Abstract
Objective
Higher body mass index (BMI) is associated with increased risk of acute kidney injury (AKI) after major trauma. Since BMI is non-specific, reflecting lean, fluid, and adipose mass, we evaluated the use of computed tomography (CT) to determine if abdominal adiposity underlies the BMI-AKI association.
Design
Prospective cohort study.
Setting
Level I Trauma Center of a university hospital.
Patients
Patients older than 13 years with an Injury Severity Score ≥16 admitted to the trauma intensive care unit were followed for development of AKI over five days. Those with isolated severe head injury or on chronic dialysis were excluded.
Interventions
None
Measurements and Main Results
Clinical, anthropometric, and demographic variables were collected prospectively. CT images at the level of the L4-5 intervertebral disc space were extracted from the medical record and used by two operators to quantitate visceral and subcutaneous adipose tissue (VAT and SAT, respectively) areas. AKI was defined by Acute Kidney Injury Network (AKIN) creatinine and dialysis criteria. Of 400 subjects, 327 (81.8%) had CT scans suitable for analysis: 264/285 (92.6%) blunt trauma subjects, 63/115 (54.8%) penetrating trauma subjects. VAT and SAT areas were highly correlated between operators (ICC>0.999, p<0.001 for each) and within operator (ICC>0.999, p<0.001 for each). In multivariable analysis, the standardized risk of AKI was 15.1% (95% CI 10.6%,19.6%), 18.1% (14%,22.2%), and 23.1% (18.3%,27.9%) at the 25th, 50th, and 75th percentiles of VAT area, respectively (p=0.001), with similar findings when using SAT area as the adiposity measure.
Conclusions
Quantitation of abdominal adiposity using CT scans obtained for clinical reasons is feasible and highly reliable in critically ill trauma patients. Abdominal adiposity is independently associated with AKI in this population, confirming that excess adipose tissue contributes to the BMI-AKI association. Further studies of the potential mechanisms linking adiposity with AKI are warranted.
Keywords: acute kidney injury, trauma, critical illness, obesity, adiposity, computed tomography
Introduction
Over 2 million people are hospitalized for injury each year in the United States. The impact of obesity on outcomes after major trauma, and critical illness in general, remains unclear, with conflicting reports regarding the association of obesity with organ dysfunction and mortality (1-4). Acute kidney injury (AKI) after major trauma is associated with a substantial increase in mortality, but its pathophysiology is incompletely understood and treatment options are limited (5). We recently reported an association of body mass index (BMI) with AKI following major trauma, an association also noted in general critical illness populations and patients with the acute respiratory distress syndrome (ARDS) (6-8). A pathophysiologic link between obesity and AKI is plausible: excess adipose tissue is associated with an inflammatory state, and circulating inflammatory mediators have been implicated in the pathogenesis of AKI (9, 10). Obesity may also predispose to AKI risk through abdominal adiposity-associated elevation in baseline intraabdominal pressure with potentiation of abdominal compartment syndrome or through subclinical obesity-related nephropathy (11).
The BMI measure is not specific for adiposity, however, as it also reflects lean and fluid mass. It is therefore not clear that adiposity underlies the association of BMI with AKI. This limitation of BMI is particularly pertinent to trauma patients—lean mass constitutes a greater percentage of total mass in this population given its lower mean age than general intensive care unit (ICU) populations, and trauma patients are routinely given rapid large volume resuscitations on presentation prior to measurement of weight (12). Failure to capture variations in body composition has been suggested as a reason for the variable association of BMI with mortality in outpatient studies, and may explain some of the conflicting results in critically ill populations (13). Computed tomography (CT)-based quantitation of abdominal adipose tissue is a precise, specific technique that distinguishes visceral adipose tissue (VAT) from subcutaneous adipose tissue (SAT), measures which have shown strong associations with mortality in outpatient cohorts (14). Abdominal VAT and SAT have different associations with circulating inflammatory markers, making such a distinction potentially important for the study of AKI and other acute organ dysfunction syndromes in which inflammation plays a role (15-17). The use of CT-defined adiposity in studies of critically ill patients has, however, been quite limited (18, 19). Factors affecting feasibility such as availability of CTs and quality issues impacting adipose quantitation are not well described, nor are associations of VAT or SAT with clinical outcomes such as organ dysfunction.
The objectives of this study were to determine the feasibility of using CT scans obtained for clinical purposes in major trauma patients to estimate abdominal VAT and SAT, and to determine the association of these adiposity measures with AKI. We quantified VAT and SAT areas at the level of the L4-5 intervertebral disc space in a well-characterized prospective trauma cohort, determined inter- and intra-operator correlation of this quantification method, identified potential pitfalls specific to application of this method to a critical illness population, and determined the association of abdominal adiposity with AKI.
Materials and Methods
Study population
Study subjects were enrolled prospectively from 2005 through 2010 from patients admitted to the Level I Trauma Center ICU at the Hospital of the University of Pennsylvania and followed for five days for the development of AKI. Full details on inclusion and exclusion have been previously reported (6). Patients ≥14 years of age with an Injury Severity Score (ISS) ≥16 were included (20). Key exclusion criteria were isolated severe head injury, death or discharge from the ICU within 24 hours of presentation, and chronic dialysis. Baseline data including demographics, medical history, trauma mechanism and severity, and transfusions were collected prospectively on each patient by review of the medical record. The Institutional Review Board of the University of Pennsylvania approved this study with a waiver of informed consent.
