Abstract
Background
The burden of coronary heart disease (CHD) worldwide is one of great concern to patients and healthcare agencies alike. Exercise-based cardiac rehabilitation aims to restore patients with heart disease to health.
Objectives
To determine the effectiveness of exercise-based cardiac rehabilitation (exercise training alone or in combination with psychosocial or educational interventions) on mortality, morbidity and health-related quality of life of patients with CHD.
Search methods
RCTs have been identified by searching CENTRAL, HTA, and DARE (using The Cochrane Library Issue 4, 2009), as well as MEDLINE (1950 to December 2009), EMBASE (1980 to December 2009), CINAHL (1982 to December 2009), and Science Citation Index Expanded (1900 to December 2009).
Selection criteria
Men and women of all ages who have had myocardial infarction (MI), coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA), or who have angina pectoris or coronary artery disease defined by angiography.
Data collection and analysis
Studies were selected and data extracted independently by two reviewers. Authors were contacted where possible to obtain missing information.
Main results
This systematic review has allowed analysis of 47 studies randomising 10,794 patients to exercise-based cardiac rehabilitation or usual care. In medium to longer term (i.e. 12 or more months follow-up) exercise-based cardiac rehabilitation reduced overall and cardiovascular mortality [RR 0.87 (95% CI 0.75, 0.99) and 0.74 (95% CI 0.63, 0.87), respectively], and hospital admissions [RR 0.69 (95% CI 0.51, 0.93)] in the shorter term (< 12 months follow-up) with no evidence of heterogeneity of effect across trials. Cardiac rehabilitation did not reduce the risk of total MI, CABG or PTCA. Given both the heterogeneity in outcome measures and methods of reporting findings, a meta-analysis was not undertaken for health-related quality of life. In seven out of 10 trials reporting health-related quality of life using validated measures was there evidence of a significantly higher level of quality of life with exercise-based cardiac rehabilitation than usual care.
Authors’ conclusions
Exercise-based cardiac rehabilitation is effective in reducing total and cardiovascular mortality (in medium to longer term studies) and hospital admissions (in shorter term studies) but not total MI or revascularisation (CABG or PTCA). Despite inclusion of more recent trials, the population studied in this review is still predominantly male, middle aged and low risk. Therefore, well-designed, and adequately reported RCTs in groups of CHD patients more representative of usual clinical practice are still needed. These trials should include validated health-related quality of life outcome measures, need to explicitly report clinical events including hospital admission, and assess costs and cost-effectiveness.
Medical Subject Headings (MeSH): *Exercise Therapy, Coronary Disease [mortality; *rehabilitation], Health Status, Myocardial Infarction [mortality; rehabilitation], Myocardial Revascularization [statistics & numerical data], Outcome Assessment (Health Care), Quality of Life, Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Male
BACKGROUND
Description of the condition
Cardiovascular disease accounts for one-third of deaths globally, with 7.22 million deaths from coronary heart disease (CHD) in 2002 (WHO 2004). In Europe, CHD is the most common cause of death and in the UK it accounts for one in five deaths in men and one in six deaths in women (British Heart Foundation 2005; Peterssen 2005). Although the mortality rate from CHD has been falling in the UK, principally due to a reduction in risk factors, particularly smoking, it has fallen less than in many other developed countries (Peterssen 2005). Treatments to individuals, including secondary prevention, explain about 42% of the decline in CHD mortality in the 1980s and 1990s (Unal 2000).
Description of the intervention
Cardiac rehabilitation has been defined as the “coordinated sum of interventions required to ensure the best physical, psychological and social conditions so that patients with chronic or post-acute cardiovascular disease may, by their own efforts, preserve or resume optimal functioning in society and, through improved health behaviours, slow or reverse progression of disease” (Fletcher 2001). It is a complex intervention that may involve a variety of therapies, including exercise, risk factor education, behaviour change, psychological support, and strategies that are aimed at targeting traditional risk factors for cardiovascular disease. Cardiac rehabilitation is an essential part of contemporary heart disease care and is considered a priority in countries with a high prevalence of CHD. International clinical guidelines consistently identify exercise therapy as a central element of cardiac rehabilitation (Balady 2007; Graham 2007; NICE 2007) i.e. ‘exercise-based cardiac rehabilitation’.
Despite the recommendations for exercise-based cardiac rehabilitation as an integral component of comprehensive cardiac care of patients with CHD (particularly those following myocardial infarction, revascularization or with angina pectoris) and heart failure, most patients do not receive it (Bethall 2008). Service provision, though predominantly hospital based, varies markedly, and referral, enrolment and completion are suboptimal, especially among women and older people (Beswick 2004). Costs of cardiac rehabilitation services vary by format of delivery. The UK survey suggests that costs can range of £50 to £712 per patient treated depending on the level of staffing, the equipment used and the intensity of the programme (Evans 2002).
Previous meta-analyses of the effects of exercise-based cardiac rehabilitation for CHD patients reported a statistically significant reduction in total and cardiac mortality, ranging from 20% to 32%, in patients receiving exercise therapy compared with usual medical care (Clark 2005; Jolliffe 2001; Oldridge 1988; O’Connor 1989). However, the evidence for psychological interventions is less convincing. A Cochrane review showed no evidence of an effect on total mortality, cardiac mortality, or revascularisation although there was a significant reduction in the number of non-fatal infarctions in the psychological intervention group (OR 0.78 [95% CI 0.67 to 0.90]) compared to usual care (Rees 2004). A Cochrane review of the effect of educational interventions for CHD is currently being undertaken (Brown 2010).
How the intervention might work
Exercise training has been shown to have direct benefits on the heart and coronary vasculature, including myocardial oxygen demand, endothelial function, autonomic tone, coagulation and clotting factors, inflammatory markers, and the development of coronary collateral vessels (Clausen 1976; Hambrecht 2000). However, findings of the original Cochrane review of exercisebased cardiac rehabilitation for CHD supported the hypothesis that reductions in mortality may also be mediated via the indirect effects of exercise through improvements in the risk factors for atherosclerotic disease (i.e. lipids, smoking and blood pressure) (Taylor 2006).
Why it is important to do this review
Our original Cochrane review published in 2001 identified a total of 35 RCTs in some 8,440 patients (Jolliffe 2001). This review reported a reduction in total mortality (random effects model, odds ratio: 0.73, 95% confidence interval: 0.54 to 0.98) with exercise intervention compared to usual care. Improvements with exercise were also seen in cardiac death, non-fatal MI, lipid profile and blood pressure. However, the authors identified a number a limitations in the evidence base:
Trials enrolled almost exclusively low-risk, middle-aged men after myocardial infarction. The exclusion or under representation of women, elderly people, and other cardiac groups (post revascularization and angina pectoris) not only limits the applicability of the evidence to contemporary cardiovascular practice but also fails to consider those who may benefit most from rehabilitation.
The widespread introduction of a variety of drug therapies as part of the routine management of CHD the cardiac patient that were not available at the time of the earliest trials may offset the magnitude of benefit associated with exercise-based rehabilitation.
It was unclear whether comprehensive (exercise plus psychosocial and/or educational interventions) cardiac rehabilitation offers incremental outcome benefits compared to exercise only interventions.
There was a lack of robust evidence for the impact on patient health-related quality of life, costs and cost-effectiveness.
Additionally, recent meta-analyses of the effects of exercise-based cardiac rehabilitation in patients with CHD have indicated an increase in the number of RCTs since the publication of the original Cochrane review (Clark 2005).
The aim of this study is to update the original Cochrane systematic review of the effects of exercise-based rehabilitation for patients with CHD.
Changes in this update review
In addition to updating the searches, this update review has: (1) formally explored the variation in exercise intervention effects using meta-regression and stratified meta-analysis and (2) not updated exercise capacity and cardiac risk outcomes (i.e. serum lipids, blood pressure, and smoking behaviour).
OBJECTIVES
To assess the effectiveness of exercise-based cardiac rehabilitation (exercise training alone or in combination with psychosocial or educational interventions) compared with usual care on mortality, morbidity and health-related quality of life in patients with CHD.
To explore the potential study level predictors of exercise-based cardiac rehabilitation in patients with CHD.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of exercise-based cardiac rehabilitation versus usual care with a follow-up period of at least six months have been sought.
Types of participants
Men and women of all ages, in both hospital-based and community-based settings, who have had a myocardial infarction (MI), or who had undergone revascularisation (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty or coronary artery stent), or who have angina pectoris or coronary artery disease defined by angiography have been included.
Studies of participants following heart valve surgery, with heart failure, with heart transplants or implanted with either cardiac-resynchronisation therapy (CRT) or implantable defibrillators (ICD) have been excluded. Studies of participants who completed a cardiac rehabilitation programme prior to randomisation have also been excluded.
Types of interventions
Exercise-based cardiac rehabilitation is defined as a supervised or unsupervised inpatient, outpatient, or community- or home-based intervention including some form of exercise training that is applied to a cardiac patient population. The intervention could be exercise training alone or exercise training in addition to psychosocial and/or educational interventions (i.e. “comprehensive cardiac rehabilitation”).
Usual care could include standard medical care, such as drug therapy, but did not receive any form of structured exercise training or advice.
Types of outcome measures
All clinical events or other outcome measures reported post-randomisation were included in this review. No maximum limit was imposed on the length of follow-up.
Primary outcomes
- Total mortality
-
○Cardiovascular mortality
-
○Non-cardiovascular mortality
-
○
- Total MI
-
○Fatal MI
-
○Non-fatal MI
-
○
- Total revascularizations
-
○CABG
-
○PTCA
-
○Restenting
-
○
- Total hospitalisations
-
○Cardiovascular hospitalisations
-
○Other hospitalisations
-
○
Secondary outcomes
Health-related quality of life assessed using validated instruments (e.g. SF-36, EQ5D)
Costs and cost-effectiveness
Search methods for identification of studies
As this review forms part of a broader review strategy, that includes updates of two other Cochrane systematic reviews addressing cardiac rehabilitation (Davies 2010a; Rees 2004) and two new Cochrane reviews - interventions for enhancing uptake and adherence to cardiac rehabilitation (Davies 2010b) and home versus centre-based cardiac rehabilitation (Taylor 2010), a generic broad search was initially undertaken. This generic search was then further updated for the purposes of this specific review.
Electronic searches
Randomized controlled trials have been identified from the previously published Cochrane review. This list of studies has been updated by the authors searching the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library Issue 4, 2009, MEDLINE (November 2000 to December 2009), EM-BASE (November 2000 to December 2009), CINAHL (November 2000 to December 2009), and Science Citation Index Expanded (SCI-Expanded, 1900 to December 2009). Health Technology Assessment (HTA) and Database of Abstracts of Reviews of Effects (DARE) databases have been searched via The Cochrane Library Issue 4, 2009. The generic (cross review) search was undertaken from 2001 (the search end date of the previous Cochrane review of exercise-based cardiac rehabilitation (Jolliffe 2001)) to January 2008 with a further update search up to December 2009 for this specific review.
Search strategies were designed with reference to those of the previous systematic review (Jolliffe 2001). MEDLINE, EMBASE and CINAHL were searched using a strategy combining selected MeSH terms and free text terms relating to exercise-based rehabilitation and coronary heart disease with RCT filters. The MED-LINE search strategy was translated into the other databases using the appropriate controlled vocabulary as applicable. Due to time and resource constraints, three databases (AMED, BIDS and SPORTSDISCUSS) included the previous review (Jolliffe 2001) were not searched in this case.
Searches have been limited to randomised controlled trials and a filter applied to limit by humans. Consideration was given to variations in terms used and spellings of terms in different countries so that studies were not missed by the search strategy because of such variations.
See Appendix 1 for a list of the search strategies used.
Searching other resources
Reference lists of retrieved articles and systematic reviews and meta-analyses published since the original Cochrane review were checked for any studies not identified by the electronic searches.
Data collection and analysis
Selection of studies
The titles and abstracts of citations identified by the electronic searches prior to 2008 were examined for possible inclusion by two reviewers (RST & Philippa Davies) working independently. The titles and abstracts of citations identified by the electronic searches from 2008 onwards were examined for possible inclusion independently by two reviewers (BSH & LF). Full publications of potentially relevant studies were retrieved (and translated into English where required) and two reviewers (BSH & JMHC) then independently determined study eligibility using a standardized inclusion form. Any disagreements about study eligibility were resolved by discussion and, if necessary, a third reviewer (RST) was asked to arbitrate.
Data extraction and management
Data from included studies were extracted by one reviewer (BSH or JMHC) using standardised data extraction forms and checked by a second reviewer (JMHC or BSH). If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were used because of possible measurement error when estimating from graphs. A second reviewer confirmed all numeric calculations and extractions from graphs or figures. Any discrepancies were resolved by consensus.
Data on patient characteristics (e.g. age, sex, CHD diagnosis) and details of the intervention (including mode of exercise, duration, frequency and intensity), nature of usual care and length of followup were also extracted.
Assessment of risk of bias in included studies
Two reviewers (BSH, JMHC) independently assessed the risk of bias in included studies using the Cochrane Collaboration’s recommended tool, which is a domain-based critical evaluation of the following domains: sequence generation; allocation concealment; blinding of outcome assessment; incomplete outcome data; and selective outcome reporting (Higgins 2011). Assessments of risk of bias are provided in the Risk of bias table for each study.
Dealing with missing data
If there were multiple reports of the same study, the duplicate publications were scanned for additional data. Outcome results have been extracted at all follow-up points post-randomisation. Study authors were contacted where necessary to provide additional information.
Assessment of heterogeneity
If there was significant statistical heterogeneity (P-value <0.10) associated with an effect estimate, a random effects model was applied. This model provides a more conservative statistical comparison of the difference between intervention and control because a confidence interval around the effect estimate is wider than a confidence interval around a fixed effect estimate. If a statistically significant difference was still present using the random effects model, the fixed effect pooled estimate and 95% CI have been reported because of the tendency of smaller trials, which are more susceptible to publication bias, to be over weighted with a random effects analysis (Heran 2008a; Heran 2008b).
Assessment of reporting biases
No language restrictions have been applied.
Data synthesis
Data have been processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data synthesis and analyses have been done using Review Manager 5.0 software and STATA version 10 (Stata Corp., College Station, Texas).
Dichotomous outcomes for each comparison have been expressed as relative risks with 95% confidence intervals (CI). Continuous outcome have been expressed as the mean (±SD) change from baseline to follow-up. Otherwise, continuous outcomes have been pooled as weighted mean difference (WMD). If there was a statistically significant absolute risk difference, the associated number needed to treat/harm was calculated.
Subgroup analysis and investigation of heterogeneity
Where possible, stratified meta-analysis (according to time of follow-up, 6 to12 months versus > 12 months) and meta-regression have been undertaken to explore heterogeneity and examine potential treatment effect modifiers. We tested five a priori hypotheses that there may be differences in the effect of exercise-based cardiac rehabilitation on total mortality, cardiovascular mortality, total MI, and revascularisation (CABG and PTCA) across particular subgroups: (1) CHD case mix (myocardial infarction-only trials versus other trials); (2) type of cardiac rehabilitation (exercise-only cardiac rehabilitation versus comprehensive cardiac rehabilitation); (3) ‘dose’ of exercise intervention [dose = duration in weeks x number of sessions x number of sessions per week] (dose ≥ 1000 units versus dose < 1000 units); (4) follow-up period (≤ 12 months versus > 12 months); and (5) year of publication (before 1995 versus 1995 or later).
Year of Publication
We included year of publication as a study level factor (pre versus post-1995) in order to assess the potential effect of a change in the standard of usual care over time, that is to reflect when pharmacologic agents became established therapies for CHD.
Heterogeneity
Heterogeneity amongst included studies was explored qualitatively (by comparing the characteristics of included studies) and quantitatively (using the chi-squared test of heterogeneity and I2 statistic). Where appropriate, data from each study have been pooled using a fixed effect model, except where substantial heterogeneity exists. We planned to pool the results for health-related quality of life using a standardised mean difference (SMD) but this was not possible due to the heterogeneity in outcome measures and methods of reporting findings.
The funnel plot and the Egger test have been used to examine small study bias (Egger 1997).
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
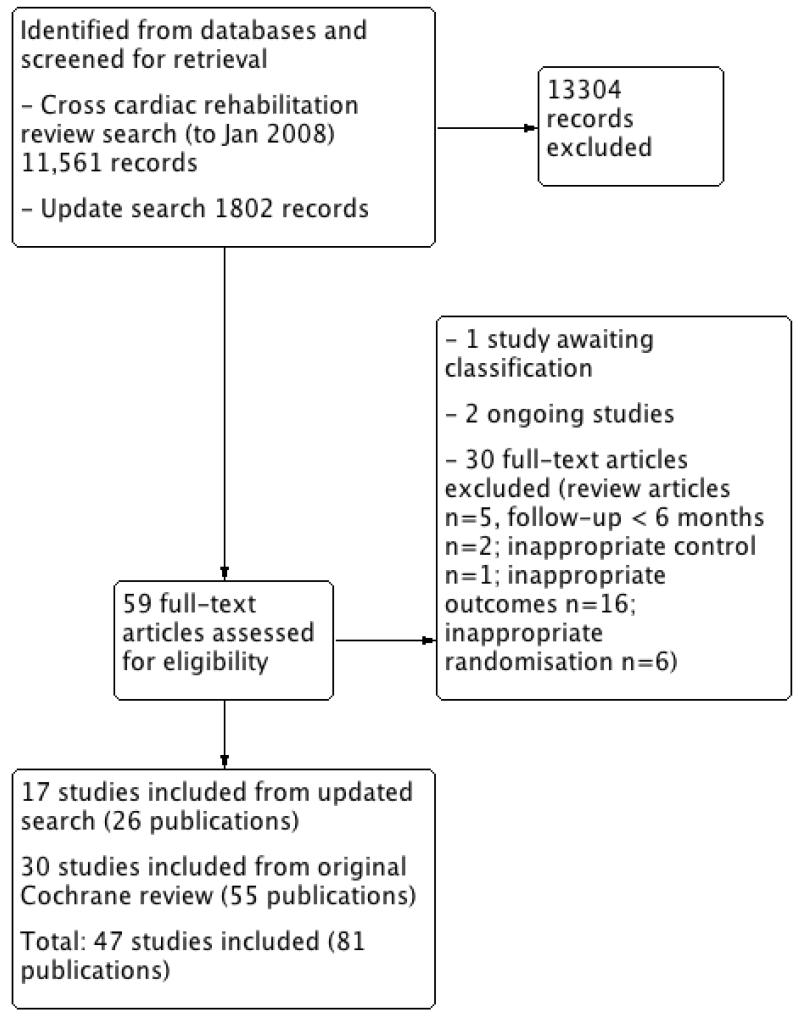
Our update cross-cardiac rehabilitation review electronic searches (to January 2008) yielded a total 11,561 titles plus 1802 titles from the update search (to December 2009). After reviewing the titles and abstracts, we retrieved 59 full-text articles for possible inclusion. A total of 30 papers were excluded: two had followup less than six months, 16 reported no useful outcomes, six had inappropriate randomisation, one had an inappropriate control, and five were review articles. In addition, one study was awaiting classification and two were ongoing studies. Seventeen studies (26 publications) met the inclusion criteria and had extractable data to assess the effects of exercise-based cardiac rehabilitation compared with usual care on mortality and morbidity in patients with CHD (Figure 1).
