Abstract
The rate of learning or memory formation varies over time for any individual, partly due to moment-to-moment fluctuation of brain state. Functional neuroimaging has revealed the neural correlates of learning and memory, but here we asked if neuroimaging can causally enhance human learning by detection of brain states that reveal when a person is prepared or not prepared to learn. The parahippocampal cortex (PHC) is essential for memory formation for scenes. Here, activation in PHC was monitored in real-time, and scene presentations were triggered when participants entered “good” or “bad” brain states for learning of novel scenes. Subsequent recognition memory was more accurate for scenes presented in “good” than “bad” brain states. These findings show that neuroimaging can identify in real-time brain states that enhance or depress learning and memory formation, and knowledge about such brain states may be useful for accelerating education and training. Further, the use of functional neuroimaging as a causal, rather than correlative, tool to study the human brain may open new insights into the neural basis of human cognition.
Introduction
The rate of learning or memory formation varies over time for any given individual, partly due to moment-to-moment fluctuation of brain state (Boly et al., 2007; Fernandez et al., 1999; Gilden et al., 1995). Varying levels of alertness, attention, and motivation likely contribute to fluctuating brain states for learning. Blood oxygenated level dependent (BOLD) signal levels in specific brain regions can be used to measure such brain state fluctuation. Although little is known about the relationship between fluctuating brain states and successful memory formation, there is ample evidence about neural systems that underlie successful learning. Humans and other mammalian species depend upon the integrity of a medial temporal lobe (MTL) system that is essential for declarative or explicit memory for events and facts (Cohen and Squire, 1980; Corkin, 2002; Graf and Schacter, 1985; Scoville and Milner, 1957; Squire, 1992). Within the MTL, there is considerable evidence that the parahippocampal cortex (PHC) is essential for successful memory formation for scenes. Patients with lesions in the PHC cannot learn new spatial environments (e.g., Epstein et al., 2001), and in healthy people there is greater PHC activation for scenes that are later remembered than for scenes that are later forgotten (Brewer et al., 1998; Gabrieli et al., 1997; Stern et al., 1996). PHC activation is also associated with successful memory formation for words (e.g., Wagner et al., 1998). The important role of PHC in learning scenes may be related to its specialization for scenes in high-level vision – a functional region within PHC has been termed the “parahippocampal place area” or PPA because it responds maximally to scenes relative to other visual categories such as faces and objects (Epstein and Kanwisher, 1998).
There is also evidence that the brain state occurring prior to stimulus presentation (in contrast to the above-reviewed studies of stimulus-evoked activation that occurs in response to a stimulus) influences memory formation for that stimulus. Pre-stimulus evoked response potentials (ERPs) correlated with memory for words (Otten et al., 2006). Within the MTL, pre-stimulus sustained entorhinal activation correlated with successful memory for words (Fernandez et al., 1999), and pre-stimulus PPA activation correlated with successful memory for scenes (Turk-Browne et al., 2006).
Knowledge of brain states that correlate with learning creates the opportunity to enhance learning itself. In animal conditioning, hippocampal theta activity (2-8 Hz oscillatory field potentials) predicts behavioral learning rate (Berry et al., 1978; Berry and Swain, 1989; Seager et al., 2002). Triggering learning trials on the basis of hippocampal theta activity enhanced learning in eyeblink conditioning in rabbits (Asaka et al., 2005; Griffin et al., 2004; Seager et al., 2002). Thus, an invasive measure of brain state could be used to enhance learning in animals by having learning occur during an optimal brain state.
Here, we asked whether human learning and memory could be increased or decreased by identifying, on a moment-to-moment basis, whether a person was in a good or bad brain state for learning scenes, and triggering presentation of each to-be-learned scene by the presence of a good or bad brain state. In Experiment 1, we examined whether such brain states that were good or bad for learning scenes could be identified in the PPA. In Experiment 2, we examined whether such brain states in the PPA, detected by real-time functional magnetic resonance imaging (fMRI) on a moment-moment basis, could be used to trigger scene presentation with the hypothesis that scenes triggered by good brain states would be better remembered than scenes triggered by bad brain states. Such a finding would offer evidence of the ability to monitor on-line whether a person is optimally prepared to learn, and the ability to use fMRI to causally enhance human learning in the sense that the real-time fMRI-measured brain state caused the scene presentation.