Adipose tissue area quantitation
For subjects with an abdominal CT available during the first 14 days of admission, axial slices at the level of the L4-5 intervertebral disc space were imported into the software system 3DVIEWNIX (21). The time window was limited to 14 days in order to reflect, as accurately as possible, VAT and SAT areas at the time of admission, prior to adipose tissue loss that might result from a prolonged hospitalization. The L4-5 level was chosen based on the observed strong correlation of L4-5 VAT area with total abdominal VAT volume as well as demonstrated associations of L4-5 VAT and SAT areas with clinical outcomes in outpatients (14, 22). If a CT image within two 5mm cuts of the L4-5 level was of clearly superior quality for adipose measurement, it was chosen in place of the L4-5 image. The only reason defined a priori for exclusion from adipose quantitation once images were viewed was the presence, at the time of imaging, of a laparotomy fascial incision left open after surgery, typically used as a “damage control” technique for trauma patients (23). Resultant alterations in adipose mass-area relationship from the exposure of abdominal contents to atmospheric pressure makes adipose quantitation in this situation difficult to compare with that performed on a CT of a closed abdomen. Adipose quantitation was performed on all other CTs, though image quality issues potentially affecting accuracy were recorded.
To determine VAT and SAT areas, the 3DVIEWNIX software employs a user-guided LiveWire tracing tool that automatically delineates the myo-subcutaneous interface to draw the boundary between subcutaneous and visceral compartments (Figure 1A) (21). Tissue area (cm2) in the adipose-attenuation range (−200 to −40 Hounsfield Units) both within (VAT) and outside of (SAT) the boundary was calculated after correcting for partial volume effects near the outer skin boundary. Two investigators (MGSS, EK) independently performed quantitation on all subjects. These investigators repeated tracings on all subjects on a subsequent occasion to evaluate intra-operator reliability. For each subject, final VAT and SAT areas were calculated as the mean of these four measurements (two from each investigator). Three investigators (MGSS, EK, JKU) reviewed all CTs with image quality issues (e.g., streak artifact). Any issues that appeared on visual inspection to have prevented accurate adipose capture were considered as additional exclusion criteria for the main analysis. The remaining CTs were considered usable. Investigators were blinded to subjects’ AKI status during adipose quantitation and image quality review.
Figure 1. CT images of study subjects.
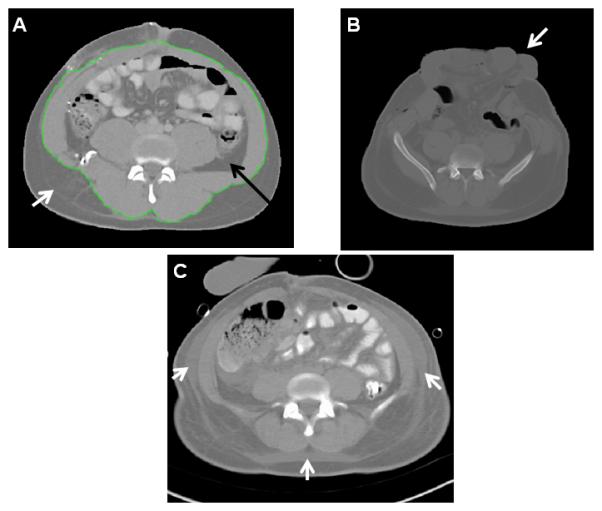
Axial images at the L4-L5 level from abdominal computed tomographic (CT) scans of three study subjects. (A) Delineation of VAT (black arrow) and SAT (white arrow) compartments with tracing (green line) around abdominal wall musculature. (B) CT with open laparotomy incision and protruding viscera (white arrow). (C) CT with extensive edema (white arrows) in subcutaneous adipose tissue. Subjects with CT scans similar to those seen in images B or C were excluded from the primary analysis due to potential inaccuracies in adipose quantitation.
Patient weight was measured on ICU admission using a calibrated electronic hospital bed scale, and height was obtained from patient or family report or was estimated by nursing staff. Body mass index was calculated using the standard World Health Organization definition (24).
Outcome definition
AKI was defined and staged according to Acute Kidney Injury Network (AKIN) creatinine and renal replacement therapy (RRT) consensus criteria (25). These criteria define AKI as a serum creatinine increase of ≥0.3 mg/dL or ≥50% from baseline over a 48-hour period or the need for acute RRT. For AKI by serum creatinine, successive 2-day time windows from day 0, the calendar day of emergency department presentation, through day 5 were tracked (e.g., days 0-2, days 1-3), using the first measured creatinine of each window as the baseline value. Data for staging were collected through day 5 or ICU discharge, whichever came first. AKIN urine output criteria, which are normalized to weight (mL/kg/h), were not included in the AKI definition as such inclusion might cause a spurious association of adipose tissue with AKI given the inclusion of weight measures in both exposure and outcome.
Statistical analysis
The difference between CT availability in the final year versus prior years of the study was determined using the test for binomial proportions. For comparison of characteristics between subjects with and without usable CT scans, as well as subjects with and without AKI, differences were tested with the unpaired t-test, Wilcoxon rank-sum test, Χ2 test, or Fisher’s exact test as appropriate. Among subjects with usable CT scans, we calculated intraclass correlations (ICC) to test inter-operator and intra-operator reliability for VAT and SAT area measurements. We used Spearman’s rho to test correlations between BMI and adipose area.
The associations with AKI of VAT and SAT areas as well as BMI were tested with the Wilcoxon rank-sum test. In order to avoid collinearity, a separate multivariable logistic regression model was constructed for each adipose measure (VAT, SAT, BMI) to adjust its association with AKI for potential confounders. We considered all baseline variables with an unadjusted association with AKI at p<0.20 as well as all hypothesized potential confounders for inclusion in the primary multivariable logistic regression models. We included confounders in the final models if they had a significant impact on the unadjusted association of adipose variables with AKI (as defined by a change in odds ratio ≥15%) or if they contributed significantly to model fit as determined by likelihood ratio tests (26). C-statistics were used to compare the fit of the three final models. Final multivariable models including all risk factors and confounders were used to compute standardized, adjusted AKI risks associated with each adiposity measure using post-estimation marginal analysis (27).
