Abstract
Background.
The coordination of steady state walking is relatively automatic in healthy humans, such that active attention to the details of task execution and performance (controlled processing) is low. Somatosensation is a crucial input to the spinal and brainstem circuits that facilitate this automaticity. Impaired somatosensation in older adults may reduce automaticity and increase controlled processing, thereby contributing to deficits in walking function. The primary objective of this study was to determine if enhancing somatosensory feedback can reduce controlled processing during walking, as assessed by prefrontal cortical activation.
Methods.
Fourteen older adults (age 77.1±5.56 years) with mild mobility deficits and mild somatosensory deficits participated in this study. Functional near-infrared spectroscopy was used to quantify metabolic activity (tissue oxygenation index, TOI) in the prefrontal cortex. Prefrontal activity and gait spatiotemporal data were measured during treadmill walking and overground walking while participants wore normal shoes and under two conditions of enhanced somatosensation: wearing textured insoles and no shoes.
Results.
Relative to walking with normal shoes, textured insoles yielded a bilateral reduction of prefrontal cortical activity for treadmill walking (ΔTOI = −0.85 and −1.19 for left and right hemispheres, respectively) and for overground walking (ΔTOI = −0.51 and −0.66 for left and right hemispheres, respectively). Relative to walking with normal shoes, no shoes yielded lower prefrontal cortical activity for treadmill walking (ΔTOI = −0.69 and −1.13 for left and right hemispheres, respectively), but not overground walking.
Conclusions.
Enhanced somatosensation reduces prefrontal activity during walking in older adults. This suggests a less intensive utilization of controlled processing during walking.
Key Words: Walking, Somatosensory disorders, Spectroscopy, near infrared, Aging
Impaired somatosensation is common in older adults and is known to be linked to decrements in walking function (1–3). Enhancing somatosensation using specialized footwear has shown promise for improving balance and walking ability. Using vibrating insoles, Priplata and colleagues (4) demonstrated reduced postural sway during standing balance in older adults. Similarly, Palluel and colleagues (5,6) found that textured insoles improved sway parameters for both walking and standing balance in older adults. Although the potential functional benefits of enhancing somatosensory feedback are encouraging, the mechanism of action remains unclear.
Somatosensory information is considered to be a crucial input to circuits in the brainstem and spinal cord that contribute to intermuscular coordination during walking (7–9). In this role, somatosensory information facilitates “automaticity” of walking. Automaticity refers to the ability to perform complex tasks without attentional effort (10). The opposite of automaticity is “controlled processing,” (10) which involves active (intentionally directed) attention to the details of task execution and performance. Accumulating evidence indicates that aging leads to a shift from automaticity to controlled processing of motor behaviors, including for walking. Specifically, a number of imaging studies have reported that older adults rely on motor control strategies that involve heightened activity in cerebral regions that process attentional, visual, vestibular, and/or somatosensory information (11–15). A heightened utilization of controlled processing for walking in older adults is also suggested by compromised walking performance with dual-tasking (16). Compared with automaticity, controlled processing is slower, more effortful, and more susceptible to interference (17). Age-related loss of automaticity and a consequent increase in controlled processing may, therefore, be a mechanism by which somatosensory impairment accounts for walking deficits in older adults.
Here we investigate the hypothesis that enhancing somatosensory feedback during walking in older adults will reduce the utilization of controlled processing, as estimated by prefrontal cortical activity. Prefrontal cortex is considered to be an important region for attentional processes contributing to motor behavior (18), including walking (19–21). We also hypothesize that enhancing somatosensory feedback will shift spatiotemporal gait characteristics toward a less cautious walking pattern.
Methods
Participants
Older adults with mild mobility deficits were recruited for this study. Inclusion criteria for this study included age between 65 and 85 years; body mass index within the range of 19–35; 400 m walking speed less than 1.1 m/s; Berg Balance Test (22) score ≥ 41; Mini-Mental State Examination (23) score ≥ 21; and agreement with the statement: “You find it physically tiring to walk a quarter mile, or climb two flights of stairs, or perform household chores.” Exclusion criteria included use of an assistive device for walking; lower extremity pain while walking; symptomatic cardiovascular disease in the past year; bone fracture in the past year; injury or illness to the central nervous system; uncontrolled hypertension; or terminal illness. Study procedures were approved by the University of Florida Institutional Review Board, and participants provided written informed consent.