Experiment 1: Identification of a Brain State of Preparedness to Learn
The goal of Experiment 1 was to define the kind of BOLD signal of brain state that we could use to control learning in Experiment 2. Further, the location of that signal had to be a region that could be defined a priori in each individual, namely a PPA region (typical subsequent memory studies define memory-related regions a posteriori on the basis of memory outcomes).
Methods
Participants
Twenty right-handed participants (mean age = 27.1 years; 9 males, 11 females) with normal or corrected-to-normal vision participated (three participants were excluded because of excessive movement during scanning; one participant was excluded because of poor behavioral performance). Participants were paid, and gave written informed consent approved by the MIT Committee on Human Subjects.
Task Materials and Procedure
Participants were told that a recognition test would follow study of scenes during scanning. Participants viewed 250 color photographs of indoor and outdoor scenes, presented one at a time for 3s, followed by a fixation cross for 9s. For each trial, they were instructed to determine whether they thought that they would remember or forget the presented scene later, and to respond by pressing one of two buttons. Participants had up to 12s (3s of stimulus duration plus 9s of fixation) to respond. Presentation of stimuli was randomized for all participants, and divided into 5 successive sessions. About 10 minutes after the scanned study phase, participants were administered on a computer a recognition memory test outside of the scanner consisting of 500 randomly presented scenes – 250 old (studied) and 250 new (unstudied foils) – with equal numbers of indoor and outdoor scenes in the studied and unstudied sets. Participants responded using a 4-button confidence scale ranging from old to new.
Image Acquisition
Functional images were acquired using MRI at 3T with a 32-channel phased-array head coil with online motion correction enabled (Thesen et al., 2000) gradient-echo, echo-planar imaging pulse sequence (MAGNETOM TIM Trio, Siemens Healthcare, Erlangen, Germany). Pulse sequence parameters were: TR = 2s, bandwidth = 2298 Hz/pixel, flip angle = 90 degrees, matrix size = 64 × 64, field of view = 200 × 200 mm2, number of slices = 32, slice thickness = 3.5 mm and slice gap = 10%.
Statistical Analyses
All participants’ imaging data were preprocessed (realigned, normalized and smoothed) and analyzed in SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). To investigate pre-stimulus BOLD signals and their correlation with successful learning, we performed a two-step subject-specific modeling analyses. First, each subject’s trials were grouped into three experimental conditions ‘remembered’ (‘hit’), ‘know’ (‘weak hit’), and ‘forgotten’ (‘strong’ and ‘weak misses’ combined). The conditions were modeled using general linear modeling (GLM) with finite impulse responses (FIR) of order 6 as basis functions to derive subject-specific empirical hemodynamic response functions for each experimental condition. The second model for each subject included 2s (1TR) pre-stimulus conditions (i.e., ‘pre-stimulus hit’, ‘pre-stimulus weak hit’, and ‘pre-stimulus miss’) in addition to 3 experimental conditions. For this GLM, a FIR of order 1 was used as a basis function for pre-stimulus conditions, and the derived empirical HRF’s were used as basis functions for experimental conditions. This modeling approach accounted for the BOLD signals associated with the onset and subsequent encoding of scenes presented. A high-pass filter of 50 seconds was applied to the data.
To minimize the influence of guessing on analyses, we considered remembered scenes as only old scenes rated high-confident old and forgotten scenes as any old scene rated as either high-confident or low-confident new. Pre-stimulus activation levels for subsequently remembered (high-confident hits) and forgotten (misses) scenes were compared in individual participants. We used a PPA probability mask created from an independent set of fMRI data collected during a functional localizer for PPA for conducting an ROI analysis where the PPA was defined by greater activation for scenes than faces and objects.
Results
Recognition performance is reported in Table 1. Pre-stimulus activation in left PPA was significantly lower for remembered scenes than forgotten scenes (t19 = 2.21, p = 0.04 two-tailed) (Fig. 1).
Table 1. Average results (N = 20) from recognition memory test in Experiment 1.
Old scenes (250 items) were categorized as either Hits or Misses with high or low confidence ratings, and foils (250 items) were categorized as correct rejections or false alarms with high or low confidence ratings.
| Mean (%) | Standard Deviation (%) | |
|---|---|---|
|
| ||
| Studied Scenes: | ||
|
|
||
| Hits (Total) | 61.3 | 13.3 |
|
|
||
| High-confident | 40.1 | 14.2 |
| Low-confident | 21.1 | 11.8 |
|
|
||
| Misses (Total) | 38.7 | 13.3 |
|
|
||
| High-confident | 22.9 | 11.0 |
| Low-confident | 15.9 | 16.6 |
|
| ||
| Foils: | ||
|
|
||
| Correct Rejections (Total) | 74.4 | 17.9 |
|
|
||
| High-confident | 36.8 | 22.4 |
| Low-confident | 37.6 | 20.2 |
|
|
||
| False Alarms (Total) | 25.6 | 17.9 |
|
|
||
| High-confident | 10.2 | 16.0 |
| Low-confident | 15.4 | 10.1 |
|
| ||
| No Responses | 0.6 | 0.9 |
Figure 1.
