Abstract
Junctional adhesion molecule A (JAM-A) is a transmembrane protein that belongs to the immunoglobulin (Ig) superfamily. Evidence determines that JAM-A plays a role in numerous cellular processes, including tight junction assembly, leukocyte migration, platelet activation, angiogenesis and virus binding. Recent research suggests that JAM-A is dysregulated in various cancers and is vital for tumor progression. JAM-A is implicated in carcinogenesis via different signal pathways such as TGF-β1 signaling. Furthermore, JAM-A expression in cancers is usually associated with certain outcome of patients and might be a prognostic indicator. In this review, the correlation between JAM-A expression and human cancers will be described.
Keywords: Tight junction, junctional adhesion molecule-A, tumor progression
Introduction
Tight junction (TJ) is a multi-protein structure and is the apical most junctional complex in certain epithelial and endothelial cells [1]. The traditional functions of TJ are barrier function and fence function. The barrier function means regulating the passage of ions, solutes, water and other molecules through paracellular spaces, and is deeply involved in edema, jaundice, diarrhea and blood-borne metastasis. The fence function divides apical and basolateral domains of plasma membranes [2], forms a fence to prevent intermixing of molecules in the apical membrane with those in the lateral membrane in order to maintain cell polarity. This function is thus related to cancer cell properties in terms of loss of cell polarity [3].
Junctional adhesion molecule (JAM), which was firstly reported in 1998, is an important component of TJs. As a member of JAMs, JAM-A is located in TJs and along the lateral membrane of endothelial and epithelial cells (Figure 1) and is involved in the barrier function of TJs in both endothelial and epithelial cells, and development of apicobasal cell polarity in epithelial cells [4-6]. The expression of JAM-A has been shown on the surface of endothelial and epithelial cells of various tissues as well as platelets and cells of the immune system [4,6-9]. JAM-A participates in diverse cell-cell adhesion processes, such as leukocyte migration, platelet activation, angiogenesis, reovirus binding and TJ assembling (Figure 2) [10-16].
Figure 1.
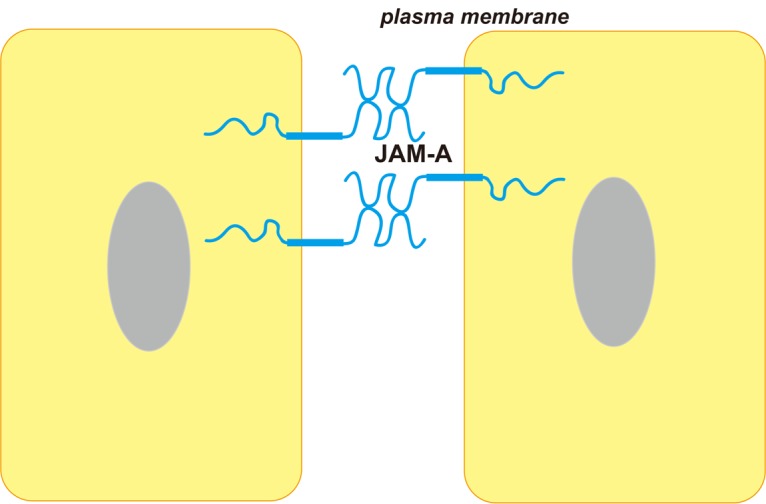
Cellular location and basic structure of JAM-A. JAM-A is component of TJ and located along the lateral membrane. JAM-A has two extracellular immunoglobulin-like domains, a single transmembrane segment and a cytoplasmic tail.
Figure 2.