To determine any differences in the association of adiposity with AKI by trauma mechanism, we tested for interaction with adiposity measure (VAT, SAT, or BMI) using likelihood ratio tests to compare multivariable models with and without interaction terms. We also qualitatively examined the final models stratified by blunt versus penetrating trauma. In order to determine if the association of any single adiposity measure with AKI was independent of the effects of the others, we constructed alternative multivariable models each including two adipose measures (VAT+SAT, VAT+BMI, SAT+BMI) in addition to confounders. Finally, we performed a sensitivity analysis including in the multivariable models subjects excluded from the primary analysis due to CT image quality issues to determine whether their inclusion affected the adiposity-AKI association. All alternative multivariable models were constructed using the methods described for the primary models.
We constructed a multivariable logistic regression model to determine characteristics independently associated with the presence of a usable CT in the overall cohort. Baseline covariates with an unadjusted association with usable CT at p<0.20 were considered for the model, and were included if likelihood ratio tests showed significant contributions to model fit.
We calculated that 300 subjects would give the study power of 0.8 to detect differences in mean adipose areas of 11cm2 (VAT) and 27cm2 (SAT) between subjects with and without AKI, less than differences reported in adipose area between normal weight and overweight healthy subjects (36cm2 (VAT), 129cm2 (SAT)) (28). All statistical analyses were done using Stata/IC 11.1 (StataCorp LP, College Station, TX 77845). A two-sided p<0.05 was considered statistically significant.
Results
Association of adiposity with AKI
During the study period, 327 subjects had abdominal CT scans usable for adipose quantitation (Figure 2). The large majority of CTs (297, 90.8%) were performed on presentation or within the first day, with the remaining evenly distributed through days 2-13. VAT and SAT area measurements were highly correlated between operators (ICC>0.99, p<0.001 for each) and within operator (ICC>0.99, p<0.001 for each). Bland-Altman plots displaying these data are in Supplemental Digital Content, Figure 1. VAT and SAT areas were moderately correlated with BMI (Spearman’s rho 0.56 and 0.70, respectively, p<0.001 for each).
Figure 2. Screening and enrollment.
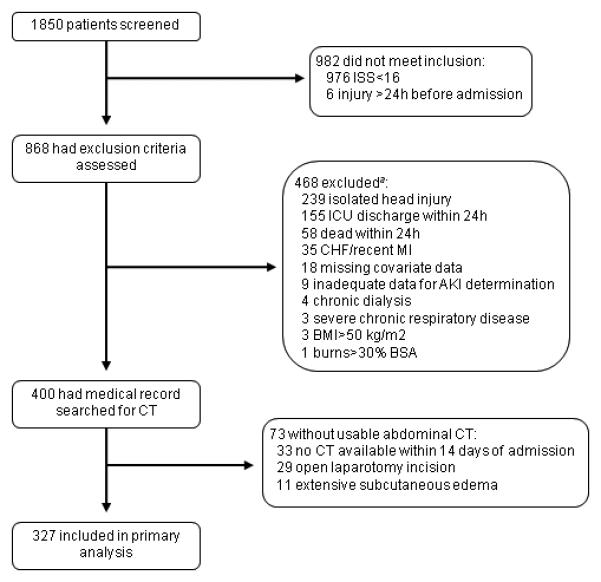
aSome subjects excluded for more than one reason.
AKI developed in 66/327 (20.2%) of subjects in the first 5 days, 32/66 (48.5%) on day 0 or 1. The majority (52/66, 78.8%) of subjects had stage 1 AKI, while 7 (10.6%) subjects each had stages 2 and 3. Subjects with AKI had significantly higher median VAT and SAT areas and BMI and were more likely to die than those who did not develop AKI (Table 1). A summary of adiposity averages by AKI stage is shown in Supplemental Digital Content, Figure 2. Separate multivariable models for VAT area, SAT area, and BMI showed that these adiposity measures were each associated with significantly increased odds of AKI after adjustment for confounders (Tables 2a-c). Comparison of C-statistics showed no significant differences in fit between the three models (p=0.801). The increases in standardized risk of AKI associated with increasing adiposity, estimated from these multivariable models, are shown in Figure 3. Intravenous contrast was received by most subjects but was less common in those with AKI (Table 1) (6). Inclusion of this covariate in multivariable models did not affect the associations of adiposity with AKI (data not shown). Only 8 subjects had underweight BMIs, and none developed AKI.
Table 1.
Among subjects with usable CT scans, comparison of characteristics of those with and without AKI.
| AKI (n=66) | No AKI (n=261) | p | |
|---|---|---|---|
|
| |||
| Age, years | 40 (26,59) | 37 (24,50) | 0.254 |
| Male sex | 48 (73) | 187 (72) | 0.862 |
| Racea | 0.423d | ||
| Caucasian | 32 (48) | 144 (55) | |
| African American | 33 (50) | 108 (41) | |
| Other | 1(2) | 9(3) | |
| Adiposity measures | |||
| VAT area (cm2) | 60 (23,111) | 41 (17,88) | 0.029 |
| SAT area (cm2) | 225 (135,377) | 171 (86,276) | 0.006 |
| BMI (kg/m2) | 28.5 (25.4,33.3) | 25.6 (22.7,29.9) | <0.001 |
| Hypertension | 15 (23) | 46 (18) | 0.342 |
| Diabetes mellitus | 9 (14) | 11 (4) | 0.004 |
| Blunt trauma mechanism | 49 (74) | 215 (82) | 0.134 |
| ISS | 26 (20,29) | 22 (19,29) | 0.037 |
| AIS score abdomen | 2 (0,3) | 2 (0,3) | 0.252 |
| AIS score extremities | 2 (1,3) | 2 (0,3) | 0.127 |
| Low SBP in ED (mmHg) | 99.5 ± 26.1 | 101.8 ± 23.5 | 0.491 |
| APACHE III (non-renal) | 53 (44,64) | 51 (41.5,61.5) | 0.222 |
| First serum Cr (mg/dL) | 1.1 (0.9,1.3) | 1.0 (0.9,1.3) | 0.080 |
| OR prior to ICU admission | 41 (62) | 121 (46) | 0.022 |
| IV contrastb | 49 (85) | 221 (92) | 0.101 |
| Crystalloid, litersc | 3.5 (1.7,6.5) | 2.9 (1.3,7.5.0) | 0.047 |
| Blood products (units)c | |||
| PRBC | 0 (0.5,5.3) | 0 (0,3) | 0.086 |
| FFP | 0 (0,4) | 0(0,1.5) | 0.004 |
| Platelets | 0 (0,0) | 0 (0,0) | 0.036 |
| Hospital mortality | 15 (23) | 12 (5) | <0.001 |
Data are shown as n (%) for categorical variables, mean ± standard deviation for normal continuous variables, and median (interquartile range) for non-normal continuous variables. Definition of abbreviations: CT=computed tomography; AKI=acute kidney injury; VAT=visceral adipose tissue; SAT=subcutaneous adipose tissue; BMI= body-mass index; ISS= Injury Severity Score; AIS= Abbreviated Injury Scale; APACHE III= Acute Physiology And Chronic Health Evaluation III; Cr=creatinine; OR=operating room; IV=intravenous; PRBC= packed red blood cells; AKI=acute kidney injury.