Figure 1. Study flow diagram.
Included studies
The original Cochrane review published in 2001 (Jolliffe 2001) included a total of 35 studies, of which five studies were judged not to meet the revised inclusion criteria of this review update (see Excluded studies).
In addition to the 30 trials (55 publications) from the original Cochrane review that met the inclusion criteria of this update review (Andersen 1981; Bell 1998; Bengtsson 1983; Bertie 1992; Bethell 1990; Carlsson 1998; Carson 1982; DeBusk 1994; Engblom 1996; Erdman 1986; Fletcher 1994; Fridlund 1991; Haskell 1994; Heller 1993; Holmbäck 1994; Kallio 1979; Leizorovicz 1991; Lewin 1992; Miller 1984; Oldridge 1991; Ornish 1990; Schuler 1992; Shaw 1981; Sivarajan 1982; Specchia 1996; Stern 1983; Vecchio 1981; Vermeulen 1983; WHO 1983; Wilhelmsen 1975), an additional 17 studies (26 publications) have been identified by the updated search and have met the revised inclusion criteria (Belardinelli 2001; Bäck 2008; Dugmore 1999; Giallauria 2008; Hofman-Bang 1999; Kovoor 2006; La Rovere 2002; Manchanda 2000; Marchionni 2003; Seki 2003; Seki 2008; Ståhle 1999; Toobert 2000; VHSG 2003; Yu 2003; Yu 2004; Zwisler 2008). Thus, a total of 47 studies reporting data for a total of 10,794 patients have been included in this review update. Details of the studies included in the review are listed in the Characteristics of included studies table. The study selection process is summarised in the PRISMA flow diagram shown in Figure 1.
Although all exercise-based cardiac rehabilitation, 17 studies were judged to be exercise-only intervention trials and 29 were judged to be comprehensive cardiac rehabilitation (exercise plus psychosocial and/or educational interventions); one trial randomly assigned patients to both exercise-only cardiac rehabilitation and comprehensive cardiac rehabilitation (Sivarajan 1982). The majority of studies were (32 studies, 68%) undertaken in Europe, either as single or multicenter studies. Trial sample sizes varied widely from 28 to 2304, with a median intervention duration of three (range 0.25 to 30) months and a follow-up of 24 (range six to 120) months. Patients with myocardial infarction alone were recruited in 30 trials (64%); the remaining trials recruited either exclusively postrevascularisation patients (i.e., CABG and PTCA) or both groups of patients. The ages of patients in the trials ranged from 46 to 84 years. Although over half of the trials (28 studies, 60%) included women, on average women accounted for only 20% of the patients recruited.
Characteristics of included interventions
Twenty nine studies compared comprehensive programmes (that is, exercise plus education or psychological management, or both), while 17 reported on an exercise only intervention. In addition, one study randomised patients to a comprehensive programme, exercise only intervention or usual care (Sivarajan 1982).
The exercise-based cardiac rehabilitation programmes differed considerably in duration (range 1-12 months), frequency (1-7 sessions/week), and session length (20-90 minutes/session). Most programmes involved the prescription of individually tailored exercise programmes, which makes it difficult to precisely quantify the amount of exercise undertaken. Most home based programmes included a short initial period of centre based intervention. Centre based programmes typically involved supervised exercise involving cycles, treadmills or weight training, while nearly all home based programmes were based on walking.
Both intervention and control patients received usual care including medication, education and advice about diet and exercise, but control patients received no formal exercise training.
Excluded studies
Five studies that had been included in the original review failed to meet the revised inclusion criteria of this review update. Of these, four studies did not report outcomes relevant to this review (Ballantyne 1982; Carlsson 1997; Krachler 1997; Wosornu 1996) and one study was not randomised (Kentala 1972). For the updated search, 24 studies (25 publications) were excluded for reasons listed in the Characteristics of excluded studies table, with the most common reason being a failure to report any of the pre-specified outcomes of this review update.
Risk of bias in included studies
Limited reporting of the methodology and outcome data in the published papers of the included trials precluded us, in most cases, from adequately performing a critical evaluation of the following domains: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other sources of bias. Nevertheless, we attempted to assess the risk of bias for each of the 47 included studies given the available information in the published trial reports.
Allocation
Nearly all the trial publications simply reported that the trial was “randomised” but did not provide any details. A total of 8/47 (17%) studies (Andersen 1981; Bell 1998; Bethell 1990;Erdman 1986; Haskell 1994; Holmbäck 1994; Wilhelmsen 1975;Zwisler 2008) reported details of appropriate generation of the random sequence and 7/47 (15%) studies (Bell 1998; Haskell 1994; Holmbäck 1994; Kovoor 2006; Schuler 1992; VHSG 2003;Zwisler 2008) reported appropriate concealment of allocation.
Blinding
For exercise-based cardiac rehabilitation trials, it is not possible to blind patients and clinicians to the intervention. For the large majority of studies, insufficient information was provided to evaluate the blinding of assessors; only 4 of 47 (9%) studies (Fletcher 1994; Ornish 1990; Wilhelmsen 1975; Zwisler 2008) reported that outcome assessors were blind to group allocation.
Incomplete outcome data
Losses to follow-up and drop out were relatively high, ranging from 21% to 48% in 12 trials. Follow-up of 80% or more was achieved in 33/47 (70%) studies (Andersen 1981; Belardinelli 2001; Bell 1998; Bethell 1990; Bäck 2008; Carlsson 1998; Dugmore 1999;Engblom 1996; Giallauria 2008; Haskell 1994; Heller 1993;Holmbäck 1994; Kallio 1979; Kovoor 2006; La Rovere 2002;Leizorovicz 1991; Lewin 1992; Manchanda 2000; Marchionni 2003; Miller 1984; Oldridge 1991; Schuler 1992; Seki 2003;Shaw 1981; Specchia 1996; Stern 1983; Ståhle 1999; Toobert 2000; Vermeulen 1983; VHSG 2003; Wilhelmsen 1975; Yu 2003;Zwisler 2008). Furthermore, reasons for loss to follow and dropout were often not reported. Two trials (Seki 2008; WHO 1983) did not report information on losses to follow-up. Several trials have excluded significant numbers of patients post-randomisation, and thus in an intention to treat analysis, these then have been regarded as dropouts.
Selective reporting
A number of the included studies were not designed to assess treatment group differences in morbidity and mortality (as these were not the primary outcomes of these trials) and, therefore, may not have fully reported all clinical events that occurred during the follow-up period. All studies collecting validated health-related quality of life outcomes fully reported these outcomes.
Other potential sources of bias
Publication bias
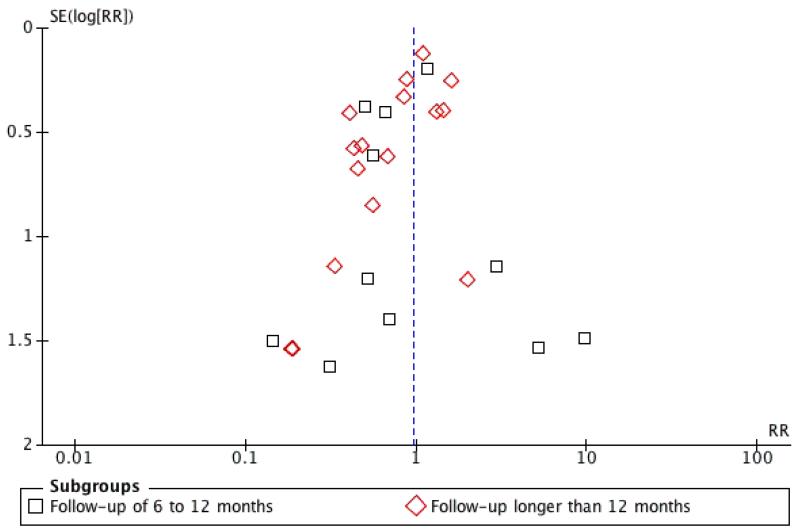
In order to test for the possibility of publication bias, the funnel plots were created for all-cause mortality, cardiovascular mortality, recurrent MI, and revascularisation (CABG and PTCA). There was no evidence of funnel plot asymmetry or significant Egger tests for all-cause mortality, cardiovascular mortality and revascularisation (CABG and PTCA). However, the funnel plot of recurrent MI suggests asymmetry and the Egger test was statistically significant (P = 0.019), which appears to be due to an absence of negative-result trials of small to medium size (Figure 2).
Figure 2. Funnel plot of exercise-based rehabilitation versus usual care for fatal and/or nonfatal MI.
Effects of interventions
Clinical Events
Mortality
Thirty (N = 8971) of the included studies reported total mortality (Analysis 1.1); two trials reported both follow-up to 12 months and longer than 12 months (Wilhelmsen 1975; WHO 1983). In studies reporting follow-up longer than 12 months, compared with control, total mortality was reduced with exercise-based cardiac rehabilitation (RR 0.87 [95% CI 0.75, 0.99]). There was no significant difference in total mortality up to 12 months follow-up.
Nineteen (N = 6583) of included studies reported cardiovascular mortality (Analysis 1.2); one trial reported both follow-up to 12 months and longer than 12 months (WHO 1983). In studies reporting follow-up longer than 12 months, compared to control, cardiovascular mortality was reduced with exercise-based cardiac rehabilitation (RR 0.74 [95% CI 0.63, 0.87]). There was no significant difference in cardiovascular mortality up to 12 months follow-up.
There was no evidence of statistical heterogeneity across trials for either total or cardiovascular mortality.
Morbidity
Twenty-five (N = 7294), 22 (N = 4392), and 11 (N = 2241) of the included studies reported total MI, CABG or PTCA, respectively (Analysis 1.3; Analysis 1.4; Analysis 1.5); follow-up to 12 months and longer than 12 months was reported by two studies for MI (Haskell 1994; WHO 1983), one study for CABG (Ståhle 1999) and two studies for PTCA (Haskell 1994; Ståhle 1999). There was no statistically significant difference between exercise-based cardiac rehabilitation and usual care for these outcome measures. The pooled risk ratios for total MI, CABG and PTCA were 0.92 (95% CI 0.70, 1.22), 0.91 (95% CI 0.67, 1.24) and 1.02 (95% CI 0.69, 1.50), respectively, up to 12 months follow-up. In studies reporting follow-up longer than 12-months, the pooled risk ratios for total MI, CABG and PTCA were 0.97 (95% CI 0.82, 1.15), 0.93 (95% CI 0.68, 1.27) and 0.89 (95% CI 0.66, 1.19) respectively. There was no evidence of statistical heterogeneity across trials for any of the morbidity outcomes.
Hospitalisations
Ten (N = 2379) of the included studies reported hospital admissions; one study reported both follow-up to 12 months and longer than 12 months (Hofman-Bang 1999). In studies reporting up to 12 months follow-up, total readmissions were reduced with exercise-based cardiac rehabilitation compared with usual care (RR 0.69, 95% CI 0.51, 0.93; Analysis 1.6). There was no significant difference in total hospitalisations in studies with follow-up longer than 12 months.
Health-related quality of life
Ten trials assessed health-related quality of life using a range of validated disease-specific (e.g. QLMI) and generic (e.g. Short-form 36) outcome measures (Table 1). Given both the heterogeneity in outcome measures and methods of reporting findings, a meta-analysis was not undertaken.
Although most trials demonstrated an improvement in baseline quality of life following exercise-based cardiac rehabilitation, a within group improvement was also often reported in control patients. Only in seven out of 10 trials was there evidence of a significantly higher level of quality of life with exercise-based cardiac rehabilitation than control at follow-up (Belardinelli 2001;Dugmore 1999; Sivarajan 1982; Yu 2004).
Costs
Three of the included studies reported limited data on costs per patient (Kovoor 2006; Marchionni 2003; Yu 2004). These results are summarised in Table 2. It was not possible to compare the costs directly across studies due to differences in currencies and the timing of studies.
In two of the three studies the total healthcare costs associated with exercise-based cardiac rehabilitation and usual care were not statistically significantly different. In Marchionni 2003, the total healthcare costs associated with exercise-based cardiac rehabilitation were higher ($4839 more per patient) than usual care.
Only Oldridge 1991 evaluated the cost-effectiveness of exercise-based cardiac rehabilitation in post-MI patients by combining cost information with time trade-off measures of health-related quality of life and data on mortality derived from a 1989 meta-analysis (O’Connor 1989). Based on their analysis, the authors concluded that rehabilitation was “an efficient use of health-care resources and may be economically justified” (Oldridge 1993).
Meta regression
Predictors of all-cause mortality, cardiovascular mortality, recurrent MI, and revascularisation (CABG and PTCA) were examined using univariate meta-regression. Covariates defined a priori included: CHD case mix (myocardial infarction-only trials versus other trials); type of cardiac rehabilitation (exercise-only versus comprehensive cardiac rehabilitation); ‘dose’ of exercise intervention (calculated as the number of weeks, multiplied by the number of sessions per week, multiplied by the duration of sessions in hours); follow-up period (≤ 12 months versus > 12 months); and publication date (before 1995 versus 1995 or later). No statistically significant associations were seen in any of these analyses (Table 3, Table 4, Table 5, Table 6, Table 7).
DISCUSSION
Summary of main results
This updated systematic review of exercise-based cardiac rehabilitation has allowed analysis of an increased number of patients from an additional 17 studies published from 2000 to 2009. A total of 47 RCTs, with 10,794 patients, have now been included. In accord with the original Cochrane review and previous metaanalyses (Clark 2005; Jolliffe 2001; O’Connor 1989; Oldridge 1988) a reduction in both total and cardiac mortality was observed in CHD patients randomised to exercise-based rehabilitation. However, this updated review shows that this mortality benefit is limited to studies with a follow-up of greater than 12 months. We also found that with exercise the rate of hospital readmissions may be reduced in studies up to 12 months follow-up (based on 4 trials with 54/254 versus 73/225 events), but not in longer term follow-up. There was no difference between exercise-based cardiac rehabilitation and usual care groups in the risk of recurrent myocardial infarction or revascularization at any duration of follow-up.
This reduction in total and cardiovascular mortality with exercise therapy appears consistent across a number of CHD groups (e.g., post-MI, post-revascularisation), as well as a range of strategies for delivery of the exercise-based intervention. We compared trials that assessed exercise therapy alone with exercise in combination with educational and psychological co-interventions and there appears to be no difference in mortality effect. In addition, there was no difference in mortality effect by exercise ‘dose’ a composite measure based on the overall duration of the exercise program plus the intensity, frequency, and length of exercise sessions.
The mechanism for reduced cardiovascular mortality in patients who have received exercise-based cardiac rehabilitation is not clear, but may be due to improved myocardial revascularisation, protection against fatal dysrhythmias, improved cardiovascular risk factor profile, improved cardiovascular fitness, or increased patient surveillance (Oldridge 1988; Taylor 2006).
There were insufficient data to definitely definitely conclude that exercise-based cardiac rehabilitation improves health-related quality of life compared to control. Only 10 of included trials reported outcomes based on a validated health-related quality of life measure. Furthermore, only three of these 10 trials randomised more than 250 patients; thus, providing relatively adequate power (80% and 5% alpha) to detect a modest difference (standardised effect size of 0.25) between exercise therapy and usual care. Heterogeneity of health-related quality of life outcome measures and their reporting precluded us from quantitatively pooling the available data across trials. Generic health-related quality of life measures that lack sensitivity to change with cardiac treatment, particularly in comparison with disease-specific measures, were used in nearly all the trials (Oldridge 2003; Taylor 1998).
All participants in the included studies had documented CHD, the majority of the participants having suffered an MI. Some participants had documented CHD having suffered angina or undergone coronary angiography, while others had undergone CABG. We have combined these different patient groups as there are insufficient data at present to stratify trials by type of CHD. The number of women participants was low and few studies mentioned the ethnic origin of their participants. The mean age of the participants was 56 years. Although most studies had an upper age limit of at least 65 years of age, this is not reflected in the mean age of the participants. The majority of the studies had exclusion criteria that would have excluded those participants who had co-morbidity, or heart failure. In some studies this may have accounted for up to 60% of the patients considered for the trial, and certainly the older patients would be more likely to be affected.
Quality of the evidence
We found no evidence of publication bias for total mortality, CV mortality, CABG or PTCA. There was evidence of small study bias for total MI.
As with the original Cochrane review, this update review has revealed limitations in the available RCT evidence, most notably the poor reporting of methodology and results in many trial publications (Jolliffe 2001). The method of randomization, allocation concealment, or blinding of outcomes assessment was rarely described. Although the quality of reporting tends to be poorer for older studies, it does not appear to have appreciably improved over the last decade. Furthermore, incomplete outcome data (primarily due to losses to follow-up or dropouts) were insufficiently addressed in most trials. Losses to follow-up were relatively high across trials (approximately one third of trials reported a greater than 20% loss to follow-up) but reasons for dropout were often not reported. Several trials excluded significant numbers of patients post-randomisation, and thus in an intention-to-treat analysis, these patients have been regarded as dropouts. This may be partly explained by the fact that the majority of trials were not designed to assess treatment group differences in mortality and morbidity but instead surrogate measures of treatment efficacy, such as exercise capacity or lipid levels.
AUTHORS’ CONCLUSIONS
Implications for practice
In medium to longer term (i.e. 12 or more months follow-up) exercise-based cardiac rehabilitation is effective in reducing overall and cardiovascular mortality and appears to reduce the risk of hospital admissions in the shorter-term (< 12 months follow-up) in patients with CHD. The available evidence does not demonstrate a reduction in the risk of total MI, CABG or PTCA with exercise-based cardiac rehabilitation as compared to usual care at any duration of follow-up. Exercise-based cardiac rehabilitation should be recommended for patients similar to those included in the randomised controlled trials - predominantly lower risk younger men who had suffered myocardial infarction or are post-revascularisation. It is a question of judgement whether evidence is sufficient to under-represented groups, particularly angina pectoris and higher risk CHD patients and those with major co-morbidities. There appears to be little to choose between exercise only or in combination with psychosocial or educational cardiac rehabilitation interventions. In the absence of definitive cost-effectiveness comparing these two approaches to exercise-based cardiac rehabilitation it would be rational to use cost considerations to determine practise.
Implications for research
In spite of inclusion of recent trial evidence including more post-revascularisation and female patients, the population of CHD patients studied in this review update remains predominately low risk middle-aged males following MI or PTCA. There has been little identification of the ethnic origin of the participants. It is possible that patients who would have benefited most from exercise-based cardiac rehabilitation were excluded from the trials e.g. those of older age or those with co-morbidity. Therefore, well-designed, and adequately reported RCTs in groups of CHD patients more representative of usual clinical practice are still needed. These trials should include validated health-related quality of life outcome measures, need to explicitly report clinical events including hospital admission, and assess costs and cost-effectiveness.
PLAIN LANGUAGE SUMMARY.