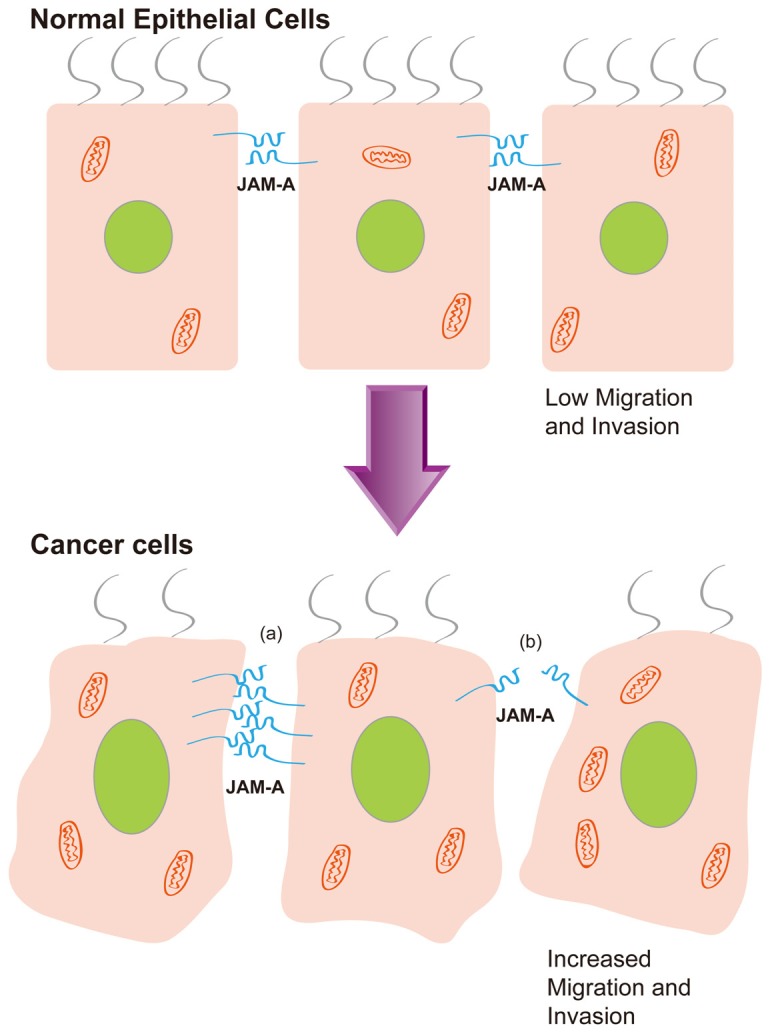
Dysregulation of JAM-A expression is required for cancer cell metastasis. Damage of JAM-A function contributes to tumor progression. In cancer cells, JAM-A overexpression (a) or underexpression (b) is related to increased migration and invasion.
Recent research reports that the TJ structure plays an important part in tumor development through the dysregulation of its barrier and fence functions. Loss of cohesion of the TJ structure can lead to invasion and ultimately to the metastasis of cancer cells [17]. As a new molecular target implicated in cell adhesion, migration and TJ formation, dysregulation of JAM-A may involved in tumor proliferation, invasion and metastasis.
In this review, we discuss the role of JAM-A involved in different cancers and potential mechanisms, especially in the breast cancer.
Roles in cancer
Cell migration is an early requirement for tumor metastasis. In order to metastasize, cancer cells must migrate out of the primary tumor then reach a distant organ and potentially proliferate into a secondary tumor [18]. TJ proteins contribute to tumor cell migration, invasion and adhesion. The loss of TJs appears to constitute an essential step in tumor development [19]. Loss of epithelial TJ barrier function has been implicated in many pathological conditions such as inflammation and cancers [20]. Damage of cell polarity is also an early indicator of carcinoma progression [21]. Dysregulation of JAM-A may, therefore, plays important roles in disease progression.
Expression of the JAM-A has been linked to proliferation and tumor progression. Goetsch et al. reported that JAM-A overexpression is detected in all other evaluated tumor tissues compared to normal tissues except of ovarian and esophagus tumor tissues [22]. The higher expression of JAM-A on tumor tissues is an obvious evidence to consider it as a potential target for tumor therapy.
Endometrial carcinoma
In endometrial carcinoma, JAM-A expression is negatively correlated with grade, myometrial invasion, or stage and low JAM-A expression is significantly associated with low overall survival rate and progression-free survival rate. Additionally, in 3-dimensional epithelial cell culture, JAM-A expression in well-differentiated adenocarcinoma is significantly higher than that in poorly differentiated adenocarcinoma. Thus, JAM-A expression is supposed to be reduced in high-grade or advanced endometrial carcinoma and may be a prognostic marker [23].