Investigator-identified; “Other” are Asian (n=9) and American Indian (n=1).
Administered prior to meeting AKI criteria.
Administered during resuscitation prior to ICU admission.
Fisher’s exact test comparing 3 categories.
Table 2.
Multivariable models of the association of adiposity measures with AKI, primary analysis (n=327).
| 2a. VAT area (C-statistic 0.730, 95% CI 0.661,0.799) |
| Characteristic | Odds ratio (95% CI) | p |
|---|---|---|
|
| ||
| VAT area, per SD | 1.60 (1.20,2.14) | 0.001 |
| African American race | 1.68 (0.84,3.36) | 0.142 |
| Diabetes | 3.39 (1.23,9.31) | 0.018 |
| Blunt trauma mechanism | 0.62 (0.26,1.48) | 0.283 |
| ISS, per SD | 1.44 (1.07,1.93) | 0.016 |
| PRBC, per unitb | 1.11 (1.04,1.19) | 0.002 |
| Crystalloid, per literb | 1.03 (0.92,1.15) | 0.619 |
| 2b. SAT area (C-statistic 0.730, 95% CI 0.663,0.797) |
| Characteristic | Odds ratio (95% CI) | p |
|---|---|---|
|
| ||
| SAT area, per SD | 1.45 (1.08,1.93) | 0.012 |
| Age, per 10 years | 1.12 (0.94,1.34) | 0.207 |
| Diabetes | 2.95 (1.07,8.11) | 0.038 |
| Blunt trauma mechanism | 0.43 (0.19,0.99) | 0.046 |
| ISS, per SD | 1.43 (1.07,1.92) | 0.016 |
| PRBC, per unitb | 1.11 (1.04,1.19) | 0.003 |
| Crystalloid, per literb | 1.02 (0.92,1.15) | 0.673 |
| 2c. BMIa (C-statistic 0.738, 95% CI 0.672,0.804) |
| Characteristic | Odds ratio (95% CI) | p |
|---|---|---|
|
| ||
| BMI, per SD | 1.55 (1.17,2.07) | 0.003 |
| Age, per 10 years | 1.11 (0.92,1.33) | 0.266 |
| Diabetes | 3.24 (1.17,8.99) | 0.024 |
| Blunt trauma mechanism | 0.52 (0.23,1.18) | 0.119 |
| ISS, per SD | 1.40 (1.04,1.88) | 0.025 |
| PRBC, per unitb | 1.10 (1.03,1.18) | 0.004 |
| Crystalloid, per literb | 1.02 (0.91,1.14) | 0.751 |
BMI model n=326 due to unavailable height and weight for one subject.
Administered during resuscitation prior to ICU admission.
Definition of abbreviations: AKI=acute kidney injury; CI= confidence interval; VAT=visceral adipose tissue; SD=standard deviation ; ISS= Injury Severity Score; PRBC= packed red blood cells; SAT=subcutaneous adipose tissue; BMI= body-mass index.
Figure 3. Adjusted risk of AKI increases with increasing adiposity.
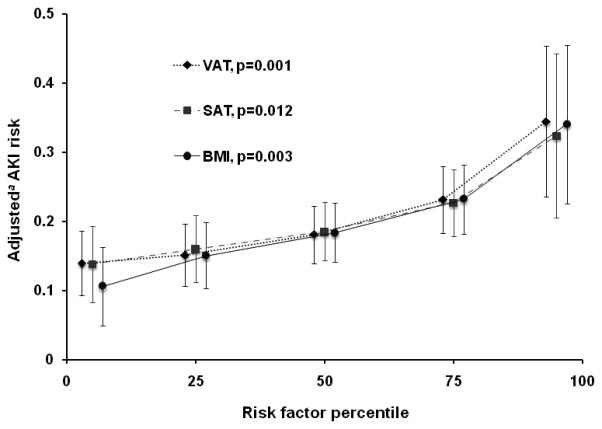
The adjusted risk of AKI associated with each adiposity measure is estimated at the 5th, 25th, 50th, 75th, and 95th percentiles of each measure. Points on the graph at each percentile have been spaced horizontally for purposes of visual clarity only. 95% confidence intervals for each risk estimate are shown with vertical error bars. P-values are for the adjusted association of each adiposity measure with AKI. Abbreviations—VAT: visceral adipose tissue area; SAT: subcutaneous adipose tissue area; BMI: body mass index. aUsing post-estimation marginal analysis. See Table 2 for list of covariates included in the multivariable models.