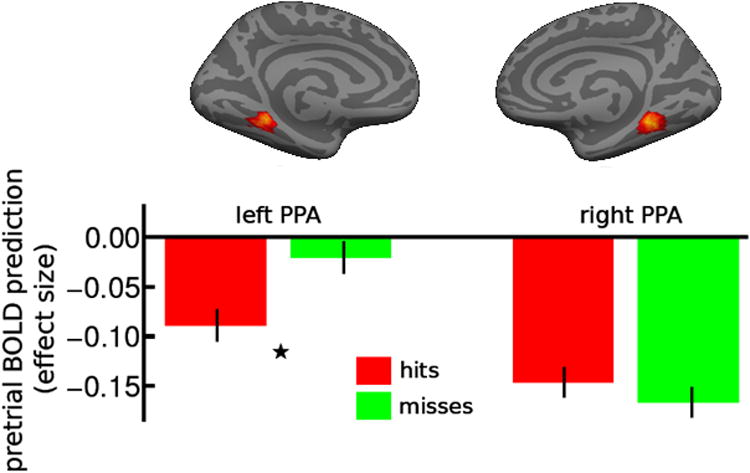
Pre-stimulus activation level in parahippocampal place area (PPA) predicts learning of scenes in Experiment 1. Pre-stimulus activation levels in PPA were measured in effect sizes (i.e., estimated parameter values of the general linear model) and plotted in arbitrary units on the Y axis for subsequently remembered (hits) and forgotten (misses) scenes in bilateral PPA in Experiment 1. Left PPA BOLD signal 2s (1 repetition time) prior to stimulus onset for remembered scenes is significantly lower than signal associated with forgotten scenes (*: p < 0.05). Error bars represent 95% confidence intervals for within-subjects design (Cousineau, 2005).
A prior report of a positive correlation between pre-stimulus PPA activation and subsequent memory noted that pre-stimulus PPA activation predicted subsequent memory when no filter was applied, but did not predict subsequent memory using a 128 s period cutoff (Turk-Browne et al., 2006). Because stimuli are categorized a posteriori on the basis of memory outcome, the selection of a high-pass filter is somewhat arbitrary. Data were reanalyzed with no high-pass filtering or with a longer (100 seconds) high-pass filter. Without high-pass filtering, there was no significant PPA activation difference between remembered and forgotten scenes. With the 100s filter, the left PPA showed a marginally significant reduction in activation for remembered relative to forgotten scenes (t19 = 1.97, p = 0.06 two-tailed). Again, there was not a reliable difference in right PPA activation. In general, fMRI studies use a high-pass filter to reduce low-frequency noise, but it is of interest that these findings are sensitive to the choice of the high-pass filtering.
Discussion
The finding that lower pre-stimulus activation in the PPA correlated with subsequent memory for that stimulus is similar but opposite in direction from a previous study that reported a positive correlation between pre-stimulus PPA activation and subsequent memory for scenes (Turk-Browne et al., 2006). The difference in direction may be related to differences in inter-stimulus intervals (9 sec in the present study to minimize overlap of hemodynamic responses between a stimulus-evoked trial and the subsequent pre-stimulus period vs. 2-6 sec in the prior study) or the use of subject- and condition-specific empirical hemodynamic response functions (HRFs) in the present study to discount residual activation from the prior stimulus versus a canonical HRF in the prior study. Further, as noted previously (Turk-Browne et al., 2006), the selection of a high-pass filter value influences these findings.
Although the activation difference for subsequently remembered and forgotten scenes was significant only in the left PPA in Experiment 1, we used both left and right PPAs as ROIs in Experiment 2 because most studies examining memory for scenes have reported bilateral PHC activations associated with successful memory formation (Brewer et al., 1998; Ofen et al., 2007; Stern et al., 1996; Turk-Browne et al., 2006).
Experiment 2: Controlling Human Learning Via Real-time Monitoring of Brain State
Methods
Participants
Sixteen right-handed participants (mean age = 21.5 years; 9 males, 7 females; all different participants from Experiment 1) with normal or corrected-to-normal vision participated.