Pancreatic cancer
Low levels of JAM-A expression is an independent prognostic marker for adverse clinical outcome and is linked to tumor aggressiveness in pancreatic cancer. Depletion of JAM-A is related to positive lymph node status, the presence of distant metastasis and tumor grade, which suggests it may be involved in tumor progression [24]. Moreover, an important role of ZO-2, another member of the JAM-associated protein complex has participated in the pathogenesis on pancreatic cancer [25].
Testicular cancer
JAM-A is expressed on germ cells in normal testis, but only in spermatogonia and spermatocytes. Besides JAM-A, TJ proteins are disorganized in testicular cancer. JAM-A localization is disorganized in tubules with testicular carcinoma in situ (CIS) and there is an aberrant high expression of JAM-A in seminoma. The expression of JAM-A by seminoma cells may promote tumor cell migration and infiltration [26]. However, further studies are needed to demonstrate the relevance of JAM-A overexpression to the disease process.
Melanoma
JAM-A inhibits melanoma transendothelial migration. It has been provided in melanoma that inhibition of JAM-A expression promotes the transmigration efficiency of the melanoma cell lines (SLM8 cells). While the inhibition of JAM-C protein diminishes the efficiency of the A375 cell line to cross the endothelial cells, which means that JAM proteins may play opposite roles in melanoma cells migration. Nevertheless, the JAM-A expression level between melanoma and normal tissue remains unknown [27].
Renal cell cancer
JAM-A protein expression is significantly downregulated in patients with clear cell renal cell carcinoma. Depletion of JAM-A is an early event in the development of renal cancer and increases the migration of renal cancer cells. Underexpression of JAM-A is associated with a more aggressive phenotype but no prognostic value of JAM-A could be demonstrated [28].
Lung cancer
JAM-A mRNA in squamous cell carcinoma is about the same level compared to lung parenchyma while lower than in bronchial cells. On the other side, JAM-A mRNA level in adenocarcinoma is higher than those in lung parenchyma but slightly lower than in bronchial cells [29]. Goetsch et al. [22] investigated JAM-A expression in the lung tumor and normal tissues, JAM-A overexpression is detected in lung cancer tissues compared to normal tissues.
Recently JAM-A expression is determined by Zhang et al. [30] in non-small cell lung cancer (NSCLC) tissues and cell lines by immunohistochemistry, the results showed that JAM-A is expressed relatively high amounts in NSCLC tissues and some types of NSCLC cell lines, and that cell membrane-associated JAM-A levels are correlated with tumor aggressiveness. High expression of JAM-A is significantly associated with TNM stage, lymph node metastasis and shorten overall survival. Furthermore, the results also suggested that suppression of JAM-A expression induces cell cycle arrest in the G1-phase and inhibits lung cancer cell growth. From the above, JAM-A promotes NSCLC cells proliferation by modulation of cell cycle-related molecules.
Breast cancer
Recent research of JAM-A involved in breast cancer and potential mechanisms is quite detailed. However, the role of JAM-A in tumor growth and dissemination remains controversial. Naik et al. [31] first reported that JAM-A expression is down-regulated in metastatic breast cancer. Expression of JAM-A is inversely correlated to the ability of the breast cancer cells to migrate in their study of tumor cell lines. Overexpression of JAM-A in highly migratory cells (MDA-MB-231 cells) affects their morphology and inhibits both migration and invasion through collagen gels. Furthermore, knockdown of JAM-A using short interfering RNAs enhance the invasiveness of breast cancer cells [31].