In alternative models that included two measures of adiposity, no adiposity measure had an association with AKI that was independent of the other (Supplemental Digital Content, Table 1a). Stratifying multivariable analyses by trauma mechanism (Supplemental Digital Content, Table 1b) demonstrated that VAT and SAT areas and BMI maintained significant associations with AKI among blunt trauma subjects (n=264). The adjusted associations of VAT and SAT areas and BMI with AKI were not statistically significant in the subgroup with penetrating trauma (n=63).
A sensitivity analysis including those subjects who had been excluded due to extensive edema on CT (n=11) resulted in a slight decrease in point estimates of the adjusted associations of adiposity measures with AKI, though all associations remained statistically significant (Supplemental Digital Content, Table 1c). Including all subjects with usable and unusable CTs, the adjusted association of BMI with AKI remained significant (OR 1.44 per standard deviation, 95% CI 1.12,1.86, p=0.005)
CT availability and quality
Subjects with usable CT scans constituted 81.8% (327/400) of all enrolled in the prospective trauma cohort during the study period. Those without usable CTs included 33 (8.3%) without an abdominal CT performed within 14 days of presentation or with images that could not be retrieved from the computerized radiology system, 29 (7.3%) with CTs available but with open laparotomy incisions at the time of imaging (Figure 1B), and 11 (2.8%) with extensive subcutaneous edema evident on CT limiting adipose measurement accuracy (Figure 1C). CT availability was more common in the final year than the first 3 years of the study (95/97, 97.9% v. 272/303, 89.8%, respectively, p=0.011). Minor quality issues (Supplemental Digital Content, Figure 3) which did not preclude adequate adipose quantitation were present in 121/327 (37%) subjects in the main analysis.
The baseline characteristics of subjects with and without usable CTs are compared in Table 3. Of note, there was no significant difference in BMI between these groups. A multivariable model including all baseline covariates associated with usable CTs showed that blunt trauma was the predominant distinguishing characteristic associated with having a usable CT (Supplemental Digital Content, Table 2). Usable CTs were available in 63/115 (54.8%) subjects with penetrating trauma compared with 264/285 (92.6%) with blunt trauma. This difference was largely due to decreased CT availability (94/115 (81.7%) v. 273/285 (95.8%), p<0.001) and more frequent open laparotomy incisions (24/115 (20.9%), v. 5/285 (1.8%), p<0.001) in penetrating versus blunt trauma subjects.
Table 3.
Comparison of characteristics of subjects with and without usable CT scans.
| Usable CT (n=327) | No usable CT (n=73) |
p | |
|---|---|---|---|
|
| |||
| Age, years | 38 (24,52) | 28 (22,44) | 0.008 |
| Male sex | 235 (72) | 62 (85) | 0.021 |
| Racea | <0.001c | ||
| Caucasian | 176 (54) | 12 (16) | |
| African American | 141 (43) | 60 (82) | |
| Other | 10 (3) | 1 (1) | |
| BMI (kg/m2) | 26.5 (23.1,30.8) | 26.0 (23.5,29.2) | 0.753 |
| Hypertension | 61 (19) | 6 (8) | 0.031 |
| Diabetes mellitus | 20 (6) | 4 (5) | >0.999 |
| Blunt trauma mechanism | 264 (81) | 21 (29) | <0.001 |
| ISS | 22 (19,29) | 20 (18,25) | 0.003 |
| AIS score abdomen | 2 (0,3) | 3 (0,4) | <0.001 |
| APACHE III | 63 (52,76) | 66 (55,83) | 0.104 |
| First serum Cr (mg/dL) | 1.0 (0.9,1.3) | 1.3 (1.1,1.4) | <0.001 |
| Crystalloid, litersb | 3.0 (1.4,5.5) | 5.5 (3.0,7.8) | <0.001 |
| PRBC, unitsb | 0 (0,3) | 4 (0,9) | <0.001 |
| AKI | 66 (20) | 28 (38) | 0.001 |
| Hospital mortality | 27 (8) | 5 (7) | 0.815 |
Data are shown as n (%) for categorical variables, mean ± standard deviation for normal continuous variables, and median (interquartile range) for non-normal continuous variables. Definition of abbreviations: CT=computed tomography; BMI= body-mass index; ISS= Injury Severity Score; AIS= Abbreviated Injury Scale; APACHE III= Acute Physiology And Chronic Health Evaluation III; PRBC= packed red blood cells; AKI=acute kidney injury.
Investigator-identified; “Other” are Asian (n=10) and American Indian (n=1).
Total amount during resuscitation prior to ICU admission.
Fisher’s exact test comparing 3 categories.
Discussion
Our primary objective in this study was to determine if CT-defined abdominal adipose tissue underlies the association of BMI with AKI in critically ill trauma patients. Abdominal VAT and SAT areas demonstrated significant associations with AKI after adjustment for previously identified risk factors such as fluid and blood product resuscitation, diabetes, and injury severity (6). Several recent studies have reported the association of obesity, determined by BMI, with AKI in trauma and other ICU populations (6-8). Our findings establish that adiposity, and not simply lean mass-associated differences in creatinine rise or excess positive fluid balance, contributes to the BMI-AKI association. This CT-based finding adds a level of specificity that strengthens the case for further investigation of adipose tissue as a risk factor in the causal pathway between traumatic insult and AKI.
Our demonstration that abdominal adipose tissue is an independent risk factor for AKI after trauma is a novel finding. Direct adipose quantitation has rarely been used in studies of obesity in critical illness. Collier et al determined the association of visceral-predominant adiposity with inflammatory markers in 281 obese trauma patients (18), while a study by Ferguson et al aimed to quantify obesity in 162 trauma patients based on adipose volume without the need to obtain height and weight (19). In addition to demonstrating an association of adiposity with organ injury, our study adds to these prior investigations by characterizing the subset of trauma patients with usable CTs and detailing qualitative challenges to accurate adipose quantitation in this population. Over 90% of subjects in our cohort had an abdominal CT within 14 days of admission, similar to the 87% rate in a recent study of nearly 2000 critically ill blunt trauma patients (29). VAT and SAT quantification was feasible in the large majority, particularly in those with blunt trauma.
