Abstract
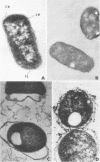
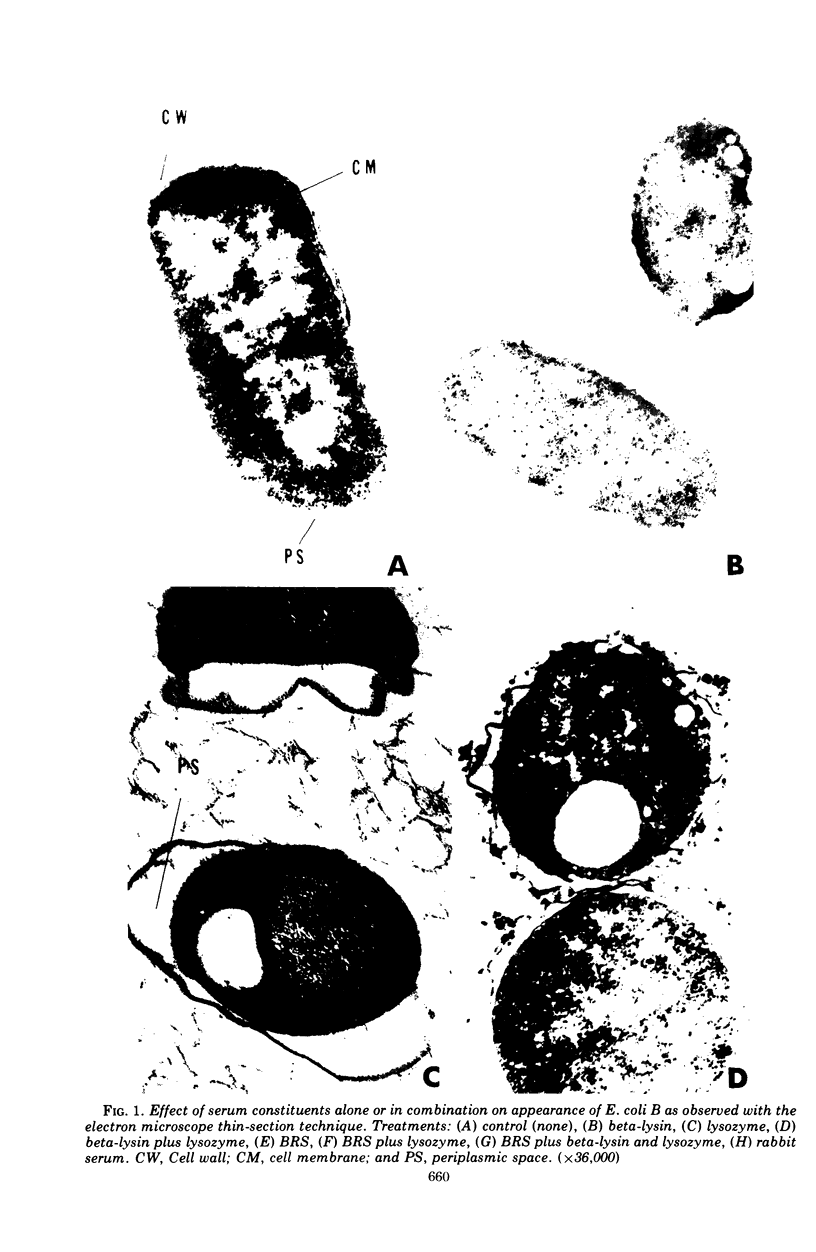
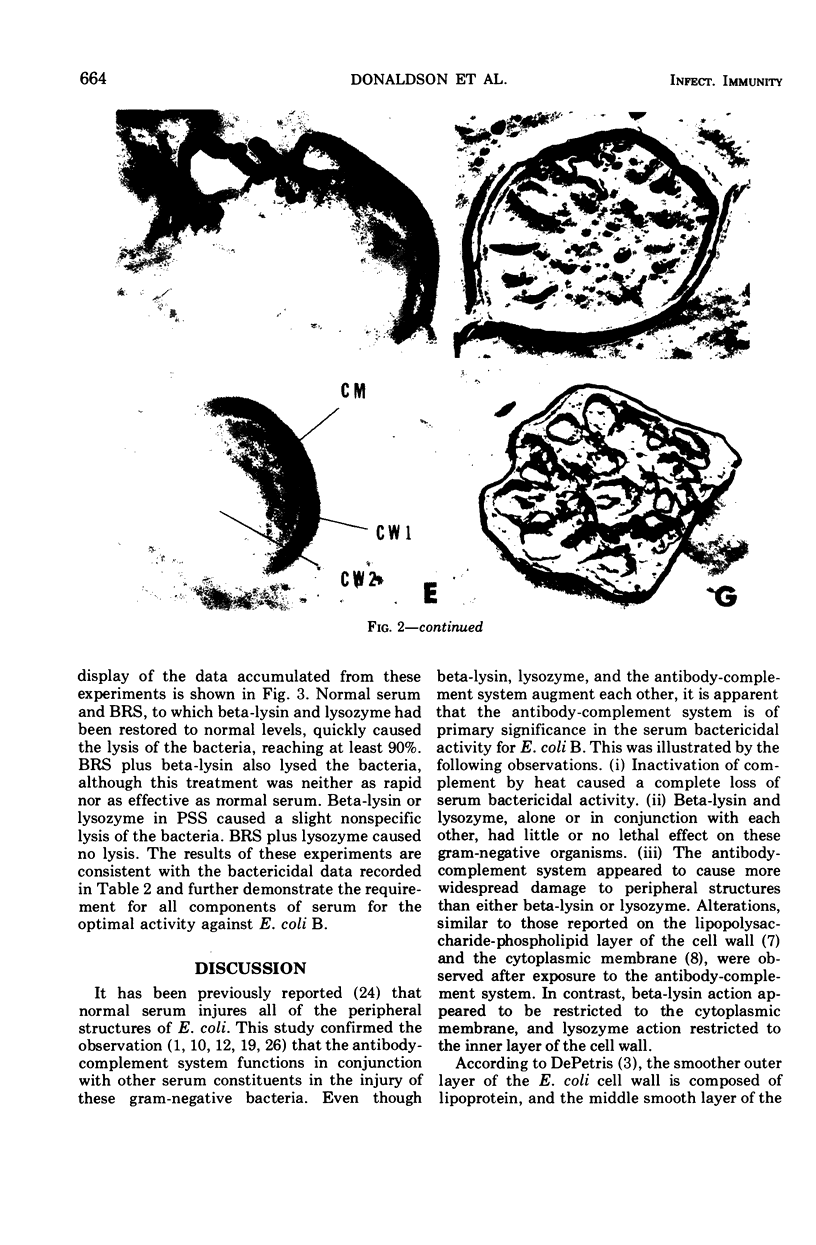
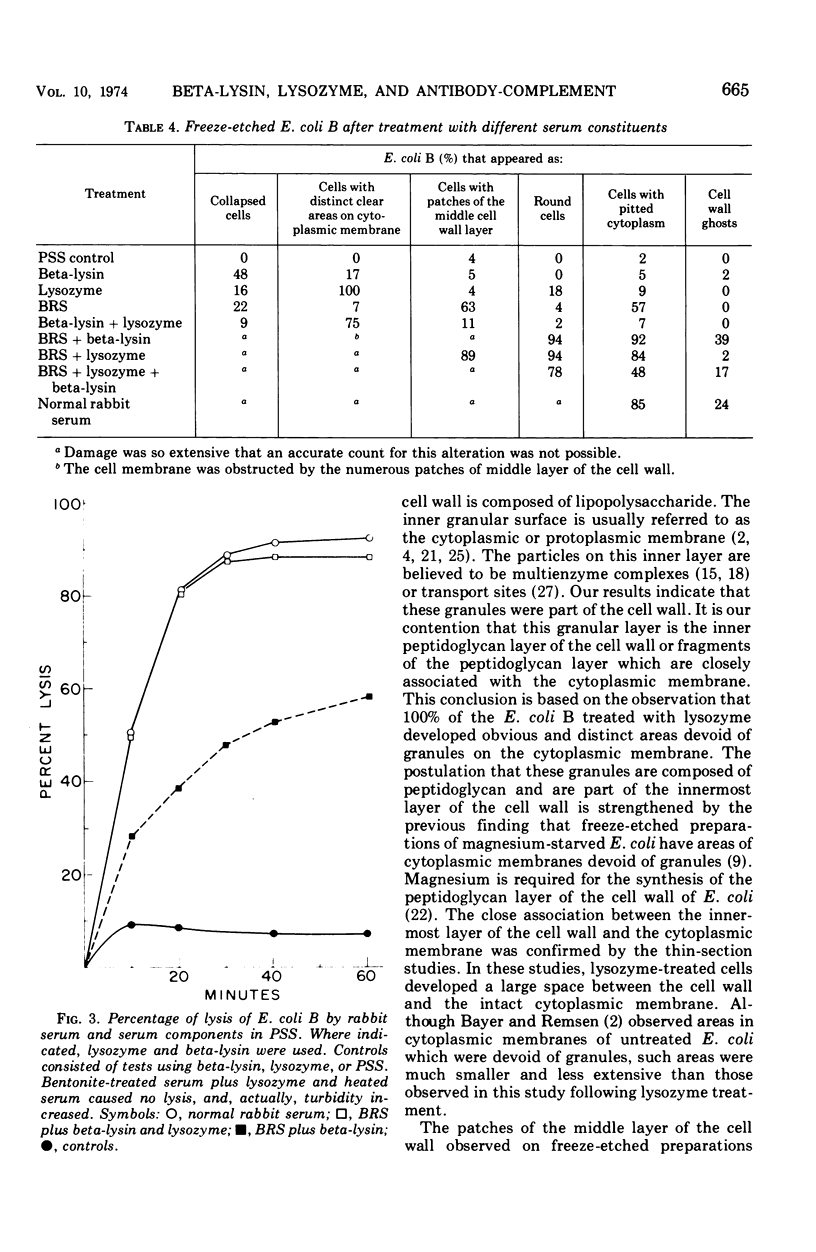
The effects of different serum components alone and in conjunction with each other on Escherichia coli B were investigated. In general, the viability, turbidity, and electron microscope results were compatible with the following conclusions. The most efficient killing and destruction of E. coli B occurred when beta-lysin, lysozyme, and the antibody-complement system functioned in cooperation with each other at the serum concentration in isotonic solutions. The addition of sucrose protected the bacteria from the lethal and lytic action of these agents. Elimination of lysozyme from serum had the least effect on bactericidal activity, even though lysozyme treatment caused the cell wall to separate from the cytoplasmic membrane and caused clear areas to appear in the inner granular layer of the cell wall. Beta-lysin removal had an intermediate effect on the serum bactericidal activity. Beta-lysin treatment caused cell walls to collapse, allowed cytoplasmic contents to leak out of the cells, and stopped the separation of cell wall and cytoplasmic membrane, which normally takes place in 0.5 M sucrose solution. Inactivation of the complement eliminated the serum bactericidal activity against E. coli B. After treatment with antibody and complement, the cell walls became thick and indistinct, a