Xia X., Wang Y., Wang H., Wang W. E., Zeng C. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059020.e59020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H. O., Choi S. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Engineering and Regenerative Medicine. 2013;10(3):93–101. doi: 10.1007/s13770-013-0010-7. [DOI] [Google Scholar]
- 3.Fukuoka H., Suga H., Narita K., Watanabe R., Shintani S. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. American Journal of Cosmetic Surgery. 2012;29(4):273–282. [Google Scholar]
- 4.Zhou B. R., Xu Y., Guo S. L., Wang Y., Zhu F., Permatasari F., Wu D., Yin Z. Q., Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Research International. 2013;2013 doi: 10.1155/2013/519126.519126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park B. S., Kim W. S., Choi J. S., Kim H. K., Won J. H., Ohkubo F., Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomedical Research. 2010;31(1):27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 6.Bhang S. H., Lee S., Shin J. Y., Lee T. J., Jang H. K., Kim B. S. Efficacious and clinically relevant conditioned-medium of human adipose-derived stem cells for therapeutic angiogenesis. Molecular Therapy. 2014;22(4):862. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho J. C. Y., Lai W., Li M., Au K., Yip M., Wong N. L. Y., Ng E. S. K., Lam F. F. Y., Siu C., Tse H. Reversal of endothelial progenitor cell dysfunction in patients with type 2 diabetes using a conditioned medium of human embryonic stem cell-derived endothelial cells. Diabetes/Metabolism Research and Reviews. 2012;28(5):462–473. doi: 10.1002/dmrr.2304. [DOI] [PubMed] [Google Scholar]
- 8.di Santo S., Yang Z., von Ballmoos M. W., Voelzmann J., Diehm N., Baumgartner I., Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005643.e5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirabella T., Cilli M., Carlone S., Cancedda R., Gentili C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials. 2011;32(15):3689–3699. doi: 10.1016/j.biomaterials.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Lee J. H., Yeo S. M., Chung H. M., Chae J. I. Stem cell recruitment factors secreted from cord blood-derived stem cells that are not secreted from mature endothelial cells enhance wound healing. In Vitro Cellular & Developmental Biology: Animal. 2014;50(2):146–154. doi: 10.1007/s11626-013-9687-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim J. Y., Song S. H., Kim K. L., Ko J. L., Im J. J., Yie S. W., Ahn Y. K., Kim D. K., Suh W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplantation. 2010;19(12):1635–1644. doi: 10.3727/096368910X516637. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha C., Zhao L., Chen K., He H., Mo Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology. 2013;2013 doi: 10.1155/2013/592454.592454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee M. J., Kim J., Lee K. I., Shin J. M., Chae J. I., Chung H. M. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy. 2011;13(2):165–178. doi: 10.3109/14653249.2010.512632. [DOI] [PubMed] [Google Scholar]
- 14.Mishra P. J., Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World Journal of Stem Cells. 2012;4(5):35–43. doi: 10.4252/wjsc.v4.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes B., Kumar A. H. S., O'Sullivan J., Klein Buneker C., Leblond A., Weiss S., Schmeckpeper J., Martin K., Caplice N. M. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. European Heart Journal. 2013;34(10):782–789. doi: 10.1093/eurheartj/ehr435. [DOI] [PubMed] [Google Scholar]