However, the results from McSherry et al. [32] and Murakami et al. [33] studies are divergent from those reported by Naik et al. [31]. McSherry et al. [32] revealed a novel association between JAM-A protein upregulation and poor prognosis in the 270 cases of invasive breast cancer tissues. Murakami et al. also found that a strong correlation between high JAM-A expression and reduced patient survival in the 273 cases of breast cancer clinical dataset [33]. It should be noted that Naik et al. [31] used a small commercially available TMA (112 cases) and several breast cell lines while McSherry et al. [31] studied a larger patient dataset and had the additional advantage of associated clinicopathological and survival data. Another possibility to explain the divergence of data could be that the type/grade of population analyzed are different among these studies [22]. The downregulation of JAM-A in carcinoma cells may be detrimental to the survival of breast cancer patients. Thus, JAM-A could be used as a prognostic marker for metastatic breast cancer [34]. Some contradictory results of JAM-A expression in breast cancer may be attributed to the diverse cell lines used, different cell density, growth factors containing or not [30].
Additionally, overexpression of JAM-A in breast cancer may increase cell motility via downstream effects on β1-integrin, which is one of several proteins used by cells migration [32]. Another study provides further evidence that JAM-A knockdown or inhibition in breast cancer cells significantly decrease the activity of Rap1 GTPase, a known activator of β1-integrin as well as a regulator of cellular adhesion. This reveals a novel role for JAM-A in driving breast cancer cell migration via activation of Rap1 GTPase and β1-integrin [35]. A research of signal pathway identified that JAM-A as a downstream target of TGF-β1 represents a crucial mechanism in cancer progression. It was concluded that TGF-β1 down-regulate JAM-A expression via its effect on both transcriptional and post-translational regulations of JAM-A, thus inducing cell invasion [36]. The study in vitro evaluation and xenograft models suggests that JAM-A can interfere with tumor proliferation and JAM-A is a potential novel target in oncology [22].
As a correct organization of cell to cell junctions induces cell resistance to apoptotic stimuli through activation of different pathways, downregulation of JAM-A reduces breast cancer aggressive behavior by increasing cell susceptibility to apoptosis [33]. Recently, JAM-A is co-expressed with HER2 and related to aggressive breast cancer phenotypes. Furthermore, JAM-A may regulate HER2 proteasomal degradation and activity, probably offering a promise as an important therapeutic target in HER2-positive breast cancers [37].
Summary
JAM-A is an integral protein of TJs that is important for the assembling and maintenance of cell–cell adhesion. Disorder of JAM-A expression is required for cancer cell metastasis (Figure 2). The fact might be that low expression of JAM-A may impair cellular adhesion and polarity and favor tumor initiation while its overexpression may promote integrin-mediated migratory events and favor tumor progression [38]. Together with the study of different tumors, JAM-A expression seems to be cell type-dependent, its expression level in diverse tumors and relationship with clinical outcome may be different or even contradictory (Table 1). Owing to the complicated cellular genetic background and microenvironment, JAM-A may play different roles in various types of cancer tissue. The diverse mechanisms in regulating these processes remain to be determined. Activation or depletion of JAM-A might be mediated by numerous signal pathways as well as a variety of signal molecules. The interaction with other TJ proteins may also contribute to the final balance of JAM-A function in tumor progression.
Table 1.
JAM-A expression level in different tumors and correlation with clinical outcome
| Type of tumor | JAM-A expression level | Correlation with poor prognosis |
|---|---|---|
| Endometrial carcinoma | ↓ | - |
| Pancreatic cancer | ↓ | - |
| Testicular cancer | ↑ | * |
| Renal cell cancer | ↓ | 0 |
| NSCLC | ↑ | + |
| Breast cancer | ↑↓ | + |
NSCLC: non-small cell lung cancer.
JAM-A is lower expressed in tumor tissue than in corresponding normal tissue.
JAM-A is overexpressed in tumor tissue than in corresponding normal tissue.
JAM-A expression in tumor is controversial.
JAM-A expression positively correlates with poor prognosis.
JAM-A expression negatively correlates with poor prognosis.
no prognostic value of JAM-A could be demonstrated.
unknown.
In conclusion, JAM-A is a new emerging target in carcinogenesis and the influence of JAM-A on tumor development and progression is complex. The role of JAM-A in tumor progression may be regulated in a tissue-dependent manner. Much research still needs to be done to elucidate the tumor type–specific and complex functional roles of JAM-A in tumor progression. In the future, based on better understanding of the molecular mechanisms of regulation and functions, JAM-A may become a prognostic indicator and beneficial target in tumor therapy.