Equipment and Materials
A split-belt treadmill with embedded force plates (Bertec Corporation, Columbus, OH) and a GAITRite instrumented walkway (CIR Systems, Sparta, NJ) were used for acquiring spatiotemporal gait measurements. Participants wore a safety harness to protect against falling. Overground walking was performed by taking five consecutive laps around an ~20-m course, for a total walking distance of almost 100 m. The floor surface on the course was smooth tile, with the exception of the 5.2-m instrumented walkway which has the texture of firm foam. Enhanced somatosensory feedback was provided by two separate walking tasks: walking with no shoes (socks were permitted) and walking with a textured insole inside each person’s normal shoes. The textured insole was made of thin semi-rigid plastic with firm raised (1.5 mm) nodules spaced 1.5 cm apart in a grid pattern (Figure 1). An averaged sized insole had about 60 nodules.
Figure 1.
Textured insole. Enhanced somatosensory feedback was provided by small, firm protrusions on a flexible plastic insole.
Metabolic activity of the left and right prefrontal cortices was assessed with a commercially available fNIRS monitor (Niro 200NX, Hamamatsu Photonics, Japan). Each set of optodes (ie, for left and right prefrontal cortex) was placed high on the forehead (but not over hair) to avoid the temporalis muscle and sufficiently lateral from the midline to avoid the superior sagittal sinus (24). Spacing between source and detector optodes was 3cm. Cortical activity was quantified as the tissue oxygenation index (TOI), which is the ratio of oxygenated hemoglobin to total hemoglobin (sum of oxygenated and deoxygenated hemoglobin). The TOI provides a real time measure of the balance between cerebral oxygen delivery and utilization (24). For each walking trial, TOI was averaged over the period of steady state walking. Exemplar TOI and hemoglobin data during a walking trial are presented in Figure 2.
Figure 2.
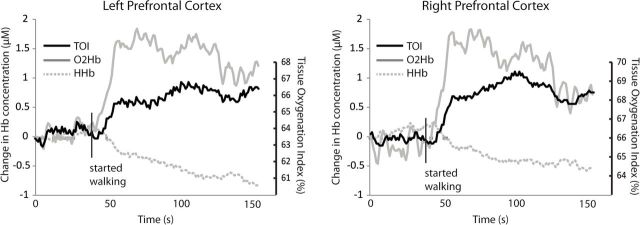
Exemplar data for tissue oxygenation index (TOI) and hemoglobin content during walking. Changes in brain metabolic activity in response to walking are indicated by altered TOI (black solid line), oxygenated hemoglobin (O2Hb; gray solid line), and deoxygenated hemoglobin (HHb, gray dashed line). The participant is standing still for a short period of time at the start of the trial and then walks at steady state preferred speed until nearly the end of the trial.
Protocol
Testing was conducted over two separate visits to our research center. Functional and somatosensory assessments occurred during the first visit. Cutaneous tactile perception was tested using a Semmes–Weinstein 5.07 (10 g) monofilament at four sites on the plantar surface of each foot: great toe, first metatarsal head, heel, and fifth metatarsal head. Vibratory perception was tested on the dorsal surface of each great toe just proximal to the nail bed using a 128-Hz tuning fork. We have reported previously detailed methodology for somatosensory testing (1).
All fNIRS and spatiotemporal measurements were obtained during the second visit to our research center. For all trials, participants walked at their own preferred speed for 60–120 seconds. Preferred walking speed on the treadmill was determined by first increasing belt speed in 0.1 m/s increments until it was faster than the participant’s preferred speed, then reducing belt speed until preferred speed was achieved. For each participant, treadmill speed remained identical across all walking trials. Both treadmill and overground walking were performed under the following tasks: (i) with normal shoes, (ii) with no shoes, (iii) with a textured insole, and (iv) with normal shoes while performing a verbal fluency task (dual-tasking). The normal shoe task was conducted at both the beginning and the end of the treadmill testing period to assess whether treadmill familiarization may account for any observed changes in cortical activity. Dual-tasking was used to provide context for the magnitude and direction of the hypothesized changes in cortical activity. The verbal fluency task required the participant to name as many words as possible that began with a particular letter assigned by the examiner.
Gait variables that were assessed for overground and treadmill tasks included walking speed, step length, double support time, variability of step length, and variability of double support time. These variables have been shown previously to be sensitive to age-related changes in walking performance (25,26).