Regular exercise or exercise with education and psychological support can reduce the likelihood of dying from heart disease
Coronary heart disease (CHD) is one of the most common forms of heart disease. It affects the heart by restricting or blocking the flow of blood around it. This can lead to a feeling of tightness in the chest (angina) or a heart attack. Exercise-based cardiac rehabilitation aims to restore people with CHD to health through either regular exercise alone or a combination of exercise with education and psychological support. The findings of this review indicate that exercise-based rehabilitation reduces the likelihood of dying from heart disease and there is moderate evidence of an improvement in quality of life in the predominantly middle aged, male patients included in these studies. More research is needed to assess the overall health impact of exercise-based rehabilitation in a broader range of patients.
ACKNOWLEDGEMENTS
We would like to thank Lambert Felix and Philippa Davies for examining the titles and abstracts of citations identified by the electronic searches for possible inclusion.
We would also like to thank Sue Whiffen for her administrative assistance and Nizar Abazid, Ela Gohil, Ellen Ingham, Cornelia Junghans, Joey Kwong, Dan Manzari, Fenicia Vescio, and Gavin Wong for their translation services.
We would like to thank all the authors who provided additional information about their trials.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
NIHR, UK Cochrane Collaboration Programme Grant, UK.
This review was supported by a National Institute for Health Research (NIHR) Cochrane Collaboration Programme Grant (CPGS10).
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Post MI randomised four weeks after discharge. 88 participants were randomised, but 13 failed to follow up. Therefore 75 took part in the study | |
| Participants | 75 men < 66 yrs with 1st MI. Mean age I = 52.2 (+/−7.5), C= 55.6 (+/−6.3). |
|
| Interventions | Aerobic activity e.g. running, cycling, skipping + weights for 1 hour × 2 weekly for 2 months, then × 1 week for 10 months. Then continue at home. F/U @ 1, 13, 25, & 37 months post discharge. |
|
| Outcomes | Total & CHD mortality and non fatal MI. | |
| Notes | Several participants in C trained on own initiative, but were analysed as intention to treat. Authors concluded that PT after MI appears to reduce consequences and to improve PWC, but PWC declines once participant on their own. PT had no effect on period of convalescence or return to work, but age and previous occupation were of significance |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 15% lost to follow-up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT, single centre in Italy 33 (SD 7) months |
|
| Participants |
N Randomised: Total:118 (99 males, 19 females); EX: 59 (49 males, 10 females) UC: 59 (50 males, 9 females) Diagnosis (% of pts); Myocardial Infarction: EX 51; UC 47 Hypercholesterolemia: EX 61; UC 54 Diabetes: EX 17; UC 20 Hypertension: EX 42; UC 47 LVEF (%): EX 52 (SD 16); UC 50 (SD 14) Case mix: Age (years): EX: 53 (SD 11); UC: 59 (SD 10) Percentage male: EX 83.1%; UC 84.8% Percentage white: Not reported Inclusion/exclusion criteria: Inclusion: successful procedure of coronary angioplasty in 1 or 2 native epicardial coronary arteries and ability to exercise Exclusion: previous coronary artery procedures, cardiogenic shock, unsuccessful angioplasty (defined as residual stenosis>30% of initial value), complex ventricular arrhythmias, uncontrolled hypertension and diabetes mellitus, creatinine ?2.5 mg/dl, orthopedic or neurological limitations to exercise or unstable angina after procedure and before enrolment |
|
| Interventions |
Exercise:
Total duration: six months aerobic/resistance/mix: exercise sessions were performedat the hospital gym and were supervised by a cardiologist frequency: 3 sessions/week duration: 15 min of stretching and callisthenics; 5 min of loadless warm-up; 30 min of pedaling on electronically braked cycle ergometer at target work rate; 3 min of unloaded cool-down pedaling intensity: 60% of peak oxygen uptake (VO2) modality: electronically braked cycle ergometer Usual care: “Control patients were recommended to perform basic daily mild physical activities but to avoid any physical training.” |
|
| Outcomes | Cardiac mortality; myocardial infarction; coronary angioplasty (percutaneous translu-minal coronary angioplasty, coronary stent); coronary artery bypass graft; health-related quality of life: MOS Short-Form General Health Survey | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “All studies were performed by
experienced operators and evaluated
by two independent observers blinded
to treatment arm and to each otherls interpretation. ” Comment: This only applied to exercise test & angiography only so assessment of events and health-related quality of life (although patient self complete) not necessarily blinded |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Cardiac events of 12 patients who were excluded not accounted for |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Post MI Randomised 4-6 days post event. |
|
| Participants | 311 men / 89 women < 65 yrs. Mean ages for women 60.7 (+/− 7.2) to 64.3 (+/−7.3), for men 57.8(+/− 8.9) to 59.4 (+/− 9.4). 2 comparisons conventional CR v: the Heart Manual (HM) and HM v: control |
|
| Interventions | Conventional CR - 1 to 2 group classes per week, walking etc other days for 8-12 weeks with multidisciplinary team HM - individual - walking programme up to 6 weeks post MI, facilitator and written text. F/U - 1 year. |
|
| Outcomes | Total mortality, health-related quality of life: Nottingham Health Profile | |
| Notes | “Heart Manual is a comprehensive home based programme which included an exercise regimen, relaxation and stress management techniques, specific self-help treatments for psychological problems commonly experienced by MI patients and advice on coronary risk-related behaviours.” Hospital readmissions significantly reduced in Heart Manual group compared with conventional CR and control in initial 6 month period |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was achieved by providing each hospital with a series of sealed envelopes containing cards evenly distributed between conditions. The envelopes were taken sequentially and, before opening the envelope, the patient’s surname was written diagonally across the sealed flap, in such a way that when the envelope was opened the name was ‘torn in two’. Opened envelopes were retained and returned to the trial coordinator. The importance of remaining neutral when advising the patients of the outcome of randomisation was emphasised in the written protocol and was reinforced during the sessions which were held to familiarise facilitators with the protocol.”” |
| Allocation concealment (selection bias) | Low risk | “Randomisation was achieved by providing each hospital with a series of sealed envelopes containing cards evenly distributed between conditions. The envelopes were taken sequentially and, before opening the envelope, the patient’s surname was written diagonally across the sealed flap, in such a way that when the envelope was opened the name was ‘torn in two’. Opened envelopes were retained and returned to the trial coordinator. The importance of remaining neutral when advising the patients of the outcome ofrandomisation was emphasised in the written protocol and was reinforced during the sessions which were held to familiarise facilitators with the protocol.” Comment: Patients were informed of outcome of randomisation. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1.5% lost to follow up and reported description of withdrawals and/or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT; single centre Sweden; F/U 14 months average | |
| Participants | N=87 (EX n= 44; CON n=43) Gender: 74 men / 13 women Mean age: EX = 55.3 +/− 6.6, CON = 57.1 +/− 6.6. Diagnosis: following acute MI. Ethnicity: NR Inclusion: <65 years with MI Exclusion: decisions based on cardiologist: severe cardiac failure, PMI-syndrome, aortic regurgitation, cerebral infarct hemiparesis, disease of hip, status post-poliomyelitis, amputation of lower extremity, Diabetes with retinopathy, hyper/hypo thyroidism, hyper-parathyroidism, mental illness |
|
| Interventions | Exercise intervention: Duration: 3 months; Frequency: 30 min twice weekly. Mode: physical training, interval training of large muscle groups, jogging, callisthenics Co-interventions: counselling, social measures, group and individual. Intensity: graded individually |
|
| Outcomes | Total mortality, CHD mortality, non-fatal MI up to average 14 months | |
| Notes | Most emphasis on social/ psychological aspects. 171 patients were randomised and at discharge the cardiologist decided whether the patient was fit to take part in the rehab programme - 45 patients were excluded at this point. 7 of intervention group declined to take part, but 6 of these were seen at follow up and included in the analysis because “control group probably had a comparable number who would have declined further treatment.” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “allocated at random” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Description of withdrawals & dropouts: 29% I, 33% C lost to follow up from 126 who took part. 171 were randomised and then 45 excluded by cardiologist |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised on day of discharge after MI; F/U 12-24 months. | |
| Participants | N = 110 (EX n:57; CON n:53) Gender: NR Mean age: EX = 52.1 +/− 1.3, CON = 52.7 +/− 1.3 Diagnosis: <65 yrs with acute myocardial infarction confirmed by typical symptoms, electrocardiographic changes, and a rise in cardiac creatinine kinase isoenzyme Ethnicity: NR Inclusion: Men and women with acute myocardial infarction and had been admitted to Plymouth coronary care unit Exclusion: uncontrolled heart failure; serious rhythm disturbances which persisted and required treatment at time of discharge; another disabling disease |
|
| Interventions | Exercise group: Duration: 4 weeks; Frequenty: 2 × week; Mode: standard pulse-monitored group exercise commonly used in the physiotherapy ofcardiac patients, 12 station circuit started 3 weeks post discharge Control: standard hospital care |
|
| Outcomes | Total mortality, non fatal MI, revascularisation; Assessments at day ofdischarge, 3rd week after discharge; after rehabilitation (for intervention group); four months after infarct and 12-24 months after infarct) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 24% lost to follow-up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Parallel RCT; single centre in Alton, Hampshire | |
| Participants | N: 200 (EX n=99; CON n101=) Gender: 100% men Age: EX = 54.2 (+/−7.2), CON = 53.2 (+/−7.7). Diagnosis: 5 days post MI. Ethnicity:NR Inclusion: <65 yrs post MI; history of chest pain typical of MI, progressive ECG changes, rise and fall in aspartate transaminase concentrations with at least one reading above 40 units/ml Exclusion: medical or orthopaedic problems that precluded their taking part in the exercise course; insulin dependent diabetes mellitus; atrial fibrillation; on investigator’s personal general practice list |
|
| Interventions | Exercise group: Duration: 3 months; Frequency: 3×/week; Mode: 8 stage circuit aerobic & weight training. Intensity: 70-85% predicted HRmax Control group: given a short talk on the sort of exercise that they might safely take unsupervised |
|
| Outcomes | Total mortality, CHD mortality, non fatal MI (11 year follow up published in 1999. 5 year follow up data from unpublished material used for meta analysis.) | |
| Notes | 229 patients were randomised; 14 in the intervention group and 15 in control dropped out before the first exercise test due to death, refusal or other problems. Therefore 200 took part in the study Cardiac mortality of 3% pa, once patients survived to be in the trial. Suggests more severely affected patients were not included. Significant predictors of cardiac death were pulmonary oedema on admission, complications during admission, one or more previous infarcts, increasing age and low initial fitness |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random letter sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 16% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Parallel RCT, single centre in Sweden | |
| Participants | N= 37 randomised (EX n=21; CON n=16) 86.5% male. Age 63.6 years Diagnosis: stable CAD and coronary angiographic changes. Ethnicity: NR Inclusion: coronary artery stenosis documented by angiography or previous coronary artery bypass grafting, classes I-III angina pectoris, classified according to Canadian Cardiovascular Society Exclusion: disabling disease that hindered regular exercise, or if the patient already has engaged in exercise more than 3 days/week |
|
| Interventions | Ttraining - high frequency exercise- group: 3 endurance resistance exercises and trained on a bicycle ergometer 30 min, 5 times a week for 8 months at 70% ofV02max. Duration: 8 months | |
| Outcomes | PTCA at 2 months before PCI and 6 months after PCI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 8.1% lost to follow-up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT; single-centre in Sweden; F/U 1 year | |
| Participants | N= 235 (EX n=118; CON n=117) Diagnosis: AMI or CABG (4 weeks post discharge); CABG (n = 67); AMI (n = 168) Mean age: AMI patients I = 62.2 +/−5.8, C= 61.7 +/−6, CABG patients Mean age I = 62.7 +/− 4.8, C= 59.8 +/− 4.8. Ethnicity: NR Inclusion:Acute MI; coronary artery bypass revascularization surgery less than 2 weeks prior; PTCA less than 2 weeks prior Exclusion: signs of unstable angina; signs of ST-depression at exercise test of more than 3 mm in 2 chest leads or more than 2mm in two limb leads at four weeks post discharge from hospital, signs ofCHF, severe, non-cardiac disease; drinking problems, not Swedish spoken |
|
| Interventions | Exercise programme: Duration: 2-3 months; Frequency: 2-3 × weekly Session duration: 60 mins; Mode: walking and jogging followed by relaxation and light stretching exercises; Nurse counselling: 9 hours of counselling in individual & group sessions over 1 year; smoking cessation 1.5, dietary management 5.5 & physical activity 2 hours Control: usual care |
|
| Outcomes | Mortality, | |
| Notes | Groups of 20 patients randomly allocated to intervention and control groups (usual care) . Randomised 4 weeks post discharge In first 3 weeks post discharge all participants ( I & C) had 2 visits by nurse & 1 by cardiologist + all participants invited to join regular exercise group × 1 per week for 30 mins information & 30 mins easy interval training |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | <20% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised 6 weeks post admission | |
| Participants | N: 303 (EX n=151; CON n=152) 100% men Mean age: EX = 50.3 (SE 0.65) years CON =52.8 (SE 0.67) years Diagnosis: MI Ethnicity: NR Inclusion: MI patients admitted to the coronary care unit; diagnosis based on ECG changes and /or elevation of serum glutamic oxaloacetic transaminase or lactic dehydro-genase taken on three consecutive days ExclusIon: >70 years; heart failure at follow-up clinic; cardio-thoracic ratio exceeding 59%; severe chronic obstructive lung disease; hypertension requiring treatment; diabetes requiring insulin; disabling angina during convalescence; orthopaedic or medical disorders likely to impede progress in the gym, personality disorders likely to render patient unsuitable for the course |
|
| Interventions | Exercise group: Duration: 12 weeks; Frequency: attended gym 2 × weekly : Mode: Exercises arranged on a circuit basis and pure isometric exercise was avoided. Control group: Did not attend gym |
|
| Outcomes | Total mortality, non fatal MI at 5 months, 1 year, 2 year and 3 year after MI (mean F/U 2.1 years) | |
| Notes | There appears to be a reduction in mortality in exercise participants with inferior MI | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 21% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised 3rd day post MI. | |
| Participants | 294 men & 8 women F <70 yrs (mean age 57+/− 8), post MI, in 5 centres | |
| Interventions | Nurse managed, home based, multifactorial risk factor intervention programme with exercise training based on De Busk/Miller. F/U 12 months | |
| Outcomes | Total mortality | |
| Notes | Levels of psychological distress dropped significantly for both groups by 12 months | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 33% lost to follow up, no description of withdrawals & dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | single-centre RCT in UK; f/u 5 yrs | |
| Participants | N=124 (EX n=62; CON n=62) Gender: 122 men Mean age: EX=54.8 y ;CON = 55.7 y Diagnosis: clinically documented MI between 1984 and 1988 Ethnicity: NR Inclusion: MI according to conventional WHO cardiac enzyme and ECG criteria ofMI Exclusion: NR |
|
| Interventions | EX : Duration: 12 months; Frequency: 3 times weekly; Mode: regular aerobic and local muscular endurance training , consisting of warm up and cool down exercises, sit ups, wall bar/bench step ups, cycle ergometry, and major component centered on training of aerobic capacity, using walking and jogging Control: “received no formal exercise training throughout the same 12 month period” |
|
| Outcomes | CV mortality; nonfatal MI; QoL at 4, 8, 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-centre open RCT in Finland | |
| Participants |
N Randomised: Total: 228 (201 males, 27 females); EX: 119 (104 males, 15 females) UC: 109 (97 males, 12 females) Baseline Characteristics: Previous unstable angina (%): EX: 29; UC: 31 Previous MI (%):EX: 42; UC: 46 Hypertension (%):EX 31;UC 23 LVEF (%):EX: 70.3 (SD 11.5); UC: 71.4 (SD 12.3) Age (years): EX: 54.1 (SD 5.9); UC: 54.3 (SD 6.2) Percentage male:88% Percentage white: Not reported Inclusion/exclusion criteria: Inclusion: patients who underwent elective CABS Exclusion: any other serious disease; ?65 years of age |
|
| Interventions | 4 stage rehab over 30 months starting pre CABG with meeting of physician, psychologist and OT/PT. 6-8 weeks post CABG - 3 weeks IP with group sessions with psychologist, aerobic physical activity, relaxation & group discussion. 8 months post CABG - 2 days meeting with OT, nutritionist, physician, physio. 30 months post CABG - one day with nutritionist, physician & exercise. |
|
| F/U I year & 6 years Usual care: no further details |
||
| Outcomes | Mortality, CABG, health-related quality of life: Nottingham Health Profile | |
| Notes | 5 years after CABG only 20% of participants were working, despite 90% of patients being in functional classes 1-2. Almost half of patients had retired pre CABG. Many other factors affect RTW post CABG - age, education, physical requirements of the job, type of occupation, self employed status, non work income, personality type, self perception of working capacity and mostly length of absence from work pre CABG | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | “open randomised trial” Data on deaths &admissions from the hospital records department |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 13% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single centre RCT in Rotterdam; Follow up 5 years. | |
| Participants | N= 80 (EX n=; CON n=) Gender: 100% male Mean age: 51years (range 35-60 years) Diagnosis: within 6 months post MI. Also with CABG/angina. Ethnicity: NR Inclusion: First MI within 6 months before the first psychologic investigation; <65 years; meet three psychologic inclusion criteria - one or more symptoms of the anxiety reaction, diminished self-esteem, positive motivation to take part in the programme Exclusion: severe cardiomyopathy, severe valvular disorders, inadequate performance on exercise, unstable angina pectoris |
|
| Interventions | Exercise intervention: duration: 6 months: Frequency: once per week; Session duration and mode: warming up period (15min), gymnastics and jogging (both 15 mins), sports such as volleyball, soccer, and hockey (30min), relaxation exercise (5min) Controls:Usual care plus educational brochure with guidelines about physical fitness training |
|
| Outcomes | Mortality, non fatal MI at 5 years | |
| Notes | Complex presentation of results. Authors conclude that patients who will benefit from rehab can be detected on psychological grounds. Those who have engaged in habitual exercise, but feel seriously disabled, yet do not feel inhibited in a group will benefit from rehab |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomly allocated by means of a table for random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 29 % lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Prospecitve, single centre RCT in the US. F/U 6 months. | |
| Participants | N= 88 (EX n=41; CON n=47) 100% male Mean age: EX= 62 +/−8, CON = 63 +/−7; (range 42 - 72) Diagnosis: CAD and a physical disability Ethnicity: NR Inclusion: ≤73 years; CAD and physical disability. CAD documented by history of MI, coronary artery bypass surgery, percutaneous transluminal coronary angioplasty or angiographically demonstrated CAD; have the functional use ofmore than 2 extremities, 1 being an arm, in order to perform the exercise test and training protocols Exclusion: uncontrolled hypertension or diabetes mellitus, clinically significant cardiac dysrhythmias, unstable angina pectoris, cognitive deficits, or other problems that would interfere with compliance to the prescribed exercise and diet protocol |
|
| Interventions | Exercise group (Home exercise training programme): Duration: 6 months; Frequency: 5 days/week; Session duration: 20mins/day; Intensity: 85% of predicted maximal heart rate Mode: stationary wheelchair ergometer Control group: routine care |
|
| Outcomes | Total mortality, non fatal MI at 6 months | |
| Notes | The treatment programme decreased myocardial oxygen demand. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “The same experienced cardiologist interpreted all echocardiograms and was unaware of randomization procedures” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 32% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single centre RCT in Sweden. F/U 1 & 5 years. | |
| Participants | N=178 (EX n=87; CON n=91) randomized N=116 (EX n=53; CON n=63) participated in the 1year F/U Gender: 101 men & 15 women Mean age: EX=55 years CON=57.6 years Ethnicity: NR Inclusion: 65 years or younger at the time of MI; independent living in the Health Care District after discharge from hospital; meaningful communication and rehabilitation that was not hindered by the MI or other serious illness Exclusion:cerebral or cardiac disorders or serious alcohol abuse |
|
| Interventions | Exercise group: Duration: 6months; Frequency: 1 weekly; Session duration: 2hrs; Mode: 1 hours exercise + 1 hours group discussion led by nurse Control: routine cardiac follow-up |