Task Materials and Procedure
The real-time design required longer inter-stimulus periods than Experiment 1, so that we could only present 40 to 80 study scenes in Experiment 2 relative to 250 study scenes in Experiment 1. To compensate for the reduced number of scenes, we shortened the stimulus duration to 1s, and conducted the recognition test two hours post-scan so as to yield enough remembered and forgotten items for fMRI analyses. The imaging session consisted of two phases – (1) individual delineation of functional PPA and reference ROIs, and (2) the use of those ROIs to measure brain state and trigger scene presentation.
ROI Definitions
Participant-specific bilateral PPAs were defined using a functional PPA localizer in which participants viewed a series of color images of indoor and outdoor scenes, objects, and faces in a block-design and responded with a button press whenever an item repeated. Each experimental block was 16s in duration and consisted of 20 trials. There were 3 repeated images in each block and each kind of block repeated 3 times over the scan. Within each trial, an image was displayed on the screen for 500ms followed by 300ms of fixation. Four 16s long fixation blocks were also included in the localizer, resulting in a scan that was just over 4 minutes with 128 measurements acquired. After image reconstruction and online motion correction, incoming images were stored to disk on a dedicated fMRI data analysis computer using a custom data sender via intranet communication. Once all images were acquired, an fMRI analysis was done using the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/) while the participant was in the scanner. The PPAs were located by a cluster of voxels more active for scenes than faces and objects (Epstein et al., 1999).
A general linear model (GLM) design matrix was constructed based on the stimulus schedule of the functional localizer with canonical hemodynamic response functions to each of the scenes, objects and faces conditions and their temporal derivatives as bases. A GLM fit was performed and the parameter estimates were then transformed into statistical images representing activation in experimental conditions. The PPAs were identified by voxels with statistically significantly higher activation levels during indoor and outdoor scenes relative to faces and objects.
Bilateral PPA ROIs were defined by thresholding the scenes versus faces and objects statistics volume until bilateral clusters in the assumed region of PPA were clearly delineated from the rest of PHC and became similar in size and shape to the PPA ROI probability mask used in Experiment 1. All voxels that survived this thresholding were included in the PPA ROI. We then performed a dilation operation by 5 voxels on the PPA ROI to slightly expand the ROI so as to anticipate potential movement in the upcoming scan.
The group average PPA was computed off-line to depict the average location of the ROIs across participants. This was done by using spatial normalization with SPM5 to compute transformation matrix for spatially warping mean functional image to MNI space, and then applying the resulting transformation to individual participant’s PPA ROIs. The PPA ROIs in MNI space were then averaged across participants to form a group average of PPA ROIs (Fig. 2).
Figure 2.
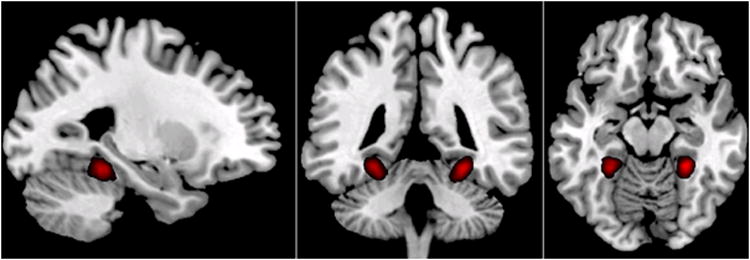
Group average of PPA ROIs used for defining brain states in Experiment 2. All individual PPA masks were spatially normalized, and averaged to create a probabilistic map of PPA ROIs. Here, p > 0.2 was used as a threshold to depict group average of PPA ROIs.
The difference between PPA and reference ROIs was used as the signal to be monitored in real time. Participant-specific reference ROIs were created so that any changes in the PPA ROI reflected specific ROI fluctuations in BOLD signal, rather than broader, non-specific changes due to physiological noise or movement. Reference ROIs were constructed by taking the participant’s whole-brain mask and subtracting regions that were active during all experimental conditions of scenes, faces, and objects (vs. fixation; threshold T = 0.1) as well as the dilated PPA ROI.
Measuring Brain States and Triggering Stimulus Presentation using rtfMRI
In the same scan session, participants passively viewed a fixation cross during scans while brain state was continuously monitored by computing BOLD signal fluctuations within the PPA and reference ROIs. Incoming images were analyzed to estimate neural activation levels using a novel real-time fMRI method (Hinds et al., 2010).