Statistics
For fNIRS TOI, two separate two-way (task × side) repeated measures analyses of variance (ANOVAs) were conducted. The first was for treadmill walking and the second for overground walking. Each contained all four walking tasks. For spatiotemporal gait variables, a separate two-way (task × side) repeated measures ANOVA with all four tasks was conducted for each variable. In the event of significant main effects, paired t tests were used for post hoc comparison of each experimental tasks (no shoes, insoles, and dual-task) to the control task (normal shoes). Experimental tasks were not compared to each other. Single-sided paired t tests were used to test the hypothesis that, relative to normal walking, TOI would be reduced for the no-shoes and insoles tasks and increased for dual-tasking. For all tasks, two-sided paired t tests were used to examine effects of task for gait spatiotemporal measurements. Statistical significance for all tests was accepted at p < .05.
Results
Participants
Fourteen older men (n = 7) and older women (n = 7) participated. All were Caucasian with the exception of one Hispanic participant. Information about physical function and cognitive status are presented in Table 1. No participants scored perfectly on the somatosensory assessments and most exhibited substantial evidence of impairment. The group mean percentage of correct responses was 83.3% ± 19.9% for tactile perception and 61.5% ± 28.6% for vibratory perception. A small amount of data was missing at random from the final analyses because: (i) a few individuals did not perform all walking tasks due to time constraints, (ii) a malfunction in the fNIRS equipment occasionally caused overwriting of data, and (iii) a malfunction in the gait mat software occasionally prevented data capture. Each comparison includes data from at least 11 of the 14 participants.
Table 1.
Participant Demographics and Functional Assessments
| Mean | Range | |
|---|---|---|
| Age (y) | 77.1±5.56 | 66–85 |
| Body mass index (kg/m2) | 28.7±3.6 | 20.0–33.8 |
| Mini-Mental State Examination score | 27.3±1.7 | 24–30 |
| Berg balance test score | 50.8±4.0 | 42–56 |
| 400m walk speed (m/s) | 0.92±0.11 | 0.66–1.08 |
Walking With Functional Near Infrared Spectroscopy
Repeated assessments of treadmill walking in normal shoes at the beginning and the end of the session yielded no significant difference in cortical activity for the left hemisphere (ΔTOI = 0.33 ± 0.28 points, p = .21) or right hemisphere (ΔTOI = 0.10 ± 0.29 points, p = .70), and thus no evidence of habituation to treadmill walking. The data for these two trials were averaged for subsequent analysis. When walking in normal shoes, treadmill walking elicited a higher level of prefrontal cortical activity than did overground walking in the right hemisphere (ΔTOI = 1.16 ± 0.46 points, p = .02), with a similar but nonsignificant trend in the left hemisphere (ΔTOI = 0.63 ± 0.51 points, p = .19). For this reason, overground and treadmill walking were evaluated in separate statistical models.
For treadmill walking, repeated measures ANOVA revealed a significant main effect for task (p = .002), with no task × side interaction (p = .72). Post hoc analysis revealed that, relative to treadmill walking with normal shoes, treadmill walking with textured insoles yielded lower prefrontal cortical activity in the left hemisphere (ΔTOI = −0.85 ± 0.35 points, p = .01) and right hemisphere (ΔTOI = −1.19 ± 0.46 points, p = .01). Similarly, treadmill walking with no shoes also yielded lower prefrontal cortical activity in the left hemisphere (ΔTOI = −0.69 ± 0.24 points, p < .004), and right hemisphere (ΔTOI = −1.13 ± 0.38 points, p < .003). There was no significant change in prefrontal activity for dual-tasking (p > .70).
For overground walking, repeated measures ANOVA revealed a significant main effect for task (p = .0005), with no task × side interaction (p = .85). Post hoc analysis revealed that, relative to overground walking with normal shoes, overground walking with textured insoles yielded lower prefrontal cortical activity in the left hemisphere (ΔTOI=−0.51 ± 0.16 points, p < .003) and right hemisphere (ΔTOI = −0.66 ± 0.26 points, p = .008). Overground walking with normal shoes and no shoes did not differ significantly (p > .81). Dual-tasking increased prefrontal cortical activity in the left hemisphere (ΔTOI = 0.64 ± 0.32 points, p < .03) and right hemisphere (ΔTOI = 0.72 ± 0.34 points, p < .02). Exemplar data showing differences between walking in normal shoes, insoles, and no shoes are presented in Figure 3. Group mean results are presented in Figure 4.