Several mechanisms could link abdominal adiposity with AKI, including potentiation by adipose tissue of the inflammatory response to acute insults. Evidence from animal models of AKI and some human studies has suggested a role for systemic inflammatory mediators in the pathogenesis of AKI (10, 16, 30, 31). It has been estimated that adipose tissue may produce approximately 20-30% of baseline circulating interleukin-6 (IL-6), and studies of humans given endotoxin challenge have shown dramatic increases in adipose expression of IL-6, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) (32, 33). Global gene expression responses to major trauma are remarkably similar to those after endotoxin challenge (34). A study of ICU patients enrolled in Acute Respiratory Distress Syndrome Network trials found conflicting evidence, however, regarding the association of BMI with plasma levels of inflammatory mediators: IL-8 levels were inversely related to BMI, whereas von Willebrand’s Factor, a marker of endothelial injury, increased proportionally with BMI (35). Immune-modulating adipokines such as leptin and resistin, which have shown associations with organ dysfunction in critically ill patients, may also play a role in the obesity-AKI association (36, 37). Further study would be needed to determine if adipose-sourced inflammation represents a mechanistic link between obesity and AKI risk after trauma.
Obesity is also a risk factor for chronic kidney disease (CKD), independent of associated hypertension and diabetes (38). Morbidly obese patients with normal glomerular filtration rates have been found to have a higher prevalence of abnormal glomerular architecture than non-obese controls (39). This raises the possibility that sub-clinical CKD could contribute to the association of abdominal adiposity with AKI.
Higher BMI is correlated with intraabdominal hypertension (IAH), which can predispose patients to AKI via renal venous congestion and an effective hypovolemic state from decreased venous return (40, 41, 41-43, 43). There was no standardized clinical protocol in our ICU for regularly measuring intraabdominal pressures during the study period, so we were unable to determine the degree to which IAH may have contributed to the adiposity-AKI association.
While two recent studies raised the question of whether obese trauma patients may be under-resuscitated relative to normal weight patients, adjustment for measures of resuscitation (systolic blood pressure, crystalloid, or blood product volume) did not alter the association of adiposity with AKI in our study (44, 45). In addition, unlike studies of obese outpatients implicating visceral-predominant adiposity in metabolic disease risk, the obesity-associated AKI risk in our study was not clearly dependent on a specific adipose compartment (14, 15, 46).
Two barriers to accurate adipose tissue quantitation may have implications for future studies. Open laparotomy incisions at the time of CT may alter the adipose mass-area relationship. Extensive subcutaneous edema, a finding likely related both to fluid resuscitation and third-spacing, might be a significant limiting factor in studies of non-trauma critical care populations in which CTs, if performed at all, are not done uniformly on admission. In our study, few subjects had extensive edema and including these in the analysis did not alter the association of adipose tissue area with AKI.
This study has several limitations. First, CT scans usable for quantifying adipose tissue were near-complete only in the blunt trauma sub-population. Had it been possible to get adipose measurement on those without usable CTs, inclusion of these subjects may have altered the adiposity-AKI association. However, the associations of VAT and SAT areas and BMI with AKI were similar, and the BMI-AKI association was persistent in the overall cohort. Second, abdominal adipose tissue was quantified by area on single CT slices rather than by estimating adipose volume three-dimensionally. VAT area at the L4-5 level, however, is known to be an excellent surrogate of abdominal VAT volume (22). Using single slices also enabled us to avoid image quality barriers (e.g., streak artifact from bullet fragments) that affected some but not all slices in a given patient. Third, the accuracy of weight and height measurements obtained from the medical record could not be verified. This limitation is common in studies of obesity in critical illness and highlights the potential use of CT as a more specific and reproducible way to measure adiposity (47). Fourth, we were unable to determine specific causes of AKI for each subject. The multiple and common renal insults experienced in the studied population (e.g., ischemia, intravenous contrast, rhabdomyolysis, blood product transfusion), however, preclude assignment of a primary etiology in most cases. Fifth, we did not have sufficient numbers of subjects with stages 2 and 3 AKI to meaningfully analyze a “dose-effect” relationship between adipose tissue and AKI severity. Despite the predominance of stage 1 AKI, mortality was still quite elevated in AKI subjects underscoring the potential clinical relevance of the association of adiposity with even mild AKI. Similarly, we did not have adequate numbers of underweight subjects (n=8) to determine whether AKI risk might rise in the setting of malnutrition. Finally, this study was not designed to determine the adjusted association of adipose tissue with mortality—with 27/327 subjects dead at hospital discharge, no meaningful multivariable analysis of risk factors for mortality was possible. Larger, multicenter studies may help to assess whether the increased risk of AKI associated with adiposity translates into increased mortality.
Conclusions
This study demonstrates that abdominal CT scans obtained for clinical purposes can feasibly be used for studies of adiposity in critically ill trauma patients, particularly those with blunt trauma. Both visceral and subcutaneous abdominal adiposity were independently associated with AKI to a similar degree as BMI. No difference was evident in the association of visceral and subcutaneous adiposity with AKI. Further study of the mechanisms underlying the adiposity-AKI relationship in trauma is warranted. The methods described in this study may also aid in future investigation of obesity’s impact in critical illness populations.