Incoming EPI volumes were sent from the scanner via TCP/IP connection to an external analysis computer immediately after image reconstruction and motion-correction (Thesen et al., 2000), processed voxel-wise to estimate activation, and finally voxels within each ROI were combined. At each timepoint, an incremental GLM fit to each voxel's timeseries was updated. The model design included bases to account for zeroth and first order temporal trends, as well as bases to account for the activation evoked by item presentation. The contribution of each of these nuisance signals was removed from the measured signal, leaving contributions from neural sources and noise. The result was converted to units of standard deviation (SD) from baseline using the estimated timeseries variance given by the GLM. The activation level within each ROI was computed as the median SD across all the voxels in the ROI.
A brain state was defined as the difference between the PPA and the reference ROI, with a “good” brain state defined as PPA activation being less than the reference ROI activation by a participant-specific threshold and a “bad” brain state defined as the PPA activation being greater than the reference ROI by the same threshold (thresholds were participant-specific so as to obtain a similar number of trials per participant). Whenever the absolute value of the difference between the PPA and reference ROI activation exceeded a predefined SD threshold, i.e. whenever participants enter their “good” or “band” brain state, a study trial was triggered and one scene was presented on the screen for one second (Fig. 3). To prevent activation evoked by item presentation from corrupting future fluctuation estimates, an event centered at the trigger TR (2s) was convolved with a canonical hemodynamic response function and added to the design matrix as a nuisance basis. No triggers were possible for 24 seconds following a trial. The participant-specific threshold was determined by manually adapting the threshold after each functional run (from the initial threshold of 1SD) so there were approximately 10 triggers per each functional run. Trial trigger thresholds were determined at the outset of each run and held constant in that run. If there were too few trials triggered in a run, the threshold was adjusted prior to the next run so that enough trials would occur in the experiment to be support a contrast between remembered and forgotten scenes. Participant-specific thresholds ranged from 0.65 SD to 1 SD.
Figure 3.

The real-time fMRI system. A real-time fMRI system was used to monitor the brain signal in individual PPA ROIs. Individual’s PPA regions were spatially normalized, and averaged to create a probabilistic map of PPA ROI across participants for this figure (regions in red are voxels that were in at least 30% of participants). Example from one participant plotted on the graph with PPA signal (red line) and the reference ROI signal (blue line). Whenever PPA ROI signal was less or more than reference ROI signal by an amount greater than the participant-specific threshold, a “good” or “bad” brain state trigger was issued to the stimulus presentation computer. When there was a “good” or “bad” trigger, a scene was presented for 1s and participants were asked to label the scene as indoor or outdoor.
Participants were told that a recognition test would follow study of scenes during scanning. After a scene was presented, participants responded by pressing one of two buttons depending on whether the presented item was an indoor or an outdoor scene. No stimuli were repeated in the PPA localizer and rtfMRI encoding session. A minimum inter-trial interval of 24s was required before the next trigger to ensure that the evoked response to the previous trial had returned to baseline. Each participant underwent between 5 and 7 brain-state triggering fMRI scans of 8 min, depending on how many good and bad triggers were accumulated so that each participant had minimum of 40 and maximum of 80 trials.
About two hours after the scanned study phase, participants were administered on a computer a recognition memory test outside of the scanner consisting of randomly presented studied scenes and foils. Unlike Experiment 1, participants were first asked if the test scene was ‘old’ or ‘new’, and when the response was ‘old’, then the participants were asked to rate their confidence using a 2-button scale.
Statistical Analyses
Recognition accuracy was calculated as correct identification of old scenes (% hits) minus incorrect identification of new scenes (% false alarms) as old.
Results
Recognition performance for “good” and “bad” brain states are presented in Table 2. Participants were significantly more accurate for remembering scenes triggered by good brain states (Hits – False Alarms X = 22.3%, SD = 14.3%) than by bad brain states (Hits – False Alarms X = 15.5%, SD = 11.8%) (t15= 2.78, p < 0.014, two-tailed t-test) (Fig 4). Hit rates in both brain states were significantly higher than false alarm rates (t15= 6.23, p < 0.001, two-tailed t-test). Memory improvements for scenes triggered by “good” relative to “bad” states were observed in 12 of 16 participants. The difference between good and bad brain states occurred for high-confident hits (t15= 1.95, p < 0.07, two-tailed t-test), with little difference for low-confident hits (t15= 0.37, p = 0.71, two-tailed t-test).