Acknowledgements
This work was supported by the National Undergraduate Innovation Project of China (No. 201410486089) and Innovation Seed Fund of Wuhan University School of Medicine (No. 2013-4).
Disclosure of conflict of interest
None.
References
- 1.Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Sawada N. Tight junction-related human diseases. Pathol Int. 2013;63:1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams L, Martin-Padura I, Dejana E, Hogg N, Simmons D. Identification and characterisation of human junctional adhesion molecule (JAM) Mol Immunol. 1999;36:1175–1188. doi: 10.1016/s0161-5890(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 6.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 8.Kang LI, Wang Y, Suckow AT, Czymmek KJ, Cooke VG, Naik UP, Duncan MK. Deletion of JAM-A causes morphological defects in the corneal epithelium. Int J Biochem Cell Biol. 2007;39:576–585. doi: 10.1016/j.biocel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Malergue F, Galland F, Martin F, Mansuelle P, Aurrand-Lions M, Naquet P. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol Immunol. 1998;35:1111–1119. doi: 10.1016/s0161-5890(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 10.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–530. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 11.Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Jin B. A novel interface consisting of homologous immunoglobulin superfamily members with multiple functions. Cell Mol Immunol. 2010;7:11–19. doi: 10.1038/cmi.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cera MR, Fabbri M, Molendini C, Corada M, Orsenigo F, Rehberg M, Reichel CA, Krombach F, Pardi R, Dejana E. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J Cell Sci. 2009;122:268–277. doi: 10.1242/jcs.037127. [DOI] [PubMed] [Google Scholar]
- 14.Nava P, Capaldo CT, Koch S, Kolegraff K, Rankin CR, Farkas AE, Feasel ME, Li L, Addis C, Parkos CA, Nusrat A. JAM-A regulates epithelial proliferation through Akt/beta-catenin signalling. EMBO Rep. 2011;12:314–320. doi: 10.1038/embor.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iden S, Misselwitz S, Peddibhotla SSD, Tuncay H, Rehder D, Gerke V, Robenek H, Suzuki A, Ebnet K. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol. 2012;196:623–639. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Severson EA, Parkos CA. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr Opin Cell Biol. 2009;21:701–707. doi: 10.1016/j.ceb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci (Landmark Ed) 2011;16:898–936. doi: 10.2741/3726. [DOI] [PubMed] [Google Scholar]
- 18.Offiah G, Brennan K, Hopkins AM. Junctional Adhesion Molecules (JAMs)-New Players in Breast Cancer? In: Mehmet Gunduz EG, editor. Breast Cancer - Focusing Tumor Microenvironment, Stem cells and Metastasis. Rijeka: InTech; 2011. [Google Scholar]
- 19.Gonzalez-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer. Prog Histochem Cytochem. 2007;42:1–57. doi: 10.1016/j.proghi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Molitoris B, Nelson W. Alterations in the establishment and maintenance of epithelial cell polarity as a basis for disease processes. J Clin Invest. 1990;85:3–9. doi: 10.1172/JCI114427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetsch L, Haeuw JF, Beau-Larvor C, Gonzalez A, Zanna L, Malissard M, Lepecquet AM, Robert A, Bailly C, Broussas M, Corvaia N. A novel role for junctional adhesion molecule-A in tumor proliferation: modulation by an anti-JAM-A monoclonal antibody. Int J Cancer. 2013;132:1463–1474. doi: 10.1002/ijc.27772. [DOI] [PubMed] [Google Scholar]
- 23.Koshiba H, Hosokawa K, Kubo A, Tokumitsu N, Watanabe A, Honjo H. Junctional adhesion molecule A [corrected] expression in human endometrial carcinoma. Int J Gynecol Cancer. 2009;19:208–213. doi: 10.1111/IGC.0b013e31819bc6e9. [DOI] [PubMed] [Google Scholar]