|
| Outcomes | Total mortality, non fatal MI, revascularisations | |
| Notes | Positive long term effects on physical condition, life habits, cardiac health knowledge. No effects found for cardiac events or psychological condition |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly subdivided” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 32% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Parallel single centre RCT in Italy; 6 month F/U | |
| Participants | N=61 (EX n=30; CON n=31) 72.1% male. Mean age: EX=55.9 years; CON=55.1 years Diagnosis: post-infarction Ethnicity: NR Inclusion: acute ST elevation MI Exclusion: residual myocardial ischemia, severe ventricular arrhythmias, AV block, valvular disease requiring surgery, pericarditis, severe renal dysfunction (creatinine >2.5 mg/dL) |
|
| Interventions | Exercise group: Duration: 6 month; Frequency: 3×/week; Session duration: 30 min; Mode: bicycle ergometer; Intensity: target of60-70% ofVo2 peak achieved at the initial symptom-limited cardiopulmonary exercise test Control group: discharged with generic instructions to maintaining physical activity and a correct lifestyle |
|
| Outcomes | Fatal/non-fatal MI (6month F/U) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | The physician performing all Doppler-echocardiography and cardiopulmonary exercise tests was unaware of the results of blood sampling and was blinded to the patient allocation into the study protocol Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Multicentre parallel RCT (4 centres in US) ; F/U 4 years | |
| Participants | N=300 (EX n=145; CON n=155) Gender: 259 men & 41 women Mean age: EX = 58.3 =/− 9.2, CON = 56.2 +/− 8.2. Diagnosis: CAD Ethnicity: NR Inclusion: < 75 years; clinically indicated coronary arteriography. After arteriography, patients received PTCA or CABG and remained eligible if at least one major coronary artery had a segment with lumen narrowing between 5% and 69% that was unaffected by revascularization procedures Exclusion: severe congestive heart failure, pulmonary disease, intermittent claudication, or noncardiac life-threatening illnesses; no qualifying segments, medical complication occurred during angiography, left ventricular ejection fraction of less than 20%, or patient was in another research study |
|
| Interventions | Exercise group (risk reduction group): Intructed by dietitian in a low-fat, low-cholesterol, and high-carbohydrate diet with a goal of <20% of energy intake from fat, <6% from saturated fat, and <75mg of cholesterol per day. Physical activity program : increase in daily activities such as walking, climbing stairs, and household chores and a specific endurance exercise training program with the exercise intensity based on the subject’s treadmill exercise test performance. (Nurse managed, home based programme based on Miller, with specific goals to be attained) Control group: usual care F/U 4 years. |
|
| Outcomes | Total & CHD mortality, non fatal MI, revascularisation at yr 1, 2, 3 and 4 | |
| Notes | The rate of change in the minimal coronary artery diameter was 47% less in I than C. This was still significant when adjusted for age and baseline segment diameter (p=0.03) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “stratified random numbers in sealed envelopes” |
| Allocation concealment (selection bias) | Low risk | “stratified random numbers in sealed envelopes” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 18% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Cluster randomised multi-centre study (hospitals in and around Newcatle, Australia); F/U of 6 months | |
| Participants | N=450 (EX n=213; CON n=237) 71% male Mean age: EX = 59 +/− 8, CON = 58 +/− 8 years Diagnosis: Ethnicity: NR Inclusion: <70 years with a suspected heart attack registered by the Newcastle collaborating centre of the WHO MONICA Project and discharged alive from hospital Exclusion: renal failure or other special dietary requirements and those considered by their physicians to have ‘endstage’ heart disease |
|
| Interventions | Exercise group: 3 packages to participant - 1st package: Step 1“Facts on fat” kit, together with walking programmme information (also (encouragement to walk in the form of a magnetic reminder sticker), and “Quit for Life” program for smokers. 2nd package: Step 2-3 “Facts on fat” kit; exercise log. 3rd package: Step 4-5 “Facts on fat” kit, together with information regarding local “Walking for Pleasure” groups Control group: usual care |
|
| Outcomes | Total mortality, health-related quality of life: QLMI Study outcomes assessed at 6 months |
|
| Notes | Low use of preventative services (dietary, anti smoking) by both groups. 10% of patients received rehab - mostly having had CABG. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation by GP. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-center, RCT in Sweden; F/U 2 years | |
| Participants | N=87 (EX n=46; CON=41) Gender 83.9% male Mean age: EX=53 years; CON=53 years Diagnosis: treated with percutaneous transluminal angioplasty Ethnicity: NR Inclusion: at least one significant coronary stenosis suitable for PTCA and at least one additional clinically insignificant coronary atherosclerotic lesion that could be evaluated by quantitative computerized angiography; <65 years; employed; able to perform a bicycle ergometer test with a minimum capacity of 70 W following the PTCA; absence of other disease of importance for completion of the programme |
|
| Interventions | Exercise group: 12 month rehabilitation programme (intense health education and activities promoting behavioural changes - stress management, diet, exercise and smoking habits). Each subject was assigned a daily individual task including self-observation, Type A behavioural drills, relaxation training and exercise. This programme is followed by 11-month step-down period, leaving the patients on their own during the second year of follow up Control group: standard care |
|
| Outcomes | Cardiovascular mortality, MI, CABG, PTCA, health-related quality of life: AP-QLQ recorded during the 2 years F/U | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 21.8 % lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single centre RCT in Sweden; F/U 1 yr | |
| Participants | N= 69 (EX n=34; CON n=35) Gender: 67 men & 2 women Mean age 55, range 38 - 63 years Diagnosis: Post-MI Inclusion: Acute MI patients under 65 years of age Exclusion: Not stated by patients have been excluded for being incapable of performing strenuous training due to poor left ventricular function or arrhythmias, orthopaedic disorders, other incapacitating somatic diseases or mental disorders |
|
| Interventions | Exercise group: Duration: 12 weeks starting 8 weeks post MI.; Frequency: 2× per week; Session duration and mode: at least 45 mins (bicycling 10 mins, callisthenics 10min, jogging 15 min, relaxation 10min); Intensity: 70% to 85% of peak heart rate at the bicycle test for initial session and workload individually adjusted to obtain the desired maximum heart rate if possible Control group: not enrolled in the training programme |
|
| Outcomes | Total mortality, non-fatal MI & revascularisation. health-related quality of life: Self report questionnaire. Evaluations at 6 weeks and 1 year post MI |
|
| Notes | Authors found no benefit from exercise training. Outcomes were related to self-rated levels of physical and psychological well being | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed according to random numbers in sealed envelopes” |
| Allocation concealment (selection bias) | Low risk | “Randomization was performed according to random numbers in sealed envelopes” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 14.5% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT in 2 Finnish centres; F/U 3 years. | |
| Participants | N= 375 (EX n=188; CON n=187) Gender: 80.3% male Mean age: EX=54.4 years; CON=54.1 years Diagnosis; acute myocardial infarction. Ethnicity: NR Inclusion: AMI based on WHO criteria |
|
| Interventions | Exercise group (Intervention group) consisted of anti-smoking and dietary advice, and discussions on psychosocial problems as well as a physical exercise programme, tailored to the individual’s working capacity determined in a bicycle ergometer test Control group: usual care | |
| Outcomes | Total mortality; Cardiovascular mortality (F/U 3 years) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1% lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT in Australia (2 centres); F/U 6 months | |
| Participants | N=142 (EX n=70; CON n=72) Mean age: EX=56.2; CON=55.8 years Male: EX=89% vs CON=86% Diagnosis: had an AMI Ethnicity: NR Inclusion: AMI; <75 years of age; no angina; <2mm ST-segment depression with exercise and if they attained >7-METS workload; left ventricular ejection fraction >40% or no inducible ventricular tachycardia |
|
| Interventions | Exercise (conventional treatment group): 5 week rehabilitation program consisted of exercise, education, and counseling sessions that were held 2 to 4 times per week, including work at 6 weeks after AMI Control group (ERNA - early return to normal activities group): work at 2 weeks after AMI without a formal rehab program |
|
| Outcomes | Total mortality; fatal/non-fatal mortality; CABG; PTCA; HRQL Assessment at 6 weeks and at 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomisation schedules were generated by an independent investigator” Comment: no description of randomisation methods. |
| Allocation concealment (selection bias) | Low risk | “…opaque sealed envelopes. These envelopes were opened by the nurse coordinator only at randomization of a patient” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “GHPS..analysed in a blinded fashion by an independent nuclear medicine specialist” Comment: Unclear in terms of other relevant outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 20.4% lost to follow-up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Parallel RCT; single-centre f/u 10 yrs | |
| Participants | N=95 (EX: n=49; CON: n=46) Age: EX: 51 years; CON: 52 years 100% males Diagnosis: surviving first uncomplicated MI Ethnicity: NR Inclusion: post MI patients admitted at Centro Medico di Montescano in 1984 and 1985 Exclusion: atrial fibrillation or abnormal sinus node function, insuline-dependent diabetes, exercise-induced myocardial ischemia, and arterial BP > 160/90 |
|
| Interventions | EX: Duration: 4-week endurance training; session duration: 30 minutes, 5 times a week; mode: callisthenics and stationary bicycle ergometry. All patients attended sessions, held by cardiologist and psychologist, dealing with secondary prevention of cardiovascular disease and stressing dietary changes and smoking cessation UC: “no training” |
|
| Outcomes | Cardiac mortality; nonfatal MI; CABG at 3 to 4 month intervals from the time of entry into the study for the first 3 years and contacted periodically by telephone thereafter | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias)bias) All outcomes |
Low risk | All patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT in 4 participating hospitals in France F/U 2 years | |
| Participants | N=182 (EX n=61; CON n=60) n=60 for counselling group 100% male Mean age: EX = 51, CON = 49 yrs. Diagnosis: MI Ethnicity: NR Inclusion: admitted to participating CCUs with suspected MI; under 65 years old with typical MI, no major irreversible complication or disability Exclusion: contraindication to exercise testing i.e., recent stroke, disability of lower limbs, uncontrolled heart failure, severe rhythm disturbances, SBP> 180 mmHg, severe angina pectoris, or abnormalities triggered by baseline exercise test |
|
| Interventions | Exercise group (rehab programme): Duration: 6 week; Frequency 3×/week; Session duration and mode: 25min cycloergometer Intensity: 80% of maximal heart rate. Also included walking, gymnastic and respiratory physiotherapy, relaxation, recommendations on control ofcardiovascular risk factors; recommendations to continue regular physical training at the end of the 6 week programme Control: usual care |
|
| Outcomes | Non fatal MI, angina, surgery, smoking | |
| Notes | Only 14% of all MI patients admitted to the participating hospitals were randomised to the trial. Exclusion of women and patients >65 accounted for 60% of exclusions | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No losses to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | F/U 1 year | |
| Participants | 126 men & 50 women, mean age 55.8 yrs, post MI. | |
| Interventions | Heart manual: home based facilitated programme with manual and tapes, 3 stage exercise plan - home, walking and life long, graded according to patient’s ability. Control had placebo facilitator’s time. F/U 1 year |
|
| Outcomes | HRQL: HAD; GHQ | |
| Notes | Study terminated (due to expiry of funding) before all pts reached 6 or 12-month stage. Anxiety scores showed significant treatment effect @ 6 weeks and 1 year, depression @ 6 weeks. Pre hospital discharge 52% of all pts had HAD scores indicating clinically significant anxiety or depression (8+). C were significantly more anxious and depressed at all follow ups |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “allocated to the experimental or control group by use of a written pre-determined randomisation protocol” Methods not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “The medical secretary who held the list was blind to the purpose of the study and to the patients taking part, and the cardiologist and nursing staff were blind to which study group the patients were in” Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 17% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-centre RCT in India; f/u 1 yr | |
| Participants | N=42 (EX n=21; CON n=21) 100% male Mean age: EX = 51 years; CON=52 years Diagnosis: chronic stable angina and angiographically proven CAD Ethnicity: NR Inclusion: chronic stable angina and angiographically proven CAD |
|
| Exclusion: recent (within last six months) MI or unstable angina | ||
| Interventions | Exercise group: program consisting of yoga at home for average of 90 min daily, control of risk factors, diet control and moderate aerobic exercise Control: usual care = ȁCmanaged by conventional methods i.e. risk factor control and American Heart Association step I diet” |
|
| Outcomes | total mortality; CABG; PTCA Assessments are baseline and 1 yr. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “Two independent observers who were blinded to group allocation analysed all arteriograms” Blinding of other outcome assessments were not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-centre RCT in Italy; f/u 14 mos | |
| Participants | N= 270 (EX n=90; Home n=90; CON n=90) Gender: 67.8% males Mean age: 69 years Diagnosis: post-MI Ethnicity: NR Inclusion: >56 years; referred to unit for functional evaluation 4 to 6 weeks after MI Exclusion: severe cognitive impairment or physical disability, left ventricular EF <35%, contraindications to vigorous physical exercise, eligibility for myocardial revascularization because of low-effort myocardial ischemia, refusal, or living too far from the unit |
|
| Interventions | EX: Hospital-CR: program consisted of 40 exercise sessions: 24 sessions (3/wk) of endurance training on cycle ergometer (5-min warm-up, 20-min training at constant workload, 5-min cool down, 5-min post-exercise monitoring) plus 16 (2/wk) 1-hr sessions of stretching and flexibility exercises Home-CR: 4-8 supervised instruction sessions in CR unit, where taught how to perform training at home; then patients received exercise prescription similar to Hosp-CR group CON: no CR, attended single structured session on CV risk factor management with no exercise prescription and were referred back to their family physicians |
|
| Outcomes | mortality, MI, CABG, PTCA, HRQL at month 2, 8 and 14 costs over study duration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 38 (14.1%) dropped out; clinical event data for these patients not reported per treatment group |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised 3 weeks post MI | |
| Participants | 198 men < 70 yrs with MI. Mean age 52 +/−9. |
|
| Interventions | Patients divided into 5 interventions; 1a-extended home 1b-brief home 2a-extended group 2b-brief group 3-ETT but no further training 4-no ETT or training. |
|
| Home; detailed instructions + HR monitors. If free of ETT induced angina @3 weeks pts used stationary bikes for 30 mins/day, 5 days/week. If had ETT induced angina @ 3 weeks, brisk walking programme for 100 mins/week. 2× weekly telemetry to base from HR monitors. Brief intervention trained for 8 weeks, extended intervention for 23 weeks. Group intervention trained in a group with clinical supervision for 8 or 23 weeks for 3 × 1 hour/week with 100 mins/week at training rate All pts in 1a & b, 2 a & b and 3 received counselling from a physician (30-45 mins) and nurse (30-45 mins). F/U 23 weeks. |
||
| Outcomes | CHD mortality, non fatal MI and revascularisation | |
| Notes | Low rate of cardiac events reflects identification of low risk population. Group 3 were unexpectedly active, th authors concluding that ETT + good explanation may enhance physical activity in the early stages |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 5% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Stratified by status (work type and employed or not) and randomised at time of MI. All participants were depressed and/or anxious (Beck Depression Inventory <5, < 43 on Spielberger State Anxiety Inventory, or <42 on Spielberger Trait Anxiety Inventory.) | |
| Participants | 177 men & 24 women with MI. Mean age I =52.9+/− 9.5 yrs, C = 52.7 +/− 9.5 yrs. |
|
| Interventions | ET for participant & spouse. 50 minutes 2 × weekly for 8 weeks at 65% of HRmax during ETT. Plus cognitive behavioural group intervention of 8 sessions of 1.5 hours + relaxation. |
|
| CPR training offered to spouse. F/U 1 year. |
||
| Outcomes | Mortality health-related quality of life: QOLMI time trade-off. | |
| Notes | Both groups improved over 12 months, with the biggest changes occurring in the first 8 weeks | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | For the primary outcome -HRQL- 9% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Prospective RCT in US (patients recruited from 2 sites) F/U 5 years | |
| Participants | N= 48 (EX n=28; CON n=20) Gender: NR for all 48 patients Mean age: EX = 56.1 +/− 7.5; CON=59.8 +/− 9.1 years Diagnosis: moderate to severe CAD (MI, PTCA, CABG, angina) Ethnicity: NR Inclusion: 35-75 years, male or female; residence in the greater San Francisco area; no other life-threatening illnesses; no MI during the preceding 6 weeks, no history of receiving streptokinase or alteplase; not currently receiving lipid-lowering drugs; 1, 2, 3 vessel coronary artery disease (defined as any measurable coronary atherosclerosis in a non-dilated or non-bypass grafting; permission granted by patient’s cardiologist and primary care physician |
|
| Interventions | Exercise intervention: exercise (typically walking) for a minimum of 3 hours per week and 30 min per session; target training heart rate of 50-80%. Co-interventions: stress management, low fat vegetarian diet, group psychosocial support . 1 year duration Control group: usual care. |
|
| Outcomes | CHD mortality, non-fatal MI, revascularisation, Assessment at baseline and after 1 year and 5 year | |
| Notes | I had 91% reduction in reported frequency of angina after 1 year and 72% after 5, C had 186% increase in reported frequency of angina after 1 year and 36% decrease after 5. I had 7.9% relative improvement in coronary artery diameter at 5 years, C had 27.7% relative worsening at 5 years |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “…investigators carrying out all medical tests remained unaware of both patient group assignment and the order ofthe tests. Different people provided the lifestyle intervention, carried out the tests, analysed the results, and carried out statistical analyses. Coronary arteriograms were analysed without knowledge ofsequence or ofgroup assignment.” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 45/93 (48%) of randomised patients did not participate, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Participants randomised after routine angiography for angina. 66% study population had previous MI. All participants spent one week as inpatient on a metabolic ward receiving instruction on exercise and diet |
|
| Participants | 113 men with CAD, aged 35 - 68 yrs (mean 53.5) | |
| Interventions | 2 further weeks as IP, then daily exercise at home on cycle (30 mins at 75% HR max) + 2 group training sessions of 60 mins/week. Informative session held 5 times/year for participants and spouses. F/U yearly for 6 years. |
|
| Outcomes | Total and CHD mortality, non fatal MI, revascularisation, | |
| Notes | Exercise adherence in the first year was 68% (39-92%, over the next 5 years 33% (389%). Pts with regression of coronary atheroma attended exercise sessions significantly more often (54+/− 24%) than patients with no change (20+/− 24%) or progression 31+/− 20%) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 20% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single centre RCT in Japan; F/U 6 months. | |
| Participants | N= 38 (EX n=20; CON n=18) 100% male Mean age: 70 years Ethnicity: Japanese patients Diagnosis: Chronic CAD Inclusion: referred at least 6 months after a major coronary event, including acute MI, coronary artery bypass grafting or percutaneous balloon angioplasty for acute coronary syndrome |
|
| Interventions | Exercise: Duration 6 months; Frequency: weekly; Session duration and mode: 20-30min upright aerobic and dynamic exercise (walking, bicycling, jogging etc) and light isometric exercise (hand weights) and 20 min cool-down stretching and callisthenics. Intensity: prescribed individually at the anaerobic threshold level at baseline. Patients also encouraged to exercise twice a week outside the clinicCo intervention: dietary and educational program Control group: standard care |
|
| Outcomes | health-related quality of life at 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned..by envelope method” |
| Allocation concealment (selection bias) | Unclear risk | “randomly assigned..by envelope method” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All 38 patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single centre RCT in Japan; F/U months | |
| Participants | N= 39 (EX n=20; CON n=19) 100% male Mean age: 69.5 years Diagnosis: stable CAD Ethnicity: Japanese patients Inclusion: <65 years old with stable CAD Exclusion:ongoing congestive heart failure, liver dysfunction, renal dysfunction, or systemic diseases, including malignancy and collagen disease |
|