Table 2. Average results (N = 16) from the recognition memory test for the good and bad brain states in Experiment 2.
Old scenes were categorized as either Hits or Misses, and further categorized with high or low confidence ratings. Foils were categorized as correct rejections (without confidence ratings) or false alarms with high or low confidence ratings.
| Mean (%) | Standard Deviation (%) | |
|---|---|---|
|
| ||
| Good Brain State: | ||
|
|
||
| Hits (Total) | 48.8 | 16.6 |
|
|
||
| High-confident | 27.7 | 14.1 |
| Low-confident | 21.1 | 8.4 |
|
|
||
| Misses | 51.2 | 16.6 |
|
| ||
| Bad Brain State: | ||
|
|
||
| Hits (Total) | 41.9 | 15.8 |
|
|
||
| High-confident | 21.9 | 11.9 |
| Low-confident | 20.0 | 11.0 |
|
|
||
| Misses | 58.1 | 15.8 |
|
| ||
| Foils: | ||
|
|
||
| False Alarms (Total) | 26.5 | 11.3 |
|
|
||
| High-confident | 6.9 | 4.2 |
| Low-confident | 19.6 | 8.5 |
|
|
||
| Correct Rejections | 73.5 | 11.3 |
Figure 4.
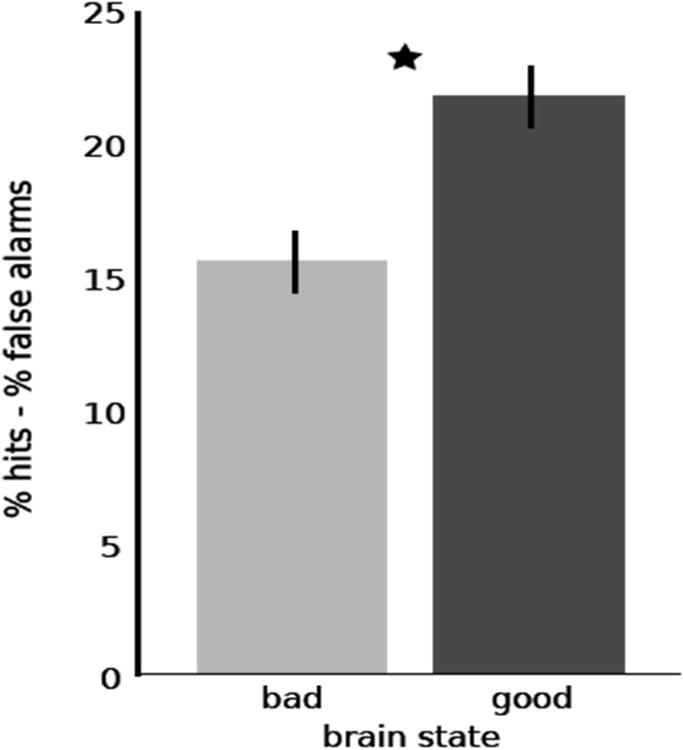
Real-time measurement of brain state alters recognition performance. Participants were significantly more accurate for scenes in which presentation was triggered by a good brain state than a bad brain state. Error bars represent 95% confidence intervals for within-subjects design (Cousineau, 2005).
We examined the consequence of using the difference between PPA and reference-ROI activations as triggers for scene presentation relative to only using the PPA activation values as triggers for scene presentation. Using PPA values only, there was still significantly better memory for scenes triggered by low than by high PPA activations (t15= 3.34, p < 0.005, two-tailed t-test).
Mean item positions across the good and bad brain states did not differ across participants (t15=0.08, p = 0.94, two-tailed). We also examined the distribution of good and bad brain states in a repeated measures ANOVA of brain state (good/bad) × run (Figure 5), and also the distributions within runs (i.e., item positions within each run). The effects of state, run, and item positions within runs and their interactions were not significant (p’s > 0.79), suggesting that the differences in brain states were not related to factors such as primacy or recency effects or fatigue across the experiment.
Figure 5.