Supplementary Material
Supplemental Digital Content, Figure 1. Bland-Altman plots of intra- and inter-operator agreement for adiposity measurements. (A) Comparison of two reads of visceral adipose tissue (VAT) area performed by one operator. Mean difference 0.059 cm2 (95% limits of agreement −0.92,1.04 cm2). Average VAT area lies between 0.19 cm2 and 339.05 cm2. (B) Comparison of reads of visceral adipose tissue (VAT) area performed by two different operators. Mean difference 0.14 cm2 (95% limits of agreement −0.93, 1.22 cm2). Average VAT area lies between 0.19 cm2 and 338.80 cm2. (C) Comparison of two reads of subcutaneous adipose tissue (SAT) area performed by one operator. Mean difference -0.059 cm2 (95% limits of agreement -1.04, 0.92 cm2). Average SAT area lies between 0.08 cm2 and 861.84 cm2. (D) Comparison of reads of subcutaneous adipose tissue (SAT) area performed by two different operators. Mean difference −0.11 cm2 (95% limits of agreement −1.90, 1.68 cm2). Average SAT area lies between 0.08 cm2 and 863.10 cm2.
Table 2. Multivariable model of the association of baseline characteristics with presence versus absence of a usable CT.
Supplemental Digital Content, Figure 2. Adiposity by AKI stage. Box plots of (A) visceral adipose tissue (VAT) area, (B) subcutaneous adipose tissue (SAT) area, and (C) body mass index (BMI, in kg/m2) by stage of acute kidney injury (AKI) according to Acute Kidney Injury Network criteria (25). aNon-parametric test of trend.
Supplemental Digital Content, Figure 3. Minor CT quality issues. Minor quality issues seen in computed tomography (CT) scans, demonstrated by examples from study subjects (white arrows highlight areas of interest): (A) Minor scatter artifact. (B) Major scatter artifact. (C) Minor flank cut-off. (D) Major flank cut-off. (E) Subcutaneous hematoma. (F) Minor subcutaneous edema. (G) Upper extremities (with minimal adipose tissue) present in field of adipose quantitation. (H) Subcutaneous air.
Acknowledgments
The authors would like to thank Dewey Odhner for his technical assistance with utilizing 3DVIEWNIX adipose quantification software.
Drs. Shashaty, Bellamy, John Reilly, Holena, Lanken, Feldman, Muredach Reilly, and Christie report institutional funding from National Institutes of Health grants. Dr. Lanken reports textbook royalties from Elsevier. Dr. Feldman reports payment for lectures at Case Western Reserve University and George Washington University and travel/accommodation/meeting expenses from the American College of Epidemiology, Dr. Muredach Reilly reports individual and institutional funding from Merck and GlaxoSmithKline grants as well as payment for lectures by Merck. Dr. Christie reports payments from various law firms for asbestos litigation as well as institutional funding from GlaxoSmithKline grants.
This study was funded by NIH grants P50-HL60290, P01-HL079063, K24-HL115354, K12-HL090021, K12-HL109009, and K23-DK097307.
Dr. Shashaty received support for article research from NIH. His institution received grant support from NIH (K12 and K23 career development awards. Dr. Bellamy received support for article research from NIH. Her institution received grant support from NIH. Dr. JP Reilly received support for article research from NIH. His institution received grant support from NIH. Dr. Cummins received support for article research from NIH. Her institution received grant support from NIH. Dr. Lanken received support for article research from NIH. His institution received grant support from NIH and received royalties from Elsevier (ICU textbook). Dr. Feldman lectured for Case Western University and George Washington University and received support for travel from the American College of Epidemiology. His institution received grant support from an NIH training grant (Funding for Dr. Shashaty). Dr. MP Reilly and his institution received grant support from Merck and GSK (research grants). Dr. MP Reilly lectured for Merck. Dr. Christie provided expert testimony for various law firms and received support for article research from NIH. His institution received grant support from NIH and GAK.
Footnotes
Institution where work was performed:
Perelman School of Medicine, University of Pennsylvania
Conflicts of Interest and Source of Funding:
Drs. Kalkan and Udupa and Ms. Cummins have declared no relevant conflicts of interest.
Copyright form disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: Weighing the impact. J Am Coll Surg. 2007;204:1056–1061. doi: 10.1016/j.jamcollsurg.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Byrnes MC, McDaniel MD, Moore MB, et al. The effect of obesity on outcomes among injured patients. Journal of Trauma - Injury, Infection and Critical Care. 2005;58:232–237. doi: 10.1097/01.ta.0000152081.67588.10. [DOI] [PubMed] [Google Scholar]
- 3.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anzueto A, Frutos-Vivar F, Esteban A, et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66:66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, George C, Gibney RTN, et al. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. 2008;30:581–589. doi: 10.1080/08860220802134649. [DOI] [PubMed] [Google Scholar]
- 6.Shashaty MGS, Meyer NJ, Localio AR, et al. African american race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care. 2012;27:496–504. doi: 10.1016/j.jcrc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druml W, Metnitz B, Schaden E, et al. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med. 2010;36:1221–1228. doi: 10.1007/s00134-010-1844-2. [DOI] [PubMed] [Google Scholar]
- 8.Soto GJ, Frank AJ, Christiani DC, et al. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med. 2012;40:2601–2608. doi: 10.1097/CCM.0b013e3182591ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honiden S, McArdle JR. Obesity in the intensive care unit. Clin Chest Med. 2009;30:581–599. doi: 10.1016/j.ccm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 11.Bucaloiu ID, Perkins RM, DiFilippo W, et al. Acute kidney injury in the critically ill, morbidly obese patient: Diagnostic and therapeutic challenges in a unique patient population. Crit Care Clin. 2010;26:607–624. doi: 10.1016/j.ccc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Hughes VA, Frontera WR, Roubenoff R, et al. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 15.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The framingham heart study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 17.Levi M, Schultz M. The inflammation-coagulation axis as an important intermediate pathway in acute lung injury. Critical Care. 2008;12:144. doi: 10.1186/cc6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collier B, Dossett L, Shipman J, et al. Visceral adiposity is not associated with inflammatory markers in trauma patients. Journal of Trauma - Injury, Infection and Critical Care. 2010;68:57–61. doi: 10.1097/TA.0b013e3181c40262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson DF, Busenlehner BJ, Rahm MD, et al. The use of routine thoracoabdominal CT scans in the polytrauma patient to estimate obesity. Obesity. 2013;21:997–1003. doi: 10.1002/oby.20283. [DOI] [PubMed] [Google Scholar]