portion of the cytoplasmic contents escaped, and patches of the middle layer of the cell wall appeared in freeze-etch preparations. Beta-lysin damaged the cytoplasmic membrane, lysozyme damaged the inner peptidoglycan layer of the cell wall, and the antibody-complement system damaged both the middle lipopolysaccharide layer of the cell wall and the cytoplasmic membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON D. M., ELLSWORTH B., MATHESON A. SEPARATION AND PURIFICATION OF BETA-LYSIN FROM NORMAL SERUM. J Immunol. 1964 Jun;92:896–901. [PubMed] [Google Scholar]
- DONALDSON D. M., JENSEN R. S., JENSEN B. M., MATHESON A. SEROLOGICAL RELATIONSHIPS AMONG BETA-LYSIN, PLAKIN, AND LEUKIN. J Bacteriol. 1964 Oct;88:1049–1055. doi: 10.1128/jb.88.4.1049-1055.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Milne C. M. A kinetic study of the bacteriolytic and bactericidal action of human serum. Immunology. 1967 Jun;12(6):639–653. [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A. The complement lysozyme sequence in immune bacteriolysis. Immunology. 1969 Apr;16(4):463–471. [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- MOOR H., MUHLETHALER K., WALDNER H., FREY-WYSSLING A. A new freezing-ultramicrotome. J Biophys Biochem Cytol. 1961 May;10:1–13. doi: 10.1083/jcb.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHEL L. H., CAREY W. F., BARON L. S. Formation of bacterial protoplasts by serum components. J Immunol. 1959 Jan;82(1):38–42. [PubMed] [Google Scholar]