| Interventions | EX:exercise training Duration 6 months; Frequency: weekly; Session duration and mode: 20-60min upright aerobic and dynamic exercise (walking, bicycling, jogging etc) and light isometric exercise (hand weights) and 15 min cool-down stretching and callisthenics. Intensity: prescribed individually at the anaerobic threshold level as measured by a treadmill exercise test. Patients also encouraged to perform aerobic exercise twice weekly (≥30 min) at home. Co-intervention: diet therapy, and weekly counselling Control: usual outpatient care |
|
| Outcomes | Total mortality; non-fatal/fatal mortality. See notes below. | |
| Notes | “No subject in either group showed any worsening of symptoms or had clinical events during this study.” | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No information reported. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Participants treated at one of 5 participating centres. Participants randomised after participating in low level exercise course for 6 weeks | |
| Participants | 651 men aged 30 - 64 yrs with MI between 8 weeks and 3 years prior to start of study (mean 14 months). Mean age I = 51.5+/− 7.4, C= 52.1 +/− 7.2 |
|
| Interventions | ET- 1 hour/day, 3 days/week for 8 weeks. 6 station circuit + gym exercises or swimming and games. F/U 3 years. Long term follow up to 19 years published in 1999, but not used for meta analysis |
|
| Outcomes | Total & CHD mortality, non fatal MI | |
| Notes | 90% of ET attended 90% of 24 scheduled sessions post randomisation, only 48% attending > 50% of sessions at 18 months. 30% of control alleged exercising regularly, on own initiative. At 19 years any protective effect form the programme had decreased over time, but an increase with PWC from the beginning to the end of the trial was associates with a consistent reduction in mortality throughout the 19 years of follow up |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 6.5% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Multicentre study. Random allocation of individuals to two intervention groups (exercise only or exercise plus teaching and counselling) and a control group (usual care) | |
| Participants | 258 patients (>80% men) aged <71 yrs. Mean age I = 55.6+/− 9.3, 56.3 +/− 8.3, C = 57.1 +/− 7.3. Following acute MI. |
|
| Interventions | All patients exercise whilst in hospital. Ex only: Weekly clinic appointments 3 months post discharge for progressive callisthenics and walking. Exercise 2 × daily until RTW and then × 1 daily. Ex + T&C: Same exercise programme + 8 × 1 hour teaching/ counselling sessions with family & friends F/U 6 months. |
|
| Outcomes | Total mortality; health-related quality of life: Sickness Impact Profile | |
| Notes | Several reports of the same trial all with various bits of information. Authors conclude that multiple intervention trial ofthis short duration did not change patient’s behaviour. MI itself acts as a strong stimulus to alter behaviour with respect to risk factors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 24% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised at hospital discharge. All participants went to a rehab centre for 3 weeks for ETT, 24 hour tape. All participants had sessions with cardiologist & psychologist for secondary prevention advice |
|
| Participants | 182 men & 18 women < 65 yrs with MI. Mean age I = 51.5 +/−7, C= 54.3+/−8. |
|
| Interventions | 4 weeks supervised cycling for 30 mins 5 days/week + callisthenics @ 75% max work capacity. After discharge to walk for 30 minutes every 2 days. F/U 34 months. |
|
| Outcomes | CHD mortality, revascularisations | |
| Notes | Ejection fraction was the only prognostic factor. Among 51 patients with EF <41%, relative risk for the 27 untrained participants was 8. 63 times higher than for 24 trained ones. (p=0.04) If EF > 40%, estimated risk for untrained participant was 1.07 times higher than for trained |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No losses to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomized by blocks of 6 into one of three groups: exercise, group counselling & control. Eligibility - work capacity <7 METs (men), <6 METs (women), Taylor Manifest Anxiety Scale raw score of 19+ and/or Zung self rating Depression Scale raw score of 40+ |
|
| Participants | 91 men & 15 women aged 30-60 yrs with MI between 6 weeks and 1 year prior to entry to study | |
| Interventions | 3×1 hour sessions/week over 12 week period for 36 sessions. All exercises dynamic against resistance, exercising upper limb and lower limb alternately for 4 minutes with 2 mins rest in between. Target HR 85% of HRmax at ETT. F/U 1 year. |
|
| Outcomes | Mortality, non fatal MII | |
| Notes | Minimal differences between groups at one year. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 7.7% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-centre RCT in Sweden; f/u 1 y | |
| Participants | 109 patients ?65 years (80% males) admitted to hospital because of acute coronary event (defined as either acute MI, n=64; or episode of unstable angina, n=45) EX: n=56 (mean age = 71 y, range 64-84; 41 men) UC : n=53 (mean age = 68 y, 65-83; 40 men) |
|
| Interventions | EX : 50 min aerobic outpatient group-training programme (including warm-up and cool-down) 3 times a week for 3 mos. Complete programme was supervised by specialized physiotherapist and supported by music which guided intensity of performance during session). Training followed by 10 min of music-supported relaxation. After 3 mos, patients had possibility of participating in programme once a week for another 3 mos UC: encouraged to re-start usual/prior physical activity as soon as they felt fit |
|
| Outcomes | total mortality, CABG, PTCA, health-related quality of life; Karolinska Questionnaire at 12-months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Clinical event data for 8 (7%) who withdrew before 3 months were not accounted for at 1 yr |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised controlled trial with follow-up of 24 months. | |
| Participants | 28 postmenopausal women with coronary heart disease, defined as atherosclerosis, MI, percutaneous transluminal coronary angioplasty, and/or coronary bypass graft surgery. Mean age: 64 years |
|
| Interventions | Randomised to PrimeTime program (very low-fat vegetarian diet, stress-management training, exercise, group support, and smoking cessation) or to usual care n=17 for PrimeTime program and n=11 for usual care | |
| Outcomes | health-related quality of life: SF-36 at 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 3/28 (10.7%) patients lost to follow-up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised after ETT, 30 days after MI. | |
| Participants | 50 patients aged 40 to 60 yrs with MI (mean 50.1). | |
| Interventions | 6 weeks physical training programme. F/U 1 year. |
|
| Outcomes | CV mortality | |
| Notes | Trained patients showed a better mid term prognosis than controls, but this could not be explained by the physical training procedure | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 24% lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised 4-6 weeks post MI after ETT. | |
| Participants | 98 men aged 40- 55 yrs with MI. Mean age I = 49.4 +/− 3.7, C = 49.1 +/− 4.5. |
|
| Interventions | Rehabilitation programme. F/U 5 years |
|
| Outcomes | Mortality, non fatal MI, | |
| Notes | Authors conclude that cardiac rehab benefits patients after MI due to direct effect on myocardial perfusion and to lowering of cholesterol levels | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No losses to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | RCT of 2 years duration | |
| Participants | 197 patients admitted to hospital for acute MI, unstable angina pectoris or after coronary artery bypass grafting. 82.2% male. Mean age: 55 years n=98 for intervention group and n=99 for usual care group. |
|
| Interventions | EX: lifestyles intervention program (low fat diet, regular exercise, smoking cessation, psychosocial support and education, delivered by nurses on the rationale for pharmacological and lifestyle measures) Usual care |
|
| Outcomes | Total mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ȁrandomised’ |
| Allocation concealment (selection bias) | Low risk | ‘[Randomization] was performed with pre-prepared sealed opaque envelopes containing details on group allocation. The patients opened the envelopes themselves so that their allocation to IP or UC was revealed to them without the prior knowledge of the study investigators’ |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 17.8 % lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | 24 centre, pan European study conducted between 1973 and 1978. Randomised on discharge from hospital. 12 centres accepted for meta analysis | |
| Participants | 160 Men < 65 yrs with first or consecutive MI. Mean age for all participants I = 52.3, C= 53.5. |
|
| Interventions | Comprehensive programme dependent on local provision. Physical training was not compulsory but was strongly recommended. F/U 3 years Local training for 6 weeks |
|
| Outcomes | Total mortality, CVD, CHD & sudden death. Fatal & non fatal re-infarction. |
|
| Notes | Methodological problems with the execution of the study allowed only death and rein-farction to be successfully used as endpoints | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Individually randomised, but method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms of assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | No description of withdrawals or dropouts. Varied greatly from site to site |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Randomised on discharge. All patients received information on increasing physical activity during convalescence | |
| Participants | 280 men & 35 women < 55 yrs with MI. Mean age 50.6. | |
| Interventions | Training programme 3 months after MI, 3 × half hour sessions per week based in hospital, at home or in workplace. F/U 5 years | |
| Outcomes | Mortality, re-infarction. | |
| Notes | 1 year post MI, 39% of those who started training were training at the hospital. A further 21% trained at home or at work | |
| Risk of bias | ||
| Bias | Authors judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “By the use of a random number table the patients were allocated…” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “The exercise test 1 yr after the MI followed the same protocol but was conducted by another physician, who did not know if the patients belonged to the experimental or the control group.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No losses to follow up for clinical events. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Unblinded, single-centre RCT in China; f/u 2 y | |
| Participants | 112 obese patients with CHD who had either recent AMI (n=72) or had undergone elective PCI (n=40) within 6 wks EX: n=72 (mean age = 62.3 y; 59 men, 13 women) UC : n=40 (mean age = 61.2 y; 30 men, 10 women) |
|
| Interventions | EX : Phase 1 was impatient ambulatory program that lasted 7-14 d; phase 2 was 16-session, twice weekly, outpatient exercise and education program lasting for 8 weeks, each session included 1 hr of education class followed by 2 hrs of exercise training, 1st hour oftraining was conducted by physiotherapist; phase 3 was community-based home exercise program for another 6 mos; phase 4 was long-term follow-up program until end of 2 years which stressed importance of regular exercise and risk factor modification UC: attended 2-hr talk that explained CHD, importance of risk factor modification, and potential benefits of physical activity, but without undergoing outpatient exercise training program |
|
| Outcomes | health-related quality of life: 3F-36 at 8 & 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unclear in terms ofassessment ofother outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Single-center, unblinded, single-centre RCT in China; f/u 2 y | |
| Participants | 269 patients (76% men; mean age 64 y) with recent AMI (n=193) or after elective percutaneous coronary intervention (n=76) EX: n=181 (mean age, 64 SD 11 y; 138 males, 43 females) UC: n=88 (mean age, 64 SD 11 y; 66 males, 22 females) |
|
| Interventions | EX : Phase 1 was impatient ambulatory program that lasted 7-14 d; phase 2 was 16-session, twice weekly, outpatient exercise and education program lasting for 8 weeks, each session included 1 hr of education class followed by 2 hrs of exercise training, 1st hour oftraining was conducted by physiotherapist; phase 3 was community-based home exercise program for another 6 mos; phase 4 was long-term follow-up program until end of 2 years which stressed importance of regular exercise and risk factor modification UC: attended 2-hr talk that explained CHD, importance of risk factor modification, and potential benefits of physical activity, but without undergoing outpatient exercise training program |
|
| Outcomes | Total mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “The QOL assessments were performed on all patients in all 4 phases by a trained social worker who was unaware of the randomization” Unclear in terms ofassessment ofother outcomes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 24 % lost to follow up, no description of withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Methods | Pragmatic, open-label, single-centre RCT in Denmark; f/u 1 y | |
| Participants | 446 patients having IHD (MI or angina pectoris in accordance with European guidelines) EX: n=227 (mean age 67 y) UC: n=219 (mean age 67 y) |
|
| Interventions | EX : 6-week intensive rehabilitation program including patient education, 12 exercise training sessions, dietary counseling, smoking cessation, psychosocial support, risk factor management and clinical assessment UC: attended 2-hr talk that explained CHD, importance of risk factor modification, and potential benefits of physical activity, but without undergoing outpatient exercise training program |
|
| Outcomes | Total mortality, MI, CABG, PTCA, health-related quality of life: SF-36 at 1-yr follow up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The Copenhagen Trial Unit computer generated the allocation sequence and provided central secretary-staffed telephone randomization” |
| Allocation concealment (selection bias) | Low risk | “The essential patient data were registered, and the result of the randomization as delivered to the research nurse, who informed the CCR team and the patient about the allocation” |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “The interventions were open to the patients and investigators. Investigator-independent outcome data from registries were chosen to ensure blinded outcome assessment. The scientific team and CCR team collected secondary outcome measures blinded to intervention at baseline and without blinding at 12 months” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All IHD patients accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
EX: exercise based cardiac rehabilitation
UC: usual care
MI: Myocardial infarction
CHD: Coronary heart disease
SBP: Systolic blood pressure
DBP: Diastolic blood pressure
HDL: High density lipoprotein
LDL: Low density lipoprotein
QoL: Quality of life
V02max: Maximum oxygen uptake
CV: Cardiovascular
PWC: physical work capacity.
ET: exercise training
RTW: return to work
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Agren 1989 | Improper method of randomisation (based on date of birth). |
| Aronov 2006 | No useful outcome data reported. |
| Ballantyne 1982 | No useful outcome data reported. |
| Belardinelli 2007 | Abstract only with incomplete reporting of study characteristics and outcome data. Full trial report not published |
| Bettencourt 2005 | Only a small subset of randomised patients responded via questionnaire. Incomplete outcome data |
| Björntorp 1972 | Not a randomised study. Participants divided alternately after admission |
| Blumenthal 1997 | Control group was not randomised, but selected on geographical basis |
| Bär 1992 | Method of randomisation was inadequate; of a study population of 265 across 5 centres only one centre randomised their patients, leaving a control group of 50 and an intervention group of 215 |
| Carlsson 1997 | No useful outcome data reported. |
| Gao 2007 | No useful outcome data reported. Duration of follow-up not reported |
| Giannuzzi 2008 | All patients (treatment and control) participated in 3-6 week cardiac rehabilitation programme (including supervised exercise sessions) prior to randomization. Control group was not “usual care” |
| Gielen 2003 | No useful outcome data reported. |
| Heldal 2000 | No useful outcome data reported. |
| Higgins 2001 | No useful outcome data reported. |
| Jiang 2007 | No useful outcome data reported. |
| Kentala 1972 | Quote: “On admission the patients were divided up according to their year of birth into a control group and a training group…” Not a randomised study. |
| Krachler 1997 | No useful outcome data reported. |
| Li 2004 | Follow-up <6 months. |
| Liao 2003 | Follow-up too short (3-4 weeks) and no useful outcome data reported |
| Mezey 2008 | Not a randomised study. |
| Peschel 2007 | No useful outcome data reported. |
| Piestrzeniewicz 2004 | No useful outcome data reported. |
| Roviaro 1984 | Not a randomised study. Assigned to treatment group according to geographic location |
| Schumacher 2006 | No useful outcome data reported. |
| Stenlund 2005 | No useful outcome data reported. |
| Takeyama 2000 | No useful outcome data reported. |
| Tokmakidis 2003 | No useful outcome data reported. |
| Wosornu 1996 | No useful outcome data reported. |
| Zheng 2008 | No useful outcome data reported. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unclear if randomized study. |
| Participants | Subjects consisted of 58 CAD patients who underwent PCI (experimental group: 30, control group: 28) |
| Interventions | The experimental group participated in an integrated symptom management program for 6 months which was composed of tailored education, stress management, exercise, diet, deep breathing, music therapy, periodical telephone monitoring and a daily log The control group received usual care. |
| Outcomes | Recurrent cardiac events, self care activity, quality of life |
| Notes | Article in Korean. Unable to find translator to answer following questions to determine study inclusion:
|
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Understanding Prognostic Benefits of Exercise and Antidepressant Therapy for Persons with Depression and Heart Disease (UPBEAT) Study |
| Methods | 5-year, single-site randomised clinical trial sponsored by the National Heart, Lung, and Blood Institute |
| Participants | 200 clinically depressed patients (with scores of Beck Depression Inventory ≥9) with stable CHD, including a previous (>60 days) myocardial infarction, revascularisation procedure, such as a PTCA or CABG, or a cardiac catheterization demonstrating significant coronary artery stenosis |
| Interventions | 4 months of treatment with supervised aerobic exercise, sertraline, or placebo |
| Outcomes | Depressive symptoms, heart rate variability, baroreflex control, vascular function (i.e., flow-mediated dilation), measures of inflammation and platelet aggregation |
| Starting date | Not reported. |
| Contact information | Blume003@mc.duke.edu |
| Notes | “This study is not powered to assess treatment group differences in CHD morbidity and mortality.” |
| Trial name or title | Akershus Comprehensive Cardiac Rehabilitation Trial (the CORE Study) |
| Methods | Randomized, controlled, parallel-group design, single centre trial, driven by the Medical Department of the Akershus Central Hospital in Oslo, Norway |
| Participants | 500 patients, men and women, aged 40-85 years, who have sustained at least one of the following: myocardial infarction, acute coronary syndrome, percutaneous transluminal coronary angioplasty and coronary artery bypass grafting |
| Interventions | Intervention: 8 weeks of supervised, structured physical training of three periods of 20 min per week, targeting a heart rate of 60-70% of the individual’s maximum; home-based physical exercise training with the same basic schedule as in the supervised period; quantification of patients’ compliance with the exercise programme by the use of wristwatches, information stored in the watch memory being retrieved once a month during the 3-year follow-up period; and life-style modification with an emphasis on the cessation of smoking and on healthy nutrition and weight control Control: Conventional care. |
| Outcomes | Primary: Quality of life. Secondary: total mortality, cardiovascular mortality, morbidity and recurrence rates of coronary events throughout a 3-year follow-up period |
| Starting date | Originally states as April 2000 with follow up complete by April 2004. No sign of publication to date. Contacted author with no reply |
| Contact information | drcornelpater@aol.com |
| Notes | Study design described at http://cvm.controlled-trials.com/content/1/3/177 |
DATA AND ANALYSES
Comparison 1. Exercise-based rehabilitation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 33 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow-up of 6 to 12 months | 19 | 6000 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.67, 1.01] |
| 1.2 Follow-up longer than 12 months | 16 | 5790 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.75, 0.99] |
| 2 Cardiovascular mortality | 19 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow-up of 6 to 12 months | 9 | 4130 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.71, 1.21] |
| 2.2 Follow-up longer than 12 months | 12 | 4757 | Risk Ratio (M-H, Fixed, 95% CI) | 0.74 [0.63, 0.87] |
| 3 Fatal and/or nonfatal MI | 26 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Follow-up of 6 to 12 months | 12 | 4216 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 3.2 Follow-up longer than 12 months | 16 | 5682 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 4 CABG | 21 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Follow-up of 6 to 12 months | 14 | 2312 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 4.2 Follow-up longer than 12 months | 9 | 2189 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.68, 1.27] |
| 5 PTCA | 11 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Follow-up of 6 to 12 months | 7 | 1328 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.69, 1.50] |
| 5.2 Follow-up longer than 12 months | 6 | 1322 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.66, 1.19] |
| 6 Hospital Admissions | 10 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Follow-up of 6 to 12 months | 4 | 463 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 6.2 Follow-up longer than 12 months | 7 | 2009 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.87, 1.11] |
ADDITIONAL TABLES
Table 1. Summary of health related quality of life (HRQL) scores at follow-up.