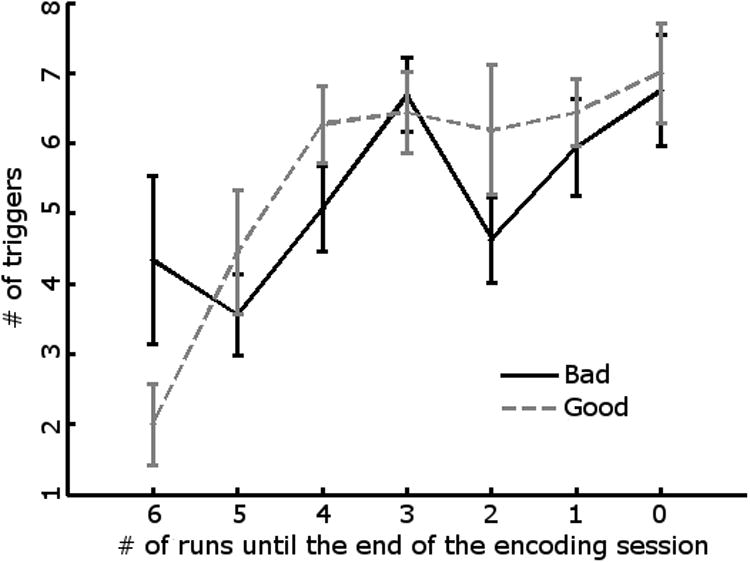
Distribution of good and bad brain states across Experiment 2. Mean numbers of good and bad brain-state trials are plotted from beginning (Run 6) through the end (Run 0) of Experiment 2 for all 16 participants. Error bars represent standard errors computed over subjects for each run.
Discussion
We identified a brain state in the PHG, specifically the PPA, that was associated with better or worse memory formation (Experiment 1), and then used real-time fMRI to monitor that brain state and to present information to-be-learned in either a good or bad brain state for learning (Experiment 2). Real-time, dynamic measurement of brain state prior to stimulus presentation resulted in either increased or decreased learning depending upon whether the brain was in a good or bad state for learning. Indeed, successful formation for memories increased by over a third when the PPA was in a prepared-to-learn state. Thus, human brain preparedness to learn can be measured and used to control the rate of learning or memory acquisition. There are, presently, no on-line behavioral measures of preparedness to learn that can be made prior to learning itself; brain measures may offer a unique window onto human preparedness to learn.
An unexpected finding was that decreased, rather than increased, pre-stimulus activation in the PPA predicted successful memory formation in both Experiments 1 and 2. This contrasts with the general finding that greater stimulus-evoked activation is associated with successful memory formation for scenes (e.g., Brewer et al., 1998; Gabrieli et al., 1997; Stern et al., 1996), and also with the prior observation that under different experimental circumstances greater pre-stimulus activation was associated with successful memory formation (Turk-Browne et al., 2006). The finding in this study that lower pre-stimulus PPA activation predicted superior learning appears convincing because it was not only replicated for the left PPA across Experiments 1 and 2, but also it was causal for superior memory formation in Experiment 2. It may be speculated that lower activation reflects a lack of processing activity in the PPA, and that more resources are available for memory encoding during such an ebb of PPA activity. Further studies will needed, however, to delineate the circumstances under which lesser or greater PPA activation is associated with superior memory formation for scenes.
One limitation of this study is that because ROIs were required to be defined during the same scan session as the real-time fMRI component of the study, the process of ROI delineation involved some observer-dependent decision making. Efforts were made to keep the number of voxels in ROI to be as consistent as possible across participants. Future studies will be needed to better automate ROI definition, but because the identical ROI was used within each participant to define good and bad brain states per subject, ROI definition did not bias the behavioral outcome. A second limitation was that because there was no neutral baseline, we cannot know whether the increased learning or decreased learning that occurred in good or bad brain states, respectively, reflect only gains, only losses, or both relative to baseline.
We measured brain state in a functional brain region, the PPA, specifically associated with learning scenes, and future studies can determine whether preparedness to learn can be measured in other brain regions associated with either other kinds of domain-specific knowledge (e.g., fusiform cortex for faces, amygdala for emotional material) or with domain-general learning (e.g., hippocampus). The use of a contrast between the PPA and a reference ROI was made to minimize the possibility that the PPA signal would reflect movement or broad physiological noise or broad arousal state of the brain. Domain-specific attentional or arousal mechanisms may be relevant, and future studies could manipulate attention and arousal to test this possibility. However, in a similar real-time triggering study involving reaction time to an unpredictable visual target (Hinds et al., 2011), greater pre-stimulus activation in motor cortex (SMA) and lesser brain activation in default brain regions were found to be related to faster performance. These findings suggest that pre-stimulus measures of optimal brain states occur in neural systems that are associated with specific functions.