- Matheson A., Donaldson D. M. Alterations in the morphology of Bacillus subtilis after exposure to beta-lysin and ultraviolet light. J Bacteriol. 1968 May;95(5):1892–1902. doi: 10.1128/jb.95.5.1892-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor H. Use of freeze-etching in the study of biological ultrastructure. Int Rev Exp Pathol. 1966;5:179–216. [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. The reactivity of serum against protoplasts and spheroplasts. J Immunol. 1966 Jul;97(1):46–51. [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. Chemical structure and biosynthesis of bacterial cell walls. Bacteriol Rev. 1963 Mar;27:18–55. doi: 10.1128/br.27.1.18-55.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Wilson L. A. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. I. Loss of label, death, and ultrastructural damage. J Bacteriol. 1966 Jan;91(1):393–400. doi: 10.1128/jb.91.1.393-400.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R. S., Koo V. M. Penetration of red cell membranes by some membrane-associated particles. Proc Soc Exp Biol Med. 1968 Jun;128(2):353–357. doi: 10.3181/00379727-128-33013. [DOI] [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Molecular and structural damage to Escherichia coli produced by antibody, complement, and lysozyme systems. J Bacteriol. 1968 Oct;96(4):1339–1348. doi: 10.1128/jb.96.4.1339-1348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]