Figure 3.
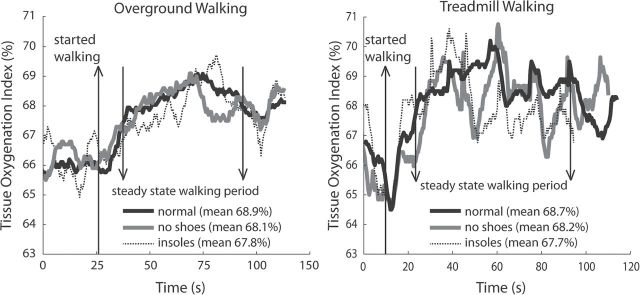
Exemplar data demonstrating task-dependent differences in tissue oxygenation index (TOI). Changes in prefrontal metabolic activity are indicated by TOI during normal walking (black solid line), walking with no shoes (gray solid line) and walking with textured insoles (gray dashed line). The participant is standing still for a short period of time at the start of the trial and then walks at preferred speed until nearly the end of the trial.
Figure 4.
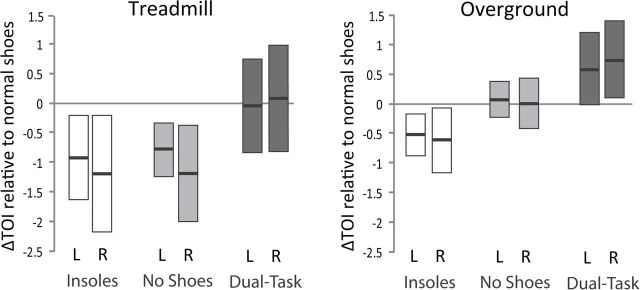
Change in tissue oxygenation index (TOI) relative to walking in normal shoes for left (L) and right (R) prefrontal cortex. When walking with textured insoles, TOI was reduced for both treadmill and overground walking. When walking with no shoes, TOI was reduced during treadmill walking but not overground walking. Dual-tasking increased TOI for overground but not treadmill walking. For each bar, group means are indicated by the bold central lines, and 95% confidence intervals are indicated by the upper and lower margins.
Gait Spatiotemporal Data
On average, 6.7 ± 0.8 steps were captured for each pass over the instrumented walkway. Since each task included five laps on the course, ~30 steps of data were attained per task for each participant. For treadmill walking, the average number of steps was 60.4 ± 9.6. Repeated assessment of walking with normal shoes yielded no statistically significant differences in any spatiotemporal variable (p range from .12 to .89). The data for these two trials were averaged for subsequent analysis. The repeated measures ANOVA models for each treadmill gait variable indicated a significant main effect of task (p < .05) but not side (p > .05). Relative to treadmill walking in normal shoes, treadmill walking with no shoes resulted in a slightly shorter step length (−0.9 ± 0.4cm or −2%, p = .0030), less time spent in double limb support (−20 ± 3ms or −7%, p < .0001) and a smaller percentage of the gait cycle spent in double support (−1.0% ± 0.2%, p < .001). No differences were observed for percent of gait cycle spent in double support or for variability of step length or double support time. For treadmill walking with textured insoles, no significant differences were observed for any variable relative to treadmill walking in normal shoes. Treadmill dual-tasking led to a small reduction in step length (−1.0 ± 0.6cm or −2.5%, p = .03) and increased variability of step length (17% ± 8%, p = .03) and variability of double support time (40% ± 12%, p = .01).
The repeated measures ANOVA models for each overground gait variable indicated a significant main effect of task (p < .05) but not side (p > .05). Relative to overground walking with normal shoes, overground walking with no shoes led to small reductions in walking speed (−0.04 ± 0.01 m/s, or −4%, p = .01) and step length (−4.7 ± 0.5cm or −8.5%, p < .001). Also reduced were double support time (−56 ± 7ms or −15%, p < .001) and percent (−12% ± 2%, p < .001). For walking with insoles, only walking speed (−0.08 ± 0.02 m/s, or −8%, p = .02) and step length (−2.3 ± 0.5cm or −4%, p < .001) were reduced relative to walking with normal shoes. Dual tasking resulted in considerable changes for all spatiotemporal measures, including slower walking speed (−0.14 ± 0.02 m/s, or −14%, p < .001), shorter step length (−4.5 ± 0.9cm or −8%, p < .001), increased double support time (55 ± 9ms or +14%, p < .001) and percent of gait cycle (7% ± 2%, p < .001), and increased variability of step length (21% ± 9%, p = .03) and double support time (24% ± 9%, p = .03).
