Abstract
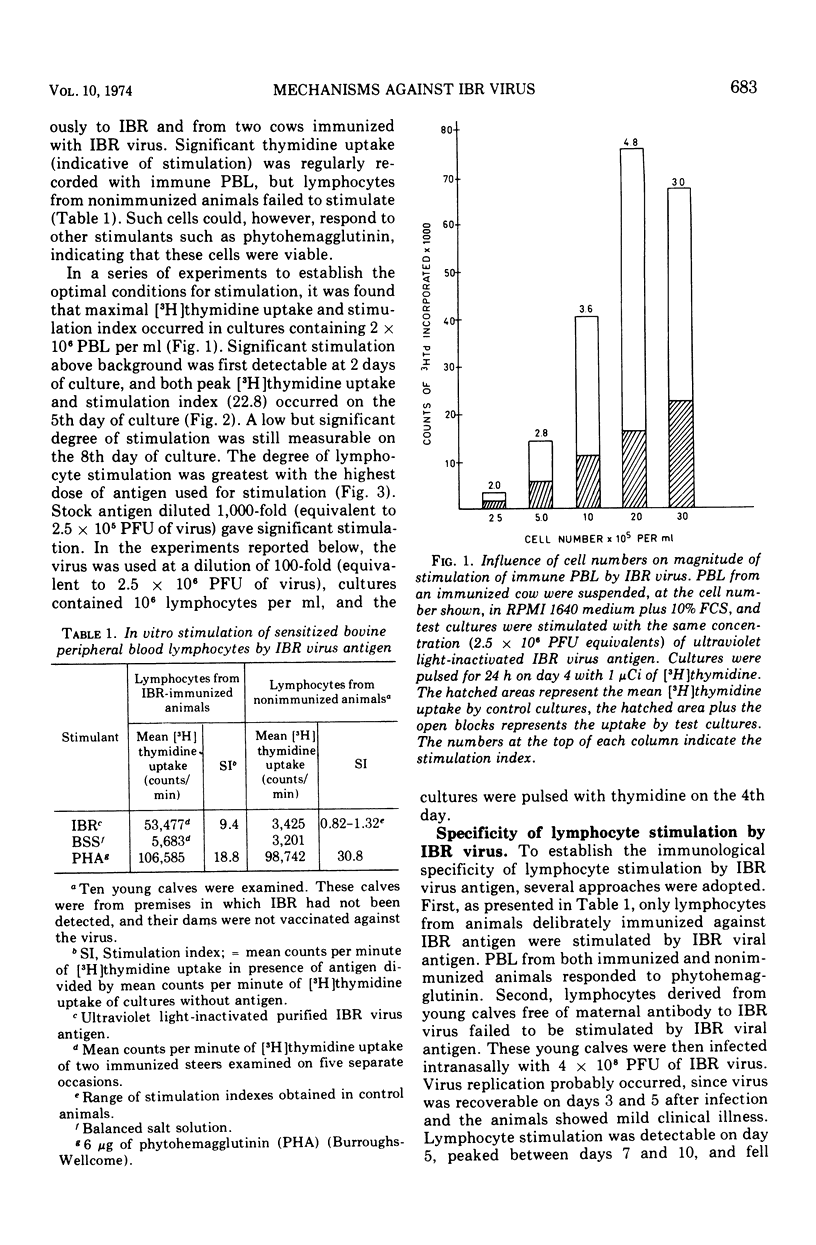
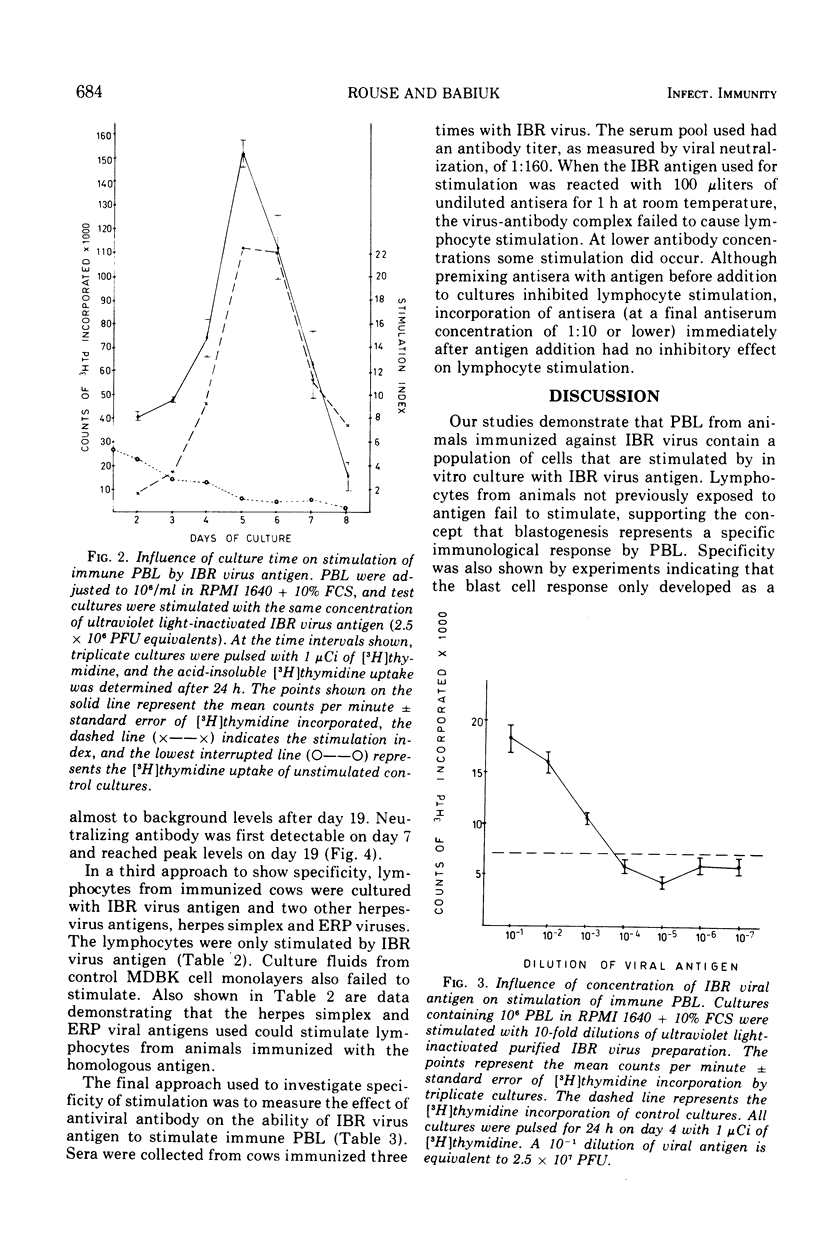