This study demonstrated that fMRI can not only identify the neural correlates of human behaviors, but also provide a kind of causal relation between brain activation and human behavior. Functional neuroimaging studies, including fMRI, are typically correlative in nature. In such studies, task conditions or materials are the independent variables used to drive variable kinds of behaviors, and the fMRI signal is the correlate of those behaviors. This correlative approach is fruitful in neuroscience, from single-cell animal neurophysiology to human brain imaging, but causal methods are a valuable complement for the study of brain function. In the present study, the fMRI measure of PPA activation, relative to a reference ROI, was the independent variable that drove stimulus presentation, so the fMRI signal was the cause of behavior (good or bad learning). Thus, this study shows that fMRI can be used as a causal tool to study the functional organization of the human brain. Such causal activation, however, does not fully define a brain region as being causal or necessary for a behavior. For example, pre-stimulus fluctuations in the PPA may have correlated with brain functions in other brain regions that were causal for the memory formation.
Many fMRI studies have focused on impaired functions in neurological and psychiatric disease with the aim of understanding how impaired brain functions contribute to those diseases, but few, if any, fMRI studies have explored whether fMRI methods could enhance or optimize typical human abilities. The ability to identify when the brain is prepared to learn could, in theory, be useful for education and training. Either students could improve their ability to be prepared to learn, or teachers could recognize when students were prepared or not prepared to learn; in both cases learning would proceed more efficiently. Practical application of the newfound ability to identify brain preparedness to learn will require some sort of translation from fMRI to a method suitable for wider, practical use. How fMRI findings may be further optimized and translated into uses beyond a scanner is currently unknown, but these findings indicate that progress is possible in enhancing human learning by measuring brain states and using those measurements to guide effective learning.
Acknowledgments
This research was supported by The Defense Advanced Research Projects Agency (government contract no. NBCHC070105), and in part by an appointment to the Postgraduate Research Participation Program at the U.S. Army Medical Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
Contributor Information
Oliver Hinds, Email: ohinds@mit.edu, 43 Vassar Street, 46-4033 Cambridge, MA 02139 Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Noa Ofen, Email: noa@mit.edu, 43 Vassar Street, 46-4033 Cambridge, MA 02139, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Todd W. Thompson, Email: toddt@mit.edu, 43 Vassar Street, 46-4033 Cambridge, MA 02139, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology. Thompson.
Susan Whitfield-Gabrieli, Email: swg@mit.edu, 43 Vassar Street, 46-4041 Cambridge, MA 02139, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Christina Triantafyllou, Email: ctrianta@mit.edu, 43 Vassar Street, 46-1165 Cambridge, MA 02139, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, Massachusetts Institute of Technology.
John D. E. Gabrieli, Email: gabrieli@mit.edu, 43 Vassar Street, 46-4033B Cambridge, MA 02139, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, Massachusetts Institute of Technology.
References
- Asaka Y, Mauldin KN, Griffin AL, Seager MA, Shurell E, Berry SD. Nonpharmacological amelioration of age-related learning deficits: the impact of hippocampal theta-triggered training. Proc Natl Acad Sci U S A. 2005;102:13284–13288. doi: 10.1073/pnas.0506515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SD, Rinaldi PC, Thompson RF, Verzeano M. Analysis of temporal relations among units and slow waves in rabbit hippocampus. Brain Res Bull. 1978;3:509–518. doi: 10.1016/0361-9230(78)90080-1. [DOI] [PubMed] [Google Scholar]
- Berry SD, Swain RA. Water deprivation optimizes hippocampal activity and facilitates nictitating membrane conditioning. Behav Neurosci. 1989;103:71–76. doi: 10.1037//0735-7044.103.1.71. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorial in Quantitative Methods for Psychology. 2005;1:42–45. [Google Scholar]
- Epstein R, DeYoe E, Press D, Rosen A, Kanwisher N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cognitive Neuropsychology. 2001;18:481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gilden DL, Thornton T, Mallon MW. 1/f noise in human cognition. Science. 1995;267:1837–1839. doi: 10.1126/science.7892611. [DOI] [PubMed] [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. J Exp Psychol Learn Mem Cogn. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci. 2004;118:403–411. doi: 10.1037/0735-7044.118.2.403. [DOI] [PubMed] [Google Scholar]
- Hinds O, Ghosh S, Thompson TW, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. Computing moment to moment BOLD activation for real-time neurofeedback. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Thompson TW, Ghosh S, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. Evidence from Real-Time fMRI. 2011. Causal Roles of Default-Mode Network and Supplementary Motor Area in Human Vigilance. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci U S A. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]