Discussion
The primary finding of this study is that enhanced somatosensory feedback during walking reduced metabolic activity of the prefrontal cortex. We propose that this finding is due to lower utilization of attention-demanding controlled processing during walking. We did not observe any clear improvement in spatiotemporal gait measures. It may be that the short duration of walking with enhanced somatosensation was insufficient for inducing altered coordination.
When walking with textured insoles, a reduction in prefrontal cortical activity was observed relative to walking in normal shoes during both overground and treadmill walking. A reduction in cortical activity was also observed when walking without shoes during treadmill walking, but not overground walking. Overall, the effect of enhanced somatosensory feedback on cortical activity was more pronounced for treadmill walking than for overground walking. This may be due, at least in part, to the finding that normal walking on the treadmill required greater cortical activity than did normal overground walking. Because of this greater activation level, we propose that treadmill walking provided the better opportunity for a potent benefit from enhanced somatosensory feedback. In addition, we note that almost one third of the overground walking course consisted of an instrumented walkway that had a softer texture. Perhaps this attenuated the somatosensory feedback when walking with no shoes, contributing to our finding of an absence of change in cortical activity for that task.
A dual-task paradigm (walking plus a verbal fluency task) was also conducted to provide a reference task by which to interpret the magnitude and direction of changes in prefrontal cortical activity. We anticipated increased metabolic activity during the dual-tasking paradigm relative to normal walking because the verbal fluency task requires attentional processing resources in the prefrontal cortex (27,28). This finding was confirmed for overground walking, in which prefrontal TOI increased ~0.68 points (average of left and right hemispheres). The magnitude of this change is comparable, but in the opposite direction, to the ~0.58 points reduction of TOI observed when walking with textured insoles. This result supports the assertion of a meaningful reduction in prefrontal cortical activity during overground walking with textured insoles. However, we did not observe the same result for treadmill walking because cortical activity was unaffected by dual-tasking. Heightened cortical activity during normal treadmill walking (as discussed earlier) may have occluded our ability to detect a further increase in response to the additional attentional demands of the verbal fluency task.
Further interpretation of the magnitude of change for TOI can be made from prior literature. One study demonstrated that clamping of the internal carotid artery led to a reduction in prefrontal TOI of 8.2 points (29). The authors further suggested that a reduction in TOI of 13 points indicates severe cerebral ischemia. A separate study showed that prefrontal TOI decreased 7.1 points under induced hypoxemia and 2.1 points under induced hypocapnia (which causes hypoxia). (24) Based on these earlier findings, fluctuations in TOI under normal physiological conditions could be expected to change only a small amount, such as by a few percentage points. Cumulatively, this evidence suggests that the changes in TOI observed in our study could be meaningful.
It is well known that somatosensory impairment is linked to compromised mobility function. This is concerning due to the high prevalence of somatosensory impairment in older adults. As quantified by clinical assessment methods (ie, monofilament and tuning fork assessments of tactile and vibratory perception), bilateral somatosensory deficits are present in ~26% of individuals 65–74 years of age, 36% of individuals 75–84 years and 54% of individuals ≥85 years of age (2). However, it is not clear whether enhancing somatosensory feedback enhances mobility. Prior studies have provided enticing evidence suggesting improvements on measures of postural stability during walking and standing balance with the use of vibrating or textured insoles (4–6). Furthermore, temporary reduction of somatosensation on the plantar surface of the foot (using local anesthesia or cold water immersion) has been shown to adversely affect the kinematics of gait (30,31). We sought to expand this area of research by examining the extent to which enhanced somatosensory feedback alters gait. The focus of our analysis was on variables that are typically used to infer the presence of a cautious gait pattern in older adults. These include factors such as slower speed, shorter step length, longer double support phase, and increased variability (32,33). Relative to normal walking, the dual-task condition clearly exhibited all of these features. This is consistent with prior literature on the consequences of dual-tasking in older adults (34,35). However, for the enhanced somatosensory feedback tasks, we observed no major evidence of an immediate benefit for the temporal characteristics of walking. Contrary to our hypothesis, walking with enhanced somatosensory feedback led to small but statistically significant reductions in walking speed and/or step length. These changes are generally in the direction of a more cautious gait pattern, although the small magnitude probably does not constitute a clinically meaningful change (36). Walking with no shoes did provide some evidence of a less cautious gait pattern, as there was less time spent in the double support phase of gait. However, it is not clear whether this change was due to altered somatosensation or to a preference for walking with modified biomechanics when not wearing shoes (37). Future studies should be conducted to determine whether longer exposures to enhanced somatosensory feedback would allow the nervous system to better use this information to enhance coordination of walking and yield improvements in spatiotemporal measures of gait. It may also be the case that more challenging mobility tasks (ie, requiring greater attentional demand) might be more likely to show improvement.