| Measure of HRQL | Mean (SD) outcome values at follow-up | P value | Difference between groups | |
|---|---|---|---|---|
| Exercise | Usual Care | |||
| Bell 1998 | ||||
| Nottingham health profile at 10.5 months follow-up: | ||||
| Energy | 17.6 (27.1) | 18.3 (29.8) | 0.87** | Exercise = Usual care |
| Pain | 2.8 (8.8) | 4.82 (11.9) | <0.05 | Exercise > Usual care |
| Emotional reactions | 6.4 (17.0) | 12.2 (19.9) | <0.001 | Exercise > Usual care |
| Sleep | 7.5 (18.4) | 20.5 (27.8) | <0.001 | Exercise > Usual care |
| Social isolation | 2.3 (10.6) | 4.0 (13.3) | 0.37* | Exercise = Usual care |
| Physical mobility | 8.4 (11.1) | 8.9 (14.5) | 0.82** | Exercise = Usual care |
| Belardinelli 2001 | ||||
| MOS at 6 months follow-up: | ||||
| PF | 78 (19) | 55 (20) | 0.001 | Exercise > Usual care |
| RP | 75 (13) | 65 (14) | 0.01 | Exercise > Usual care |
| BP | 4(9) | 22 (10) | 0.001 | Exercise > Usual care |
| GH | 68 (14) | 50 (19) | 0.001 | Exercise > Usual care |
| VT | NR | NR | ||
| SF | 66 (10) | 69 (12) | 0.14* | Exercise = Usual care |
| RE | NR | NR | ||
| MH | 65 (12) | 48 (15) | 0.01 | Exercise > Usual care |
| MOS at 12 months follow-up: | ||||
| PF | 82 (18) | 54 (20) | 0.001 | Exercise > Usual care |
| RP | 76 (9) | 58 (14) | 0.01 | Exercise > Usual care |
| BP | 4(9) | 32 (12) | 0.001 | Exercise > Usual care |
| GH | 70 (14) | 50 (18) | 0.001 | Exercise > Usual care |
| VT | NR | NR | ||
| SF | 68 (11) | 68 (12) | 1.00* | Exercise = Usual care |
| RE | NR | NR | ||
| MH | 70 (14) | 45 (15) | 0.001 | Exercise > Usual care |
| Engblom 1992 | ||||
| Nottingham health profile at 5 years follow-up: | ||||
| Energy | 18 | 25 | 0.08 | Exercise = Usual care |
| Pain | 12 | 18 | 0.07 | Exercise = Usual care |
| Emotional reactions | 14 | 21 | 0.27 | Exercise = Usual care |
| Sleep | 24 | 29 | 0.42 | Exercise = Usual care |
| Social isolation | 7 | 9 | 0.42 | Exercise = Usual care |
| Physical mobility | 6 | 14 | 0.005 | Exercise > Usual care |
| Heller 1993 | ||||
| QLMI at 6 months follow-up: | ||||
| Emotional | 5.4 (1.1) | 5.2 (1.2) | 0.04 | Exercise > Usual care |
| Physical | 5.4 (1.2) | 5.2 (1.3) | 0.17* | Exercise = Usual care |
| Social | 5.9 (1.1) | 5.8 (1.1) | 0.35* | Exercise = Usual care |
| Hofman-Bang 1999 | ||||
| AP-QLQat 12 months follow-up: | ||||
| Physical activity | 4.9 | 4.3 | <0.05 | Exercise > Usual care |
| Somatic symptoms | NR | NR | NS | Exercise = Usual care |
| Emotional distress | NR | NR | NS | Exercise = Usual care |
| Life satisfaction | NR | NR | NS | Exercise = Usual care |
| Oldridge 1991 | ||||
| QLMI at 4 months follow-up: | ||||
| Limitations | 54 | 54 | NS | Exercise = Usual care |
| Emotions | 103 | 101 | NS | Exercise = Usual care |
| QLMI at 8 months follow-up: | ||||
| Limitations | 54 | 54 | NS | Exercise = Usual care |
| Emotions | 103 | 103 | NS | Exercise = Usual care |
| QLMI at 12 months follow-up: | ||||
| Limitations | 54 | 55 | NS | Exercise = Usual care |
| Emotions | 105 | 102 | NS | Exercise = Usual care |
| Stahle 1999 | ||||
| Karolinska Questionnaire at 12 months follow-up: | ||||
| Chest pain | 0.6 (1.2) | 0.4 (1.3) | NS | Exercise = Usual care |
| Shortness of breath | 0.4 (1.1) | 0.2 (1.0) | NS | Exercise = Usual care |
| Dizziness | −0.1 (1.1) | 0.2 (0.9) | NS | Exercise = Usual care |
| Palpitation | −0.1 (1.0) | 0.1 (0.9) | NS | Exercise = Usual care |
| Cognitive ability | −0.1 (0.6) | 0.0 (0.7) | NS | Exercise = Usual care |
| Alertness | 0.0 (0.9) | 0.1 (0.8) | NS | Exercise = Usual care |
| Quality of sleep | 0.0 (0.5) | 0.1 (0.5) | NS | Exercise = Usual care |
| Physical ability | 0.2 (0.7) | 0.1 (0.4) | NS | Exercise = Usual care |
| Daily activity | 0.3 (0.5) | 0.1 (0.5) | NS | Exercise = Usual care |
| Depression | 0.1 (0.3) | 0.1 (0.2) | NS | Exercise = Usual care |
| Self perceived health | 0.5 (1.3) | 0.3 (1.0) | NS | Exercise = Usual care |
| “Ladder of Life” present | 1.2 (1.2) | 0.9 (1.8) | NS | Exercise = Usual care |
| “Ladder of Life future” | 0.8 (2.7) | 0.4 (2.3) | NS | Exercise = Usual care |
| Fitness | 0.6 (1.4) | 0.4 (1.0) | NS | Exercise = Usual care |
| Physical ability | 0.7 (1.0) | 0.4 (1.1) | NS | Exercise = Usual care |
| Toobert 2000 | ||||
| SF-36 at 24 months follow-up: | ||||
| PF | NR | NR | NS | Exercise = Usual care |
| RP | NR | NR | NS | Exercise = Usual care |
| BP | NR | NR | NS | Exercise = Usual care |
| GH | NR | NR | <0.05 | Exercise > Usual care |
| VT | NR | NR | NS | Exercise = Usual care |
| SF | NR | NR | <0.05 | Exercise > Usual care |
| RE | NR | NR | NS | Exercise = Usual care |
| MH | NR | NR | NS | Exercise = Usual care |
| Yu 2003 | ||||
| SF-36 at 8 months follow-up: | ||||
| PF | 88 (12) | 82 (17) | 0.03* | Exercise > Usual care |
| RP | 75 (33) | 66 (35) | 0.18* | Exercise = Usual care |
| BP | 80 (25) | 80 (25) | 1.00* | Exercise = Usual care |
| GH | 64 (26) | 60 (28) | 0.45* | Exercise = Usual care |
| VT | 79 (18) | 65 (17) | 0.0001 | Exercise > Usual care |
| SF | 89 (27) | 82 (28) | 0.15 | Exercise = Usual care |
| RE | 93 (18) | 83 (35) | 0.05 | Exercise = Usual care |
| MH | 84 (16) | 80 (15) | 0.20 | Exercise = Usual care |
| SF-36 at 24 months follow-up: | ||||
| PF | 88 (13) | 87 (9) | 0.67* | Exercise = Usual care |
| RP | 80 (32) | 79 (30) | 0.87* | Exercise = Usual care |
| BP | 81 (21) | 85 (20) | 0.33* | Exercise = Usual care |
| GH | 64 (20) | 61 (18) | 0.43* | Exercise = Usual care |
| VT | 73 (21) | 73 (17) | 1.00* | Exercise = Usual care |
| SF | 79 (30) | 90 (18) | 0.04* | Exercise > Usual care |
| RE | 89 (25) | 93 (25) | 0.42* | Exercise = Usual care |
| MH | 85 (14) | 85 (12) | 1.00* | Exercise = Usual care |
| Zwisler 2008 | ||||
| SF-36 at 12 months follow-up: | ||||
| PCS | 45.2 (9.8) | 46.4 (9.8) | 0.39* | Exercise = Usual care |
| MCS | 50.6 (10.8) | 48.4 (11.5) | 0.16* | Exercise = Usual care |
MOS=Medical Outcomes Study (MOS); Short Form-36 (SF-36); QLMI=Quality of Life After Myocardial Infarction questionnaire;
AP-QLQ=Angina Pectoris-Quality of Life questionnaire; PF=physical problems; RP=role limitations because of physical problems;
RE=role limitations because of emotional problems; VT=vitality; BP=bodily pain; SF=social functioning; MH=mental health; GH=general health perceptions; PCS=physical component summary; MCS=mental component summary; NR=not reported; NS=not significant
Calculated by authors of this report based on independent two group t test.
Adjusted for baseline difference between groups.
Exercise = Usual care: no statistically significant difference (P>0.05) between exercise and usual care groups at follow up
Exercise > Usual care: statistically significant difference (P=<0.05) between exercise and usual care groups at follow up
Table 2. Summary of costs of exercise-based rehabilitation and usual care.
| Variable | Kovoor 2006 | Marchionni 2003 | Yu 2004 |
|---|---|---|---|
| Follow-up (months) | 12 | 14 | 24 |
| Year of costs | 1999 ($AUD) | 2000 ($USD) | 2003 ($USD) |
| Mean cost of exercise-based rehabilitation (per patient): | |||
| Exercise | $394 | $5246 | NR |
| Usual Care | $0 | $0 | $0 |
| Mean difference (95% CI) | $394 | $5246 | NR |
| P value | NR | NR | NR |
| Costs considered | assessments, counseling, education | NR | staff salary, equipment, investigations |
| Mean total healthcare costs (per patient): | |||
| Exercise | NR | $17 272 | $15 292 |
| Usual Care | NR | $12 433 | $15 707 |
| Mean difference (95% CI) | NR | $4839 | −$415 |
| P value | NS, see below for details | NR | NS |
| Additional healthcare costs considered | phone calls (p=0.10); hospital admissions (p=0.11); gated heart pool scan (p=0.50); exercise stress test (p=0.72); other diagnostics (p=0.37); visits to general practitioner (p=0.61), specialist doctor (p=0.35), or health-care professional (p=0.31) | NR | hospitalisations; revascularisations; private clinic visit; cardiac clinic visits; public non-cardiac visits; casualty visits; drugs |
NR=not reported
Table 3. Results of univariate meta-regression analysis for total mortality.
| Explanatory variable | Exp(slope)* | 95% Confidence interval* | Proportion of variation explained | Interpretation |
|---|---|---|---|---|
| Case mix (% MI patients) | RR=0.99 | 0.99 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Dose of exercise (dose=duration in weeks × number of sessions × number of sessions per week) | RR=1.00 | 1.00 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Type of rehabilitation (exercise only vs comprehensive rehab) | RR=0.92 | 0.66 to 1.28 | 0% | No evidence that relative risk differs between types of rehabilitation |
| Follow up (months) | RR=0.99 | 0.98 to 1.01 | 0% | No evidence that relative risk is associated with case mix |
| Publication year (pre 1995 vs post 1995) | RR=0.80 | 0.54 to 1.20 | 0% | No evidence that relative risk is associated with publication year |
Table 4. Results of univariate meta-regression analysis for cardiovascular mortality.
| Explanatory variable | Exp(slope)* | 95% Confidence interval* | Proportion of variation explained | Interpretation |
|---|---|---|---|---|
| Case mix (% MI patients) | RR=1.01 | 0.98 to 1.04 | 0% | No evidence that relative risk is associated with case mix |
| Dose of exercise (dose=duration in weeks × number of sessions × number of sessions per week) | RR=1.00 | 1.00 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Type of rehabilitation (exercise only vs comprehensive rehab) | RR=0.84 | 0.57to 1.23 | 0% | No evidence that relative risk differs between types of rehabilitation |
| Follow up (months) | RR=0.99 | 0.98 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Publication year (pre 1995 vs post 1995) | RR=1.37 | 0.73 to 2.22 | 0% | No evidence that relative risk is associated with publication year |
Table 5. Results of univariate meta-regression analysis for total MI.
| Explanatory variable | Exp(slope)* | 95% Confidence interval* | Proportion of variation explained | Interpretation |
|---|---|---|---|---|
| Case mix (% MI patients) | RR=1.00 | 0.99 to 1.02 | 3.5% | No evidence that relative risk is associated with case mix |
| Dose of exercise (dose=duration in weeks × number of sessions × number of sessions per week) | RR=1,00 | 1.00 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Type of rehabilitation (exercise only vs comprehensive rehab) | RR=0.87 | 0.55 to 1.36 | 0.4% | No evidence that relative risk differs between types of rehabilitation |
| Follow up (months) | RR=0.99 | 0.98 to 1.01 | 6.3% | No evidence that relative risk is associated with case mix |
| Publication year (pre 1995 vs post 1995) | RR=1.38 | 0.82 to 2.33 | 0% | No evidence that relative risk is associated with publication year |
Table 6. Results of univariate meta-regression analysis for CABG.
| Explanatory variable | Exp(slope)* | 95% Confidence interval* | Proportion of variation explained | Interpretation |
|---|---|---|---|---|
| Case mix (% MI patients) | RR=1.01 | 1.00 to 1.02 | 3.5% | No evidence that relative risk is associated with case mix |
| Dose of exercise (dose=duration in weeks × number of sessions × number of sessions per week) | RR=1.00 | 1.00 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Type of rehabilitation (exercise only vs comprehensive rehab) | RR=1.13 | 0.67 to 1.93 | 0% | No evidence that relative risk differs between types of rehabilitation |
| Follow up (months) | RR=0.99 | 0.99 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Publication year (pre 1995 vs post 1995) | RR=0.84 | 0.50 to 1.42 | 0% | No evidence that relative risk is associated with publication year |
Table 7. Results of univariate meta-regression analysis for PTCA.
| Explanatory variable | Exp (slope)* | 95% Confidence interval* | Proportion of variation explained | Interpretation |
|---|---|---|---|---|
| Case mix (% MI patients) | RR=0.99 | 1.00 to 1.01 | 3.5% | No evidence that relative risk is associated with case mix |
| Dose of exercise (dose=duration in weeks × number of sessions × number of sessions per week) | RR=1.00 | 1.00 to 1.00 | 0% | No evidence that relative risk is associated with case mix |
| Type of rehabilitation (exercise only vs comprehensive rehab) | RR=0.99 | 0.39 to 2.54 | 0% | No evidence that relative risk differs between types of rehabilitation |
| Follow up (months) | RR=1.00 | 0.99 to 1.02 | 0% | No evidence that relative risk is associated with case mix |
| Publication year (pre 1995 vs post 1995) | RR=0.92 | 0.42 to 2.06 | 0% | No evidence that relative risk is associated with publication year |
WHAT’S NEW
Last assessed as up-to-date: 13 June 2010.
| Date | Event | Description |
|---|---|---|
| 4 July 2011 | Amended | Author (Neil Oldridge) details updated |
HISTORY
Protocol first published: Issue 3, 1999
Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 7 June 2011 | New search has been performed | The searches were updated and re-run in December 2009, identifying an additional 17 studies for inclusion. Fourty-seven trials in total have been included |
| 7 June 2011 | New citation required and conclusions have changed | The inclusion criteria have been revised for this update. Five out of the 35 formerly included studies (in the review) have therefore been excluded The conclusions have changed based on the analysis of 47 included studies and have focused more on the impact of exercise-based cardiac rehabilitation on clinical events and HRQL outcomes |
| 1 November 2000 | New citation required and conclusions have changed | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Changes in this update review
Given its policy focus, in addition to updating the original Cochrane review, this update review:
Excluded exercise capacity and cardiac risk factors outcomes and added costs.
Limited the inclusion to those studies that assess outcomes at six months or longer.
Footnotes
DECLARATIONS OF INTEREST
RST, JJ, SE, KR, NO, DT were authors of the original Cochrane review. RST has been a co-investigator on a number of trials of cardiac rehabilitation.