- 24.Fong D, Spizzo G, Mitterer M, Seeber A, Steurer M, Gastl G, Brosch I, Moser P. Low expression of junctional adhesion molecule A is associated with metastasis and poor survival in pancreatic cancer. Ann Surg Oncol. 2012;19:4330–4336. doi: 10.1245/s10434-012-2381-8. [DOI] [PubMed] [Google Scholar]
- 25.Chlenski A, Ketels KV, Tsao MS, Talamonti MS, Anderson MR, Oyasu R, Scarpelli DG. Tight junction protein ZO-2 is differentially expressed in normal pancreatic ducts compared to human pancreatic adenocarcinoma. Int J Cancer. 1999;82:137–144. doi: 10.1002/(sici)1097-0215(19990702)82:1<137::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Tarulli GA, Stanton PG, Loveland KL, Meyts ER, McLachlan RI, Meachem SJ. A survey of Sertoli cell differentiation in men after gonadotropin suppression and in testicular cancer. Spermatogenesis. 2013;3:e24014. doi: 10.4161/spmg.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghislin S, Obino D, Middendorp S, Boggetto N, Alcaide-Loridan C, Deshayes F. Junctional adhesion molecules are required for melanoma cell lines transendothelial migration in vitro. Pigment Cell Melanoma Res. 2011;24:504–511. doi: 10.1111/j.1755-148X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 28.Gutwein P, Schramme A, Voss B, Abdel-Bakky MS, Doberstein K, Ludwig A, Altevogt P, Hansmann ML, Moch H, Kristiansen G, Pfeilschifter J. Downregulation of junctional adhesion molecule-A is involved in the progression of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2009;380:387–391. doi: 10.1016/j.bbrc.2009.01.100. [DOI] [PubMed] [Google Scholar]
- 29.Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Luo W, Huang B, Liu Z, Sun L, Zhang Q, Qiu X, Xu K, Wang E. Overexpression of JAM-A in non-small cell lung cancer correlates with tumor progression. PLoS One. 2013;8:e79173. doi: 10.1371/journal.pone.0079173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik MU, Naik TU, Suckow AT, Duncan MK, Naik UP. Attenuation of junctional adhesion molecule-A is a contributing factor for breast cancer cell invasion. Cancer Res. 2008;68:2194–2203. doi: 10.1158/0008-5472.CAN-07-3057. [DOI] [PubMed] [Google Scholar]
- 32.McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM, Gallagher WM. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J Cancer. 2009;125:1343–1351. doi: 10.1002/ijc.24498. [DOI] [PubMed] [Google Scholar]
- 33.Murakami M, Giampietro C, Giannotta M, Corada M, Torselli I, Orsenigo F, Cocito A, d’Ario G, Mazzarol G, Confalonieri S, Di Fiore PP, Dejana E. Abrogation of junctional adhesion molecule-A expression induces cell apoptosis and reduces breast cancer progression. PLoS One. 2011;6:e21242. doi: 10.1371/journal.pone.0021242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik UP, Naik MU. Putting the brakes on cancer cell migration: JAM-A restrains integrin activation. Cell Adh Migr. 2008;2:249–251. doi: 10.4161/cam.2.4.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McSherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13:R31. doi: 10.1186/bcr2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Lui WY. Transforming growth factor-beta1 attenuates junctional adhesion molecule-A and contributes to breast cancer cell invasion. Eur J Cancer. 2012;48:3475–3487. doi: 10.1016/j.ejca.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Brennan K, McSherry EA, Hudson L, Kay EW, Hill ADK, Young LS, Hopkins AM. Junctional adhesion molecule-A is co-expressed with HER2 in breast tumors and acts as a novel regulator of HER2 protein degradation and signaling. Oncogene. 2013;32:2799–2804. doi: 10.1038/onc.2012.276. [DOI] [PubMed] [Google Scholar]
- 38.Brennan K, Offiah G, McSherry EA, Hopkins AM. Tight junctions: a barrier to the initiation and progression of breast cancer? J Biomed Biotechnol. 2010;2010:460607. doi: 10.1155/2010/460607. [DOI] [PMC free article] [PubMed] [Google Scholar]