- 16.Timmers L., Lim S. K., Hoefer I. E., Arslan F., Lai R. C., van Oorschot A. A. M., Goumans M. J., Strijder C., Sze S. K., Choo A., Piek J. J., Doevendans P. A., Pasterkamp G., de Kleijn D. P. V. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Research. 2011;6(3):206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 17.See F., Seki T., Psaltis P. J., Sondermeijer H. P., Gronthos S., Zannettino A. C. W., Govaert K. M., Schuster M. D., Kurlansky P. A., Kelly D. J., Krum H., Itescu S. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. Journal of Cellular and Molecular Medicine. 2011;15(10):2117–2129. doi: 10.1111/j.1582-4934.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmers L., Lim S. K., Arslan F., Armstrong J. S., Hoefer I. E., Doevendans P. A., Piek J. J., El Oakley R. M., Choo A., Lee C. N., Pasterkamp G., de Kleijn D. P. V. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research. 2008;1(2):129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Du Z., Wei C., Cheng K., Han B., Yan J., Zhang M., Peng C., Liu Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. Journal of Surgical Research. 2013;183(2):907–915. doi: 10.1016/j.jss.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Zagoura D. S., Roubelakis M. G., Bitsika V., Trohatou O., Pappa K. I., Kapelouzou A., Antsaklis A., Anagnou N. P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. 2012;61(6):894–906. doi: 10.1136/gutjnl-2011-300908. [DOI] [PubMed] [Google Scholar]
- 21.van Poll D., Parekkadan B., Cho C. H., Berthiaume F., Nahmias Y., Tilles A. W., Yarmush M. L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo . Hepatology. 2008;47(5):1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 22.Parekkadan B., Van Poll D., Suganuma K., Carter E. A., Berthiaume F., Tilles A. W., Yarmush M. L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2(9, article e941) doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue T., Sugiyama M., Hattori H., Wakita H., Wakabayashi T., Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Engineering A. 2013;19(1-2):24–29. doi: 10.1089/ten.tea.2011.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho Y. J., Song H. S., Bhang S., Lee S., Kang B. G., Lee J. C., An J., Cha C. I., Nam D., Kim B. S., Joo K. M. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. Journal of Neuroscience Research. 2012;90(9):1794–1802. doi: 10.1002/jnr.23063. [DOI] [PubMed] [Google Scholar]
- 25.Bakondi B., Shimada I. S., Perry A., Munoz J. R., Ylostalo J., Howard A. B., Gregory C. A., Spees J. L. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Molecular Therapy. 2009;17(11):1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang T. J., Lin K. C., Chio C. C., Wang C. C., Chang C. P., Kuo J. R. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. Journal of Trauma and Acute Care Surgery. 2012;73(5):1161–1167. doi: 10.1097/TA.0b013e318265d128. [DOI] [PubMed] [Google Scholar]
- 27.Chang C., Chio C., Cheong C., Chao C., Cheng B., Lin M. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clinical Science. 2013;124(3):165–176. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 28.Cantinieaux D., Quertainmont R., Blacher S., Rossi L., Wanet T., Noël A., Brook G., Schoenen J., Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0069515.e69515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Koppen A., Joles J. A., van Balkom B. W. M., Lim S. K., de Kleijn D., Giles R. H., Verhaar M. C. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038746.e38746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gheisari Y., Ahmadbeigi N., Naderi M., Nassiri S. M., Nadri S., Soleimani M. Stem cell-conditioned medium does not protect against kidney failure. Cell Biology International. 2011;35(3):209–213. doi: 10.1042/CBI20100183. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W., Huang L., Li Y., Qian H., Shan X., Yan Y., Mao F., Wu X., Xu W. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10(18):3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]
- 32.Li L., Liu Y., Yang C., Chien Y., Twu N., Wang M., Wang C., Huang C., Kao K., Hsu H., Wu C., Chiou S. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials. 2013;34(1):78–91. doi: 10.1016/j.biomaterials.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 33.Inukai T., Katagiri W., Yoshimi R., Osugi M., Kawai T., Hibi H., Ueda M. Novel application of stem cell-derived factors for periodontal regeneration. Biochemical and Biophysical Research Communications. 2013;430(2):763–768. doi: 10.1016/j.bbrc.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 34.Ivanova-Todorova E., Bochev I., Dimitrov R., Belemezova K., Mourdjeva M., Kyurkchiev S., Kinov P., Altankova I., Kyurkchiev D. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. Journal of Biomedicine and Biotechnology. 2012;2012 doi: 10.1155/2012/295167.295167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sze S. K., de Kleijn D. P. V., Lai R. C., Tan E. K. W., Zhao H., Yeo K. S., Low T. Y., Lian Q., Lee C. N., Mitchell W., El Oakley R. M., Lim S. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular and Cellular Proteomics. 2007;6(10):1680–1689. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Sadat S., Gehmert S., Song Y., Yen Y., Bai X., Gaiser S., Klein H., Alt E. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical and Biophysical Research Communications. 2007;363(3):674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 37.Goswami R., Kaplan M. H. A brief history of IL-9. The Journal of Immunology. 2011;186(6):3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun Y. R., Won J. E., Jeon E., Lee S., Kang W., Jo H., Jang J. H., Shin U. S., Kim H. W. Fibroblast growth factors: biology, function, and application for tissue regeneration. Journal of Tissue Engineering. 2010;2010 doi: 10.4061/2010/218142..218142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeki T., Tanada M., Takashima S., Saeki H., Takiyama W., Nishimoto N., Moriwaki S. Correlation between expression of platelet-derived endothelial cell growth factor (thymidine phosphorylase ) and microvessel density in early-stage human colon carcinomas. Japanese Journal of Clinical Oncology. 1997;27(4):227–230. doi: 10.1093/jjco/27.4.227. [DOI] [PubMed] [Google Scholar]
- 40.Litwack G. Growth factors and cytokines. In: Litwack G., editor. Human Biochemistry and Disease. Elsevier Academic Press; 2008. pp. 587–683. [Google Scholar]
- 41.Park J. E., Chen H. H., Winer J., Houck K. A., Ferrara N. Placenta growth factor: potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. The Journal of Biological Chemistry. 1994;269(41):25646–25654. [PubMed] [Google Scholar]
- 42.Ray P., Devaux Y., Stolz D. B., Yarlagadda M., Watkins S. C., Lu Y., Chen L., Yang X., Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6098–6103. doi: 10.1073/pnas.1031851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner J. E., Morrison P. J., Wilhelm C., Wilson M., Ahlfors H., Renauld JC., Panzer U., Helmby H., Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth—induced lung inflammation. The Journal of Experimental Medicine. 2013;210(13):2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White G. E., Greaves D. R. Fractalkine: a survivor's guide chemokines as antiapoptotic mediators. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(3):589–594. doi: 10.1161/ATVBAHA.111.237412. [DOI] [PubMed] [Google Scholar]
- 45.Mancini A., Koch A., Whetton A. D., Tamura T. The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene. 2004;23(39):6581–6589. doi: 10.1038/sj.onc.1207841. [DOI] [PubMed] [Google Scholar]
- 46.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes and Development. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wuchter P., Bieback K., Schrezenmeier H., BornhΣuser M., Mɒller LP., B÷nig H., Wagner W., Meisel R., Pavel P., Tonn T., Lang P., Mɒller I., Renner M., Malcherek G., Saffrich R., Buss EC., Horn P., Rojewski M., Schmitt A., AD Ho., Sanzenbacher R. Standardization of Good Manufacturing Practice–compliant production of bone marrow–derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy. 2014 doi: 10.1016/j.jcyt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Yde P., Mengel B., Jensen M. H., Krishna S., Trusina A. Modeling the NF-κB mediated inflammatory response predicts cytokine waves in tissue. BMC Systems Biology. 2011;5, article 115 doi: 10.1186/1752-0509-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khosravi A., Cutler C. M., Kelly M. H., Chang R., Royal R. E., Sherry R. M., Wodajo F. M., Fedarko N. S., Collins M. T. Determination of the elimination half-life of fibroblast growth factor-23. The Journal of Clinical Endocrinology & Metabolism. 2007;92(6):2374–2377. doi: 10.1210/jc.2006-2865. [DOI] [PubMed] [Google Scholar]