References to studies included in this review
- Andersen 1981 {published data only} .Andersen GS, Christiansen P, Madsen S, Schmidt G. The value of regular, supervised physical training after acute myocardial infarction [Vaerdien af regelmaessig og overvåget fysisk traening efter akut myokardieinfarkt.] Ugeskrift for Laeger. 1981;143((45):2952–5. [PubMed] [Google Scholar]
- Belardinelli 2001 {published data only} .Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: The ETICA Trial. Journal of the American College of Cardiology. 2001;37((7):1891–900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- Bell 1998 {unpublished data only} .Bell JM. PhD Thesis. University of London; 1998. A comparison of a multi-disciplinary home based cardiac rehabilitation programme with comprehensive conventional rehabilitation in post-myocardial infarction patients. [Google Scholar]
- Bengtsson 1983 {published data only} .Bengtsson K. Rehabilitation after myocardial infarction. Scandinavian Journal of Rehabilitation Medicine. 1983;15(1):1–9. [PubMed] [Google Scholar]
- Bertie 1992 {published data only} .Bertie J, King A, Reed N, Marshall AJ, Ricketts C. Benefits and weaknesses of a cardiac rehabilitation programme. Journal of the Royal College of Physicians of London. 1992;26(2):147–51. [PMC free article] [PubMed] [Google Scholar]
- Bethell 1990 {published and unpublished data} .Bethell HJN, Mullee MA. A controlled trial of community based coronary rehabilitation. British Heart Journal. 1990;64(6):370–5. doi: 10.1136/hrt.64.6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck 2008 {published data only} .Bäck M, Wennerblom B, Wittboldt S, Cider A. Effects of high frequency exercise in patients before and after elective percutaneous coronary intervention. European Journal of Cardiovascular Nursing. 2008;7((4):307–13. doi: 10.1016/j.ejcnurse.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Carlsson 1998 {published data only} .Carlsson R. Serum cholesterol, lifestyle, working capacity and quality of life in patients with coronary artery disease. Experiences from a hospital-based secondary prevention programme. Scandinavian Cardiovascular Journal. Supplement. 1998;50:1–20. doi: 10.1080/140174398427956-1. [DOI] [PubMed] [Google Scholar]
- Carson 1982 {published data only} .Carson P, Phillips R, Lloyd M, Tucker H, Neophytou M, Buch NJ, et al. Exercise after myocardial infarction: a controlled trial. Journal of the Royal College of Physicians of London. 1982;16((3):147–51. [PMC free article] [PubMed] [Google Scholar]
- DeBusk 1994 {published data only} .DeBusk RF, Miller NH, Superko HR, Dennis CA, Thomas RJ, Lew HT, et al. A case management system for coronary risk factor modification following acute myocardial infarction. Annals of Internal Medicine. 1994;120((9):721–9. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]; *
- Taylor CB, Miller NH, Smith PM, DeBusk RF. The effect of a home-based, case-managed, multifactorial risk-reduction program on reducing psychological distress in patients with cardiovascular disease. Journal of Cardiopulmonary Rehabilitation. 1997;17((3):157–62. doi: 10.1097/00008483-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Dugmore 1999 {published data only} .Dugmore LD, Tipson RJ, Phillips MH, Flint EJ, Stentiford NH, Bone MF, et al. Changes in cardiorespiratory fitness, psychological wellbeing, quality of life, and vocational status following a 12 month cardiac exercise rehabilitation programme. Heart. 1999;81((4):359–66. doi: 10.1136/hrt.81.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom 1996 {published data only} .Engblom E, Hamalainen H, Lind J, Mattlar CE, Ollila S, Kallio V, et al. Quality of life during rehabilitation after coronary bypass surgery. Quality of Life Research. 1992;1:167–75. doi: 10.1007/BF00635616. MEDLINE: 93244729. [DOI] [PubMed] [Google Scholar]
- Engblom E, Hietanen EK, Hamalainen H, Kallio V, Inberg M, Knuts L-R. Exercise habits and physical performance during comprehensive rehabilitation after coronary artery bypass surgery. European Heart Journal. 1992;13:1053–9. doi: 10.1093/oxfordjournals.eurheartj.a060313. MEDLINE: 92209581. [DOI] [PubMed] [Google Scholar]
- Engblom E, Korpilahti K, Hamalainen H, Puukka P, Ronnemaa T. Effects of five years of cardiac rehabilitation after coronary artery bypass grafting on coronary risk factors. American Journal of Cardiology. 1996;78:1428–31. doi: 10.1016/s0002-9149(96)00629-7. MEDLINE: 97125341. [DOI] [PubMed] [Google Scholar]; *
- Engblom E, Korpilahti K, Hamalainen H, Ronnemaa T, Puukka P. Quality of life and return to work 5 years after coronary artery bypass surgery. Journal of Cardiopulmonary Rehabilitation. 1997;17:29–36. doi: 10.1097/00008483-199701000-00004. MEDLINE: 97193477. [DOI] [PubMed] [Google Scholar]
- Engblom E, Rönnemaa T, Hämäläinen H, Kallio V, Vänttinen, Knuts LR. Coronary heart disease risk factors before and after bypass surgery: results of a controlled trial on multifactorial rehabilitation. European Heart Journal. 1992;13((2):232–7. doi: 10.1093/oxfordjournals.eurheartj.a060152. MEDLINE: 92209581. [DOI] [PubMed] [Google Scholar]
- Erdman 1986 {published data only} .Erdman RAM, Duivenvoorden HJ, Verhage F, Kazemier M, Hugenholtz PG. Predictability of beneficial effects in cardiac rehabilitation: A randomized clinical trial of psychosocial variables. Journal of Cardiopulmonary Rehabilitation. 1986;6(6):206–13. [Google Scholar]
- Fletcher 1994 {published data only} .Fletcher BJ, Dunbar SB, Felner JM, Jensen BE, Almon L, Cotsonis G, et al. Exercise testing and training in physically disabled men with clinical evidence of coronary artery disease. American Journal of Cardiology. 1994;73((2):170–4. doi: 10.1016/0002-9149(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Fridlund 1991 {published data only} .Fridlund B, Högstedt B, Lidell E, Larsson PA. Recovery after myocardial infarction: Effects of a caring rehabilitation programme. Scandinavian Journal of Caring Sciences. 1991;5(1):23–32. doi: 10.1111/j.1471-6712.1991.tb00078.x. [DOI] [PubMed] [Google Scholar]; *
- Fridlund B, Lidell E, Larsson PA. A caring perspective on rehabilitation after myocardial infarction: A theoretical framework and a suggestion for a rehabilitation programme. Scandinavian Journal of Caring Sciences. 1989;3((3):129–35. doi: 10.1111/j.1471-6712.1989.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Fridlund B, Pihilgren C, Wannestig LB. A supportive - educative caring rehabilitation programme: improvements of physical health after myocardial infarction. Journal of Clinical Nursing. 1992;1:141–6. [Google Scholar]
- Lidell E, Fridlund B. Long-term effects of a comprehensive rehabilitation programme after myocardial infarction. Scandinavian Journal of Caring Sciences. 1996;10:67–74. doi: 10.1111/j.1471-6712.1996.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Giallauria 2008 {published data only} .Giallauria F, Cirillo P, Lucci R, Pacileo M, De Lorenzo A, D’Agostino M, et al. Left ventricular remodelling in patients with moderate systolic dysfunction after myocardial infarction: favourable effects of exercise training and predictive role of N-terminal pro-brain natriuretic peptide. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15((1):113–8. doi: 10.1097/HJR.0b013e3282f00990. [DOI] [PubMed] [Google Scholar]
- Haskell 1994 {published data only} .Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease: The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89((3):975–90. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- Heller 1993 {published data only} .Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. American Journal of Cardiology. 1993;72((11):759–62. doi: 10.1016/0002-9149(93)91058-p. [DOI] [PubMed] [Google Scholar]
- Hofman-Bang 1999 {published data only} .Hofman-Bang C, Lisspers J, Nordlander R, Nygren Å , Sundin Ö , Öhman A, et al. Two-year results of a controlled study of residential rehabilitation for patients treated with percutaneous transluminal coronary angioplasty. A randomized study of a multifactorial programme. European Heart Journal. 1999;20((20):1465–74. doi: 10.1053/euhj.1999.1544. [DOI] [PubMed] [Google Scholar]
- Lisspers J, Sundin Ö , Hofman-Bang C, Nordlander R, Nygren Å , Rydén L, et al. Behavioral effects of a comprehensive multifactorial program for lifestyle change after percutaneous transluminal coronary angioplasty: A prospective randomized, controlled study. Journal of Psychosomatic Research. 1999;46((2):143–54. doi: 10.1016/s0022-3999(98)00074-9. [DOI] [PubMed] [Google Scholar]; *
- Lisspers J, Sundin Ö , Öhman A, Hofman-Bang C, Rydén L, Nygren Å . Long-term effects of lifestyle behavior change in coronary artery disease: Effects on recurrent coronary events after percutaneous coronary intervention. Health Psychology. 2005;24((1):41–8. doi: 10.1037/0278-6133.24.1.41. [DOI] [PubMed] [Google Scholar]
- Holmbäck 1994 {published data only} .Holmbäck AM, Säwe U, Fagher B. Training after myocardial infarction: Lack of long-term effects on physical capacity and psychological variables. Archives of Physical Medical and Rehabilitation. 1994;75((5):551–4. [PubMed] [Google Scholar]
- Kallio 1979 {published data only} .Kallio V, Hämäläinen H, Hakkila J, Luurila OJ. Reduction in sudden deaths by a multifactorial intervention programme after acute myocardial infarction. Lancet. 1979;2(8152):1091–4. doi: 10.1016/s0140-6736(79)92502-9. [DOI] [PubMed] [Google Scholar]
- Kovoor 2006 {published data only} .Kovoor P, Lee AKY, Carrozzi F, Wiseman V, Byth K, Zecchin R, et al. Return to full normal activities including work at two weeks after acute myocardial infarction. American Journal of Cardiology. 2006;97((7):952–8. doi: 10.1016/j.amjcard.2005.10.040. [DOI] [PubMed] [Google Scholar]
- La Rovere 2002 {published data only} .La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106((8):945–9. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- Leizorovicz 1991 {published data only} .Leizorovicz A, Saint-Pierre A, Vasselon C, Boissel JP, P.RE.COR. Group Comparison of a rehabilitation programme, a counselling programme and usual care after an acute myocardial infarction: Results of a long-term randomized trial. European Heart Journal. 1991;12(5):612–6. doi: 10.1093/oxfordjournals.eurheartj.a059948. [DOI] [PubMed] [Google Scholar]
- Lewin 1992 {published data only} .Lewin B, Robertson IH, Cay EL, Irving JB, Campbell M. Effects of self-help post-myocardial infarction rehabilitation on psychological adjustment and use of health services. Lancet. 1992;339((8800):1036–40. doi: 10.1016/0140-6736(92)90547-g. [DOI] [PubMed] [Google Scholar]
- Manchanda 2000 {published data only} .Manchanda SC, Narang R, Reddy KS, Sachdeva U, Prabhakaran D, Dharmanand S, et al. Retardation of coronary atherosclerosis with yoga lifestyle intervention. Journal of the Association of Physicians of India. 2000;48(7):687–94. [PubMed] [Google Scholar]
- Marchionni 2003 {published data only} .Marchionni N, Fattirolli F, Fumagalli S, Oldridge N, Del Lungo F, Morosi L, et al. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction: Results of a randomized, controlled trial. Circulation. 2003;107((17):2201–6. doi: 10.1161/01.CIR.0000066322.21016.4A. [DOI] [PubMed] [Google Scholar]
- Miller 1984 {published data only} .DeBusk RF, Haskell WL, Miller NH, Berra K, Taylor CB, Berger WE, et al. Medically directed at-home rehabilitation soon after clinically uncomplicated acute myocardial infarction: a new model for patient care. American Journal of Cardiology. 1985;55((4):251–7. doi: 10.1016/0002-9149(85)90355-8. [DOI] [PubMed] [Google Scholar]
- Miller NH, Haskell WL, Berra K, DeBusk RF. Home versus group exercise training for increasing functional capacity after myocardial infarction. Circulation. 1984;70(4):645–9. doi: 10.1161/01.cir.70.4.645. [DOI] [PubMed] [Google Scholar]; *
- Taylor CB, Houston-Miller N, Ahn DK, Haskell WL, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. Journal of Psychosomatic Research. 1986;30(5):581–7. doi: 10.1016/0022-3999(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Houston-Miller N, Haskell WL, DeBusk RF. Smoking cessation after acute myocardial infarction: The effects of exercise training. Addictive Behaviors. 1988;13(4):331–5. doi: 10.1016/0306-4603(88)90039-1. [DOI] [PubMed] [Google Scholar]
- Oldridge 1991 {published and unpublished data} .Oldridge N, Guyatt G, Jones N, Crowe J, Singer J, Feeny D, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. American Journal of Cardiology. 1991;67((13):1084–9. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]; *
- Oldridge N, Streiner D, Hoffmann R, Guyatt G. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Medicine and Science in Sports and Exercise. 1995;27(6):900–5. [PubMed] [Google Scholar]
- Ornish 1990 {published data only} .Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336((8708):129–33. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]; *
- Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280((23):2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- Pischke CR, Scherwitz L, Weidner G, Ornish D. Longterm effects of lifestyle changes on well-being and cardiac variables among coronary heart disease patients. Health Psychology. 2008;27((5):584–92. doi: 10.1037/0278-6133.27.5.584. [DOI] [PubMed] [Google Scholar]
- Schuler 1992 {published data only} .Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: Effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. Journal of the American College of Cardiology. 1993;22((2):468–77. doi: 10.1016/0735-1097(93)90051-2. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, et al. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. American Journal of Cardiology. 1995;76(11):771–5. doi: 10.1016/s0002-9149(99)80224-0. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, et al. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96((8):2534–41. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Velich T, Marburger C, Hauer K, Kreuzer J, et al. Predictive value of lipid profile for salutary coronary angiographic changes in patients on a low-fat diet and physical exercise program. American Journal of Cardiology. 1996;78((2):163–7. doi: 10.1016/s0002-9149(96)90390-2. [DOI] [PubMed] [Google Scholar]
- Nikolaus T, Schlierf G, Vogel G, Schuler G, Wagner I. Treatment of coronary heart disease with diet and exercise: problems of compliance. Annals of Nutrition and Metabolism. 1991;35:1–7. doi: 10.1159/000177615. [DOI] [PubMed] [Google Scholar]
- Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, et al. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation. 1992;86((1):1–11. doi: 10.1161/01.cir.86.1.1. [DOI] [PubMed] [Google Scholar]; *
- Seki 2003 {published data only} .Seki E, Watanabe Y, Sunayama S, Iwama Y, Shimada K, Kawakami K, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP) Circulation Journal. 2003;67((1):73–7. doi: 10.1253/circj.67.73. [DOI] [PubMed] [Google Scholar]
- Seki 2008 {published data only} .Seki E, Watanabe Y, Shimada K, Sunayama S, Onishi T, Kawakami K, et al. Effects of a phase III cardiac rehabilitation program on physical status and lipid profiles in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP) Circulation Journal. 2008;72((8):1230–4. doi: 10.1253/circj.72.1230. [DOI] [PubMed] [Google Scholar]
- Shaw 1981 {published data only} .Naughton J. The National Exercise and Heart Disease Project. The pre-randomization exercise program. Report number 2. Cardiology. 1978;63((6):352–67. doi: 10.1159/000169916. [DOI] [PubMed] [Google Scholar]
- Shaw LW, The National Exercise and Heart Disease Project Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction. American Journal of Cardiology. 1981;48((1):39–46. doi: 10.1016/0002-9149(81)90570-1. [DOI] [PubMed] [Google Scholar]; *
- Stern MJ, Cleary P. The National Exercise and Heart Disease Project: Long-term psychosocial outcome. Archives of Internal Medicine. 1982;142((6):1093–7. [PubMed] [Google Scholar]
- Sivarajan 1982 {published data only} .Ott CR, Sivarajan ES, Newton KM, Almes MJ, Bruce RA, Bergner M, et al. A controlled randomized study of early cardiac rehabilitation: The sickness impact profile as an assessment tool. Heart & Lung. 1983;12((2):162–70. [PubMed] [Google Scholar]
- Sivarajan ES, Bruce RA, Almes MJ, Green B, Belanger L, Lindskog BD, et al. In-hospital exercise after myocardial infarction does not improve treadmill performance. New England Journal of Medicine. 1981;305((7):357–62. doi: 10.1056/NEJM198108133050701. [DOI] [PubMed] [Google Scholar]
- Sivarajan ES, Bruce RA, Lindskog BD, Almes MJ, Belanger L, Green B. Treadmill test responses to an early exercise program after myocardial infarction: A randomized study. Circulation. 1982;65((7):1420–8. doi: 10.1161/01.cir.65.7.1420. [DOI] [PubMed] [Google Scholar]; *
- Sivarajan ES, Newton KM, Almes MJ, Kempf TM, Mansfield LW, Bruce RA. Limited effects of outpatient teaching and counselling after myocardial infarction: A controlled study. Heart & Lung. 1983;12((1):65–73. [PubMed] [Google Scholar]
- Specchia 1996 {published data only} .Specchia G, De Servi S, Scirè A, Assandri J, Berzuini C, Angoli L, et al. Interaction between exercise training and ejection fraction in predicting prognosis after a first myocardial infarction. Circulation. 1996;94((5):978–82. doi: 10.1161/01.cir.94.5.978. [DOI] [PubMed] [Google Scholar]
- Stern 1983 {published data only} .Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Archives of Internal Medicine. 1983;143((9):1719–25. [PubMed] [Google Scholar]
- Ståhle 1999 {published data only} .Hage C, Mattsson E, Ståhle A. Long term effects of exercise training on physical activity level and quality of life in elderly coronary patients - a three- to six-year follow-up. Physiotherapy Research International. 2003;8((1):13–22. doi: 10.1002/pri.268. [DOI] [PubMed] [Google Scholar]
- Ståhle A, Lindquist I, Mattsson E. Important factors for physical activity among elderly patients one year after an acute myocardial infarction. Scandinavian Journal of Rehabilitation Medicine. 2000;32((3):111–6. doi: 10.1080/003655000750045451. [DOI] [PubMed] [Google Scholar]
- Ståhle A, Mattsson E, Rydén L, Unden AL, Nordlander R. Improved physical fitness and quality of life following training of elderly patients after acute coronary events. A 1 year follow-up randomized controlled study. European Heart Journal. 1999;20((20):1475–84. doi: 10.1053/euhj.1999.1581. [DOI] [PubMed] [Google Scholar]; *
- Ståhle A, Nordlander R, Rydén L, Mattsson E. Effects of organized aerobic group training in elderly patients discharged after an acute coronary syndrome. A randomized controlled study. Scandinavian Journal of Rehabilitation Medicine. 1999;31((2):101–7. doi: 10.1080/003655099444614. [DOI] [PubMed] [Google Scholar]
- Ståhle A, Tollbäck A. Effects of aerobic group training on exercise capacity, muscular endurance and recovery in elderly patients with recent coronary events: A randomized, controlled study. Advances in Physiotherapy. 2001;3:29–37. [Google Scholar]
- Toobert 2000 {published data only} .Toobert DJ, Glasgow RE, Nettekoven LA, Brown JE. Behavioral and psychosocial effects of intensive lifestyle management for women with coronary heart disease. Patient Education and Counseling. 1998;35((3):177–88. doi: 10.1016/s0738-3991(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Toobert DJ, Glasgow RE, Radcliffe JL. Physiologic and related behavioral outcomes from the Women’s Lifestyle Heart Trial. Annals of Behavioral Medicine. 2000;22((1):1–9. doi: 10.1007/BF02895162. Toobert DJ. Glasgow RE. Radcliffe JL. [DOI] [PubMed] [Google Scholar]; *
- Vecchio 1981 {published data only} .Vecchio C, Cobelli F, Opasich C, Assandri J, Poggi G, Griffo R. Early functional evaluation and physical rehabilitation in patients with wide myocardial infarction [Valutazione funzionale precoce e riabilitazione fisica nei pazienti con infarto miocardico esteso] Giornale Italiano di Cardiologia. 1981;11:419–29. [PubMed] [Google Scholar]