There are a number of methodological factors that should be considered when interpreting the results of this study. We examined a cohort of older adults with mild mobility deficits and mild somatosensory impairment in the feet. Accumulating literature suggests a heightened attentional demand for walking in persons with these characteristics (38–40). For example, performance decrements during dual-tasking were shown to be worse in older adult diabetic patients with peripheral neuropathy compared with patients without peripheral neuropathy (40). Thus, our participants may be particularly good candidates for somatosensory enhancement paradigms. It is also important to note that the role of the prefrontal cortex in control of walking is not fully understood, and we did not measure activity in other brain regions that could be relevant to our research question. Although we focused on gait temporal measurement as a potential benefit, there may also be benefits for outcomes that we did not measure. For example, reducing motor processing demands in the prefrontal cortex may free up resources that are necessary for attending to hazardous features of the environment that contribute to falls.
In conclusion, our findings support the hypothesis that enhanced somatosensory feedback of the feet reduces prefrontal metabolic activity. This finding is consistent with lower utilization of attention-demanding controlled processing during walking in older adults. More research will be needed to confirm this point, such as by conducting group-wise comparisons of prefrontal activity in cohorts with different levels of somatosensory function. Additional research is also needed to better understand the implications of our findings on the mechanics and safety of mobility in older adults.
Funding
This work was supported by the North Florida/South Georgia Veterans Health System Rehabilitation Research and Development Service (B7176W), National Institute on Aging via the University of Florida Claude Pepper Older Americans Independence Center (2P30AG028740-06), and University of Florida McKnight Brain Research Foundation endowment to the Cognitive Aging and Memory Clinical Translational Research Program.
References
- 1. Cruz-Almeida Y, Black ML, Christou EA, Clark DJ. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front Aging Neurosci. 2014;6:68. :10.3389/fnagi.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318. [DOI] [PubMed] [Google Scholar]
- 3. Deshpande N, Ferrucci L, Metter J, et al. Association of lower limb cutaneous sensitivity with gait speed in the elderly: the health ABC study. Am J Phys Med Rehabil. 2008;87:921–928. :10.1097/PHM.0b013e31818a5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet. 2003;362:1123–1124. :10.1016/S0140-6736(03)14470-4 [DOI] [PubMed] [Google Scholar]
- 5. Palluel E, Nougier V, Olivier I. Do spike insoles enhance postural stability and plantar-surface cutaneous sensitivity in the elderly? Age (Disorder). 2008;30:53–61. :10.1007/s11357-008-9047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palluel E, Olivier I, Nougier V. The lasting effects of spike insoles on postural control in the elderly. Behav Neurosci. 2009;123:1141–1147. :10.1037/a0017115 [DOI] [PubMed] [Google Scholar]
- 7. Frigon A, Rossignol S. Experiments and models of sensorimotor interactions during locomotion. Biol Cybern. 2006;95:607–627. :10.1007/s00422-006-0129-x [DOI] [PubMed] [Google Scholar]
- 8. Hultborn H, Nielsen JB. Spinal control of locomotion—from cat to man. Acta Physiol (Oxf). 2007;189:111–121. [DOI] [PubMed] [Google Scholar]
- 9. Kargo WJ, Giszter SF. Afferent roles in hindlimb wipe-reflex trajectories: free-limb kinematics and motor patterns. J Neurophysiol. 2000;83:1480–1501. [DOI] [PubMed] [Google Scholar]
- 10. Schneider W, Shiffrin RM. Controlled and automatic human information processing. I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- 11. Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. :10.1523/JNEUROSCI.1263-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. :10.1523/JNEUROSCI.3300-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattay VS, Fera F, Tessitore A, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. [DOI] [PubMed] [Google Scholar]
- 14. Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33:1073–1084. :10.1016/j.neurobiolaging.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 15. Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol A Biol Sci Med Sci. 2013. :10.1093/gerona/glt207 [DOI] [PubMed] [Google Scholar]
- 16. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–3–42. :10.1002/mds.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider W, Chein JM. Controlled and automatic processing: behavior, theory, and biological mechanisms. Cognit Sci. 2003;27:525–559. [Google Scholar]
- 18. Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. [DOI] [PubMed] [Google Scholar]
- 19. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014. :10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. :10.1093/ageing/afr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atsumori H, Kiguchi M, Katura T, et al. Noninvasive imaging of prefrontal activation during attention-demanding tasks performed while walking using a wearable optical topography system. J Biomed Opt. 2010;15:046002. :10.1117/1.3462996 [DOI] [PubMed] [Google Scholar]
- 22. Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–311. [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24. Tisdall MM, Taylor C, Tachtsidis I, Leung TS, Elwell CE, Smith M. The effect on cerebral tissue oxygenation index of changes in the concentrations of inspired oxygen and end-tidal carbon dioxide in healthy adult volunteers. Anesth Analg. 2009;109:906–913. :10.1213/ane.0b013e3181aedcdc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callisaya ML, Blizzard L, McGinley JL, Schmidt MD, Srikanth VK. Sensorimotor factors affecting gait variability in older people–a population-based study. J Gerontol A Biol Sci Med Sci. 2010;65:386–392. :10.1093/gerona/glp184 [DOI] [PubMed] [Google Scholar]
- 26. Montero-Odasso M, Casas A, Hansen KT, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. :10.1186/1743-0003-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doi T, Makizako H, Shimada H, et al. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res. 2013;25:539–544. :10.1007/s40520-013-0119-5 [DOI] [PubMed] [Google Scholar]
- 28. Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66:879–887. :10.1093/gerona/glr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Rawi PG, Kirkpatrick PJ. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke. 2006;37:2720–2725. :10.1161/01.STR.0000244807.99073.ae [DOI] [PubMed] [Google Scholar]
- 30. Höhne A, Ali S, Stark C, Brüggemann GP. Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. Eur J Appl Physiol. 2012;112:3829–3838. :10.1007/s00421-012-2364-2 [DOI] [PubMed] [Google Scholar]
- 31. Eils E, Behrens S, Mers O, Thorwesten L, Völker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern. Gait Posture. 2004;20:54–60. :10.1016/S0966-6362(03)00095-X [DOI] [PubMed] [Google Scholar]
- 32. Giladi N, Herman T, Reider G, II, Gurevich T, Hausdorff JM. Clinical characteristics of elderly patients with a cautious gait of unknown origin. J Neurol. 2005;252:300–306. :10.1007/s00415-005-0641-2 [DOI] [PubMed] [Google Scholar]
- 33. Tersteeg MC, Marple-Horvat DE, Loram ID. Cautious gait in relation to knowledge and vision of height: is altered visual information the dominant influence? J Neurophysiol. 2012;107:2686–2691. :10.1152/jn.00875.2011 [DOI] [PubMed] [Google Scholar]
- 34. Bridenbaugh SA, Kressig RW. Laboratory review: the role of gait analysis in seniors’ mobility and fall prevention. Gerontology. 2011;57:256–264. :10.1159/000322194 [DOI] [PubMed] [Google Scholar]
- 35. Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. :10.1016/S0966-6362(01)00156-4 [DOI] [PubMed] [Google Scholar]
- 36. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. :10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Paquette MR, Zhang S. A comparison of gait biomechanics of flip-flops, sandals, barefoot and shoes. J Foot Ankle Res. 2013;6:45. :10.1186/1757-1146-6-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beauchet O, Annweiler C, Dubost V, et al. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–795. :10.1111/j.1468-1331.2009.02612.x [DOI] [PubMed] [Google Scholar]
- 39. Manor B, Newton E, Abduljalil A, Novak V. The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes Care. 2012;35:1907–1912. :10.2337/dc11-2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B. The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabet Med. 2009;26:234–239. :10.1111/j.1464-5491.2008.02655.x [DOI] [PubMed] [Google Scholar]