- 20.Civil ID, Schwab CW. The abbreviated injury scale, 1985 revision: A condensed chart for clinical use. J Trauma. 1988;28:87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Udupa JK, Goncalves RJ, Iyer K, et al. 3DVIEWNIX: An open, transportable software system for the visualization and analysis of multidimensional, multimodality, multiparametric images. 1993 [Google Scholar]
- 22.Schoen RE, Thaete FL, Sankey SS, et al. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes. 1998;22:338–342. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]
- 23.Rotondo MF, Schwab CW, McGonigal MD, et al. 'Damage control': An approach for improved survival in exsanguinating penetrating abdominal injury. 1993 [PubMed] [Google Scholar]
- 24.BMI classification. World Health Organization; [Google Scholar]
- 25.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11 doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 27.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 28.Weinsier RL, Hunter GR, Gower BA, et al. Body fat distribution in white and black women: Different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74:631–636. doi: 10.1093/ajcn/74.5.631. [DOI] [PubMed] [Google Scholar]
- 29.Yeguiayan J, Yap A, Freysz M, et al. Impact of whole-body computed tomography on mortality and surgical management of severe blunt trauma. Crit Care. 2012;16:R101. doi: 10.1186/cc11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Guo R, Chen P, et al. TNF induces caspase-dependent inflammation in renal endothelial cells through a rho- and myosin light chain kinase-dependent mechanism. American Journal of Physiology - Renal Physiology. 2009;297:F316–F326. doi: 10.1152/ajprenal.00089.2009. [DOI] [PubMed] [Google Scholar]
- 31.Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clinical Journal of the American Society of Nephrology. 2007;2:22–30. doi: 10.2215/CJN.02510706. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 33.Shah R, Lu Y, Hinkle CC, et al. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes. 2009;58:2211–2219. doi: 10.2337/db09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. The Journal of Experimental Medicine. 2011 doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stapleton RD, Dixon AE, Parsons PE, et al. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: The lung transplant outcomes group obesity study. American Journal of Respiratory and Critical Care Medicine. 2011;184:1055–1061. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch A, Gressner OA, Sanson E, et al. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Critical Care. 2009;13 doi: 10.1186/cc7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. J Am Med Assoc. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 39.Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 40.Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Archives of Surgery. 2003;138:637–643. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 41.Kim IB, Prowle J, Baldwin I, et al. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40:79–89. doi: 10.1177/0310057X1204000107. [DOI] [PubMed] [Google Scholar]
- 42.Frezza EE, Shebani KO, Robertson J, et al. Morbid obesity causes chronic increase of intraabdominal pressure. Dig Dis Sci. 2007;52:1038–1041. doi: 10.1007/s10620-006-9203-4. [DOI] [PubMed] [Google Scholar]
- 43.Blaser AR, Par P, Kitus R, et al. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol Scand. 2011;55:607–614. doi: 10.1111/j.1399-6576.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- 44.Winfield RD, Delano MJ, Lottenberg L, et al. Traditional resuscitative practices fail to resolve metabolic acidosis in morbidly obese patients after severe blunt trauma. Journal of Trauma - Injury, Infection and Critical Care. 2010;68:317–328. doi: 10.1097/TA.0b013e3181caab6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson J, Billeter AT, Seifert B, et al. Obese trauma patients are at increased risk of early hypovolemic shock: A retrospective cohort analysis of 1,084 severely injured patients. Critical Care. 2012;16 doi: 10.1186/cc11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obesity Reviews. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 47.Oud L. Reporting the methodology of height and weight acquisition in studies of body mass index-based prognosis in critically ill patients. J Crit Care. 2013;28:640–646. doi: 10.1016/j.jcrc.2013.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content, Figure 1. Bland-Altman plots of intra- and inter-operator agreement for adiposity measurements. (A) Comparison of two reads of visceral adipose tissue (VAT) area performed by one operator. Mean difference 0.059 cm2 (95% limits of agreement −0.92,1.04 cm2). Average VAT area lies between 0.19 cm2 and 339.05 cm2. (B) Comparison of reads of visceral adipose tissue (VAT) area performed by two different operators. Mean difference 0.14 cm2 (95% limits of agreement −0.93, 1.22 cm2). Average VAT area lies between 0.19 cm2 and 338.80 cm2. (C) Comparison of two reads of subcutaneous adipose tissue (SAT) area performed by one operator. Mean difference -0.059 cm2 (95% limits of agreement -1.04, 0.92 cm2). Average SAT area lies between 0.08 cm2 and 861.84 cm2. (D) Comparison of reads of subcutaneous adipose tissue (SAT) area performed by two different operators. Mean difference −0.11 cm2 (95% limits of agreement −1.90, 1.68 cm2). Average SAT area lies between 0.08 cm2 and 863.10 cm2.
Table 2. Multivariable model of the association of baseline characteristics with presence versus absence of a usable CT.
Supplemental Digital Content, Figure 2. Adiposity by AKI stage. Box plots of (A) visceral adipose tissue (VAT) area, (B) subcutaneous adipose tissue (SAT) area, and (C) body mass index (BMI, in kg/m2) by stage of acute kidney injury (AKI) according to Acute Kidney Injury Network criteria (25). aNon-parametric test of trend.
Supplemental Digital Content, Figure 3. Minor CT quality issues. Minor quality issues seen in computed tomography (CT) scans, demonstrated by examples from study subjects (white arrows highlight areas of interest): (A) Minor scatter artifact. (B) Major scatter artifact. (C) Minor flank cut-off. (D) Major flank cut-off. (E) Subcutaneous hematoma. (F) Minor subcutaneous edema. (G) Upper extremities (with minimal adipose tissue) present in field of adipose quantitation. (H) Subcutaneous air.