- Vermeulen 1983 {published data only} .Vermeulen A, Lie KI, Durrer D. Effects of cardiac rehabilitation after myocardial infarction: changes in coronary risk factors and long-term prognosis. American Heart Journal. 1983;105((5):798–801. doi: 10.1016/0002-8703(83)90243-0. [DOI] [PubMed] [Google Scholar]
- VHSG 2003 {published data only} .Vestfold Heartcare Study Group Influence on lifestyle measures and five-year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. European Journal of Cardiovascular Prevention and Rehabilitation. 2003;10((6):429–37. doi: 10.1097/01.hjr.0000107024.38316.6a. [DOI] [PubMed] [Google Scholar]
- WHO 1983 {published data only} .World Health Organisation . Rehabilitation and comprehensive secondary prevention after acute myocardial infarction. 1983. EURO Reports and Studies 84. [Google Scholar]
- Wilhelmsen 1975 {published data only} .Sanne H. Exercise tolerance and physical training of non-selected patients after myocardial infarction. Acta Medica Scandinavica. 1973;(Supplementum 551):1–124. [PubMed] [Google Scholar]
- Wilhelmsen L, Sanne H, Elmfeldt D, Grimby G, Tibblin G, Wedel H. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction, and death. Preventive Medicine. 1975;4(4):491–508. doi: 10.1016/0091-7435(75)90035-3. [DOI] [PubMed] [Google Scholar]; *
- Yu 2003 {published data only} .Yu CM, Li LS, Ho HH, Lau CP. Long-term changes in exercise capacity, quality of life, body anthropometry, and lipid profiles after a cardiac rehabilitation program in obese patients with coronary heart disease. American Journal of Cardiology. 2003;91((3):321–5. doi: 10.1016/s0002-9149(02)03159-4. [DOI] [PubMed] [Google Scholar]
- Yu 2004 {published data only} .Yu C, Li L, Lam M, Siu D, Miu R, Lau C. Effect of a cardiac rehabilitation program on left ventricular diastolic function and its relationship to exercise capacity in patients with coronary heart disease: experience from a randomized, controlled study. American Heart Journal. 2004;147(5):e24. doi: 10.1016/j.ahj.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Yu CM, Lau CP, Chau J, McGhee S, Kong SL, Cheung BM, et al. A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Archives of Physical Medicine and Rehabilitation. 2004;85((12):1915–22. doi: 10.1016/j.apmr.2004.05.010. [DOI] [PubMed] [Google Scholar]; *
- Zwisler 2008 {published and unpublished data} .Kruse M, Hochstrasser S, Zwisler AD, Kjellberg J. Comprehensive cardiac rehabilitation: A cost assessment based on a randomized clinical trial. International Journal of Technology Assessment in Health Care. 2006;22((4):478–83. doi: 10.1017/S0266462306051403. [DOI] [PubMed] [Google Scholar]
- Zwisler AD, Soja AM, Rasmussen S, Frederiksen M, Abedini S, Appel J, et al. Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. American Heart Journal. 2008;155(6):1106–13. doi: 10.1016/j.ahj.2007.12.033. [DOI] [PubMed] [Google Scholar]; *
References to studies excluded from this review
- Agren 1989 {published data only} .Agren B, Olin C, Castenfors J, Nilsson-Ehle P. Improvements of the lipoprotein profile after coronary bypass surgery: additional effects of an exercise training program. European Heart Journal. 1989;10((5):451–8. doi: 10.1093/oxfordjournals.eurheartj.a059509. [DOI] [PubMed] [Google Scholar]
- Aronov 2006 {published data only} .Aronov DM, Krasnitski VB, Bubnova MG, Posdniakov IuM, Ioseliani DV, Shchegol’kov AN, et al. Exercise in outpatient complex rehabilitation and secondary prophylaxis in patients with ischemic heart disease after acute coronary events (a cooperative trial in Russia) Terapevticheskii Arkhiv. 2006;78((9):33–8. [PubMed] [Google Scholar]
- Ballantyne 1982 {published data only} .Ballantyne FC, Clark RS, Simpson HS, Ballantyne D. The effect of moderate physical exercise on the plasma lipoprotein subfractions of male survivors of myocardial infarction. Circulation. 1982;65((5):913–8. doi: 10.1161/01.cir.65.5.913. [DOI] [PubMed] [Google Scholar]
- Belardinelli 2007 {published data only} .Belardinelli R, Lacalaprice F, Piccoli G, Iacobone G, Piva R. Long-term benefits of cardiac rehabilitation in patients with incomplete revascularization: 5-year follow-up. Circulation. 2007;116(16):3543. [Google Scholar]
- Bettencourt 2005 {published data only} .Bettencourt N, Dias C, Mateus P, Sampaio F, Santos L, Adao L, et al. Impact of cardiac rehabilitation on quality of life and depression after acute coronary syndrome [Impacto da reabilitacao cardiaca na qualidade-de-vida e sintomatologia depressiva apos sindroma coronaria aguda] Revista Portuguesa de Cardiologia. 2005;24((5):687–96. [PubMed] [Google Scholar]
- Björntorp 1972 {published data only} .Björntorp, Berchtold P, Grimby G, Lindholm B, Sanne H, Tibblin G, et al. Effects of physical training on glucose tolerance, plasma insulin and lipids and on body composition in men after myocardial infarction. Acta Medica Scandinavica. 1972;192(1-6):439–43. doi: 10.1111/j.0954-6820.1972.tb04843.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal 1997 {published data only} .Blumenthal JA, Wei J, Babyak MA, Krantz DS, Frid DJ, Coleman RE, et al. Stress management and exercise training in cardiac patients with myocardial ischemia: effects on prognosis and evaluation of mechanisms. Archives of Internal Medicine. 1997;157((19):2213–23. [PubMed] [Google Scholar]
- Bär 1992 {published data only} .Bär FW, Hoppener P, Diederiks J, Vonken H, Bekkers J, Hoofd W, Appels A, et al. Cardiac rehabilitation contributes to the restoration of leisure and social activities. Journal of Cardiopulmonary Rehabilitation. 1992;12((2):117–25. [Google Scholar]
- Carlsson 1997 {published data only} .Carlsson R, Lindberg G, Westin L, Israelsson B. Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart. 1997;77((3):256–9. doi: 10.1136/hrt.77.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao 2007 {published data only} .Gao WG, Hu DY, Ma WL, Tang CZ, Li J, Hasimu B, et al. Effect of health management on the rehabilitation of patients undergoing coronary artery bypass graft. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007;11(25):4874–8. [Google Scholar]
- Giannuzzi 2008 {published data only} .Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP, Balestroni G, Ceci V, et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: Results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Archives of Internal Medicine. 2008;168(20):2194–204. doi: 10.1001/archinte.168.20.2194. [DOI] [PubMed] [Google Scholar]
- Gielen 2003 {published data only} .Gielen S, Erbs S, Linke A, Mobius-Winkler S, Schuler G, Hambrecht R. Home-based versus hospital-based exercise programs in patients with coronary artery disease: effects on coronary vasomotion. American Heart Journal. 2003;145(1):e3. doi: 10.1067/mhj.2003.58a. [DOI] [PubMed] [Google Scholar]
- Heldal 2000 {published data only} .Heldal M, Sire S, Dale J. Randomised training after myocardial infarction: Short and long-term effects of exercise training after myocardial infarction in patients on beta-blocker treatment. A randomized, controlled study. Scandinavian Cardiovascular Journal. 2000;34((1):59–64. doi: 10.1080/14017430050142413. [DOI] [PubMed] [Google Scholar]
- Higgins 2001 {published data only} .Higgins HC, Hayes RL, McKenna KT. Rehabilitation outcomes following percutaneous coronary interventions (PCI) Patient Education and Counseling. 2001;43(3):219–30. doi: 10.1016/s0738-3991(00)00164-6. [DOI] [PubMed] [Google Scholar]
- Jiang 2007 {published data only} .Jiang X, Sit JW, Wong TKS. A nurse-led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: evidence from Chengdu, China. Journal of Clinical Nursing. 2007;16(10):1886–97. doi: 10.1111/j.1365-2702.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- Kentala 1972 {published data only} .Kentala E. Physical fitness and feasibility of physical rehabilitation after myocardial infarction in men of working age. Annals of Clinical Research. 1972;4(Suppl 9):1–84. [PubMed] [Google Scholar]
- Krachler 1997 {published data only} .Krachler M, Lindschinger M, Eber B, Watzinger N, Wallner S. Trace elements in coronary heart disease. Biological Trace Element Research. 1997;60((3):175–85. doi: 10.1007/BF02784438. [DOI] [PubMed] [Google Scholar]
- Li 2004 {published data only} .Li H, Guo L, Sun JZ, Feng JZ, Wang P, Wu GL, et al. Effect of exercise therapy on the quality of life in patients after successful percutaneous transluminal coronary angioplasty. Chinese Journal of Clinical Rehabilitation. 2004;8((9):1601–3. [Google Scholar]
- Liao 2003 {published data only} .Liao X, Ma H, Dong Y. Effects of early rehabilitation programme on heart rate variability and quality of life in patients with uncomplicated acute myocardial infarction. Journal of Rehabilitation Medicine. 2003;18((3):153–5. [Google Scholar]
- Mezey 2008 {published data only} .Mezey B, Kullmann L, Smith K, Sarolta B, Sandori K, Belicza E, et al. Outpatient cardiac rehabilitation: initial experience in the first Hungarian multicenter study. Orvosi Hetilap. 2008;149((8):353–9. doi: 10.1556/OH.2008.28184. [DOI] [PubMed] [Google Scholar]
- Peschel 2007 {published data only} .Peschel T, Sixt S, Beitz F, Sonnabend M, Muth G, Thiele H, et al. High, but not moderate frequency and duration of exercise training induces downregulation of the expression of inflammatory and atherogenic adhesion molecules. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14((3):476–82. doi: 10.1097/HJR.0b013e328167239d. [DOI] [PubMed] [Google Scholar]
- Piestrzeniewicz 2004 {published data only} .Piestrzeniewicz K, Navarro-Kuczborska N, Bolinska H, Jegier A, Maciejewski M. The impact of comprehensive cardiac rehabilitation in young patients after acute myocardial infarction treated with primary coronary intervention on the clinical outcome and leading again a “normal” life [Korzystne efekty kompleksowej rehabilitacji kardiologicznej u osob do 55 roku zycia, po zawale miesnia sercowego, leczonych za pomoca pierwotnej angioplastyki] Polskie Archiwum Medycyny Wewnetrznej. 2004;111(3):309–17. [PubMed] [Google Scholar]
- Roviaro 1984 {published data only} .Roviaro S, Holmes DS, Holmsten RD. Influence of a cardiac rehabilitation program on the cardiovascular, psychological, and social functioning of cardiac patients. Journal of Behavioral Medicine. 1984;7((1):61–81. doi: 10.1007/BF00845347. [DOI] [PubMed] [Google Scholar]
- Schumacher 2006 {published data only} .Schumacher A, Peersen K, Sommervoll L, Seljeflot I, Arnesen H, Otterstad JE. Physical performance is associated with markers of vascular inflammation in patients with coronary heart disease. European Journal of Cardiovascular Prevention and Rehabilitation. 2006;13((3):356–62. doi: 10.1097/01.hjr.0000188244.54287.96. [DOI] [PubMed] [Google Scholar]
- Stenlund 2005 {published data only} .Stenlund T, Lindström B, Granlund M, Burell G. Cardiac rehabilitation for the elderly: Qi Gong and group discussions. European Journal of Cardiovascular Prevention and Rehabilitation. 2005;12((1):5–11. [PubMed] [Google Scholar]
- Takeyama 2000 {published data only} .Takeyama J, Itoh H, Kato M, Koike A, Aoki K, Fu LT, et al. Effects of physical training on the recovery of the autonomic nervous activity during exercise after coronary artery bypass grafting: effects of physical training after CABG. Japanese Circulation Journal. 2000;64((11):809–13. doi: 10.1253/jcj.64.809. [DOI] [PubMed] [Google Scholar]
- Tokmakidis 2003 {published data only} .Tokmakidis SP, Volaklis KA. Training and detaining effects of a combined-strength and aerobic exercise program on blood lipids in patients with coronary artery disease. Journal of Cardiopulmonary Rehabilitation. 2003;23((3):193–200. doi: 10.1097/00008483-200305000-00006. [DOI] [PubMed] [Google Scholar]; *
- Volaklis KA, Douda HT, Kokkinos PF, Tokmakidis SP. Physiological alterations to detraining following prolonged combined strength and aerobic training in cardiac patients. European Journal of Cardiovascular Prevention and Rehabilitation. 2006;13((3):375–80. doi: 10.1097/01.hjr.0000198922.42437.39. [DOI] [PubMed] [Google Scholar]
- Wosornu 1996 {published data only} .Wosornu D, Bedford D, Ballantyne D. A comparison of the effects of strength and aerobic exercise training on exercise capacity and lipids after coronary artery bypass surgery. European Heart Journal. 1996;17((6):854–63. doi: 10.1093/oxfordjournals.eurheartj.a014966. [DOI] [PubMed] [Google Scholar]
- Zheng 2008 {published data only} .Zheng H, Luo M, Shen Y, Ma Y, Kang W. Effects of 6 months exercise training on ventricular remodelling and autonomic tone in patients with acute myocardial infarction and percutaneous coronary intervention. Journal of Rehabilitation Medicine. 2008;40((9):776–9. doi: 10.2340/16501977-0254. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Son 2008 {published data only} .Son YJ. The development and effects of an integrated symptom management program for prevention of recurrent cardiac events after percutaneous coronary intervention. Journal of Korean Academy of Nursing. 2008;38((2):217–28. doi: 10.4040/jkan.2008.38.2.217. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Blumenthal 2007 {published data only} .Blumenthal JA, Sherwood A, Rogers SD, Babyak MA, Doraiswamy PM, Watkins L, et al. Understanding prognostic benefits of exercise and antidepressant therapy for person with depression and heart disease: the UPBEAT study - rationale, design, and methodological issues. Clinical Trials. 2007;4:548–59. doi: 10.1177/1740774507083388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater 2000 {published data only} .Pater C, Jacobsen C, Rollag A, Sandvik L, Erikssen J, Kogstad E. Design of a randomized controlled trial of comprehensive rehabilitation in patients with myocardial infarction, stabilized acute coronary syndrome, percutaneous transluminal coronary angioplasty or coronary artery bypass grafting: Akershus Comprehensive Cardiac Rehabilitation Trial (the CORE Study) Current Controlled Trials in Cardiovascular Medicine. 2000;1((3):177–83. doi: 10.1186/cvm-1-3-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Balady 2007 .Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; theCouncils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–82. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- Beswick 2004 .Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups. Health Technology Assessment. 2004;8(iii-iv,ix-x):1–152. doi: 10.3310/hta8410. [DOI] [PubMed] [Google Scholar]
- Bethall 2008 .Bethell H, Lewin R, Evans J, Turner S, Allender S, Petersen S. Outpatient cardiac rehabilitation attendance in England: variability by region and clinical characteristics. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28:386–91. doi: 10.1097/HCR.0b013e31818c3b44. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation 2005 .British Heart Foundation . European Cardiovascular Disease Statistics. British Heart Foundation; London: 2005. [Google Scholar]
- Brown 2010 .Brown JPR, Clark AM, Dalal H, Welch K, Taylor RS. Patient education in the contemporary management of coronary heart disease. Cochrane Database of Systematic Reviews. 2010;(12) doi: 10.1002/14651858.CD008895. DOI: 10.1002/14651858.CD008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark 2005 .Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Annals of Internal Medicine. 2005;143(9):659–72. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- Clausen 1976 .Clausen JP, Trap-Jensen J. Heart rate and arterial blood pressure during exercise in patients with angina pectoris: effects of exercise training and of nitroglycerin. Circulation. 1976;53:436–42. doi: 10.1161/01.cir.53.3.436. [DOI] [PubMed] [Google Scholar]
- Davies 2010a .Davies EJ, Moxham T, Rees K, Singh S, Coats AJS, Ebrahim S, et al. Exercise-based rehabilitation for heart failure. Cochrane Database of Systematic Reviews. 2010;(4) doi: 10.1002/14651858.CD003331.pub3. DOI: 10.1002/14651858.CD003331. [DOI] [PubMed] [Google Scholar]
- Davies 2010b .Davies P, Taylor F, Beswick A, Wise F, Moxham T, Rees K, et al. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database of Systematic Reviews. 2010;(7) doi: 10.1002/14651858.CD007131.pub2. DOI: 10.1002/14651858.CD007131.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans 2002 .Evans JA, Turner SC, Bethell HJN. Cardiacrehabilitation: are the NSF milestones achievable? Heart. 2002;87(Suppl ii):41–4. [Google Scholar]
- Fletcher 2001 .Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- Graham 2007 .Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14(Suppl 2):1–113S. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- Hambrecht 2000 .Hambrecht R, Wolff A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. New England Journal of Medicine. 2000;342:454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- Heran 2008a .Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD003823.pub2. DOI: 10.1002/14651858.CD003823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heran 2008b .Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD003822.pub2. DOI: 10.1002/14651858.CD003822.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011 .The Cochrane Collaboration Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org.
- NICE 2007 .National Institute for Health and Clinical Excellence . Secondary prevention in primary and secondary care for patients following a myocardial infarction. NICE; [accessed 1 May 2010]. 2007. www.nice.org.uk/CG48 [Google Scholar]
- O’Connor 1989 .O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstaed EM, Paffenbarger RS, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- Oldridge 1988 .Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomised clinical trials. JAMA. 1988;260:945–50. [PubMed] [Google Scholar]
- Oldridge 1993 .Oldridge N, Furlong W, Feeny D, Torrance G, Guyatt G, Crowe J, et al. Economic evaluation of cardiac rehabilitation soon after acute myocardial infarction. American Journal of Cardiology. 1993;72:154–61. doi: 10.1016/0002-9149(93)90152-3. [DOI] [PubMed] [Google Scholar]
- Oldridge 2003 .Oldridge N. Assessing health-related quality of life: it is important when evaluating the effectiveness of cardiac rehabilitation? Journal of Cardiopulmonary Rehabilitation. 2003;23:26–8. doi: 10.1097/00008483-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Peterssen 2005 .Peterssen S, Peto V, Scarborough PRM. Coronary Heart Statistics. British Heart Foundation; London: 2005. [Google Scholar]
- Rees 2004 .Rees K, Bennett P, West R, Davey Smith G, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews. 2004;(2) doi: 10.1002/14651858.CD002902.pub2. DOI: 10.1002/14651858.CD002902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor 1998 .Taylor RS, Kirby BJ, Burdon D, Caves R. The assessment of recovery in post-myocardial infarction patients using three generic quality of life measures. Journal of Cardiopulmonary Rehabilitation. 1998;18:139–44. doi: 10.1097/00008483-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor 2006 .Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: How much can be attributed to cardiovascular risk factors improvements? European Journal of Cardiopulmonary Rehabilitation. 2006;136:369–74. doi: 10.1097/01.hjr.0000199492.00967.11. [DOI] [PubMed] [Google Scholar]
- Taylor 2010 .Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A home-based versus centre-based cardiac rehabilitation. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD007130.pub2. DOI: 10.1002/14651858.CD007130.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal 2000 .Unal B, Critchley J, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2000;109:1101–7. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- WHO 2004 .World Health Organization . Atlas of Heart Disease and Stroke. WHO; Geneva: 2004. [Google Scholar]
References to other published versions of this review
- Jolliffe 2001 .Jolliffe J, Rees K, Taylor RRS, Thompson DR, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews. 2001;(1) doi: 10.1002/14651858.CD001800. DOI: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- Taylor 2004 .Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. American Journal of Medicine. 2004;116((110):682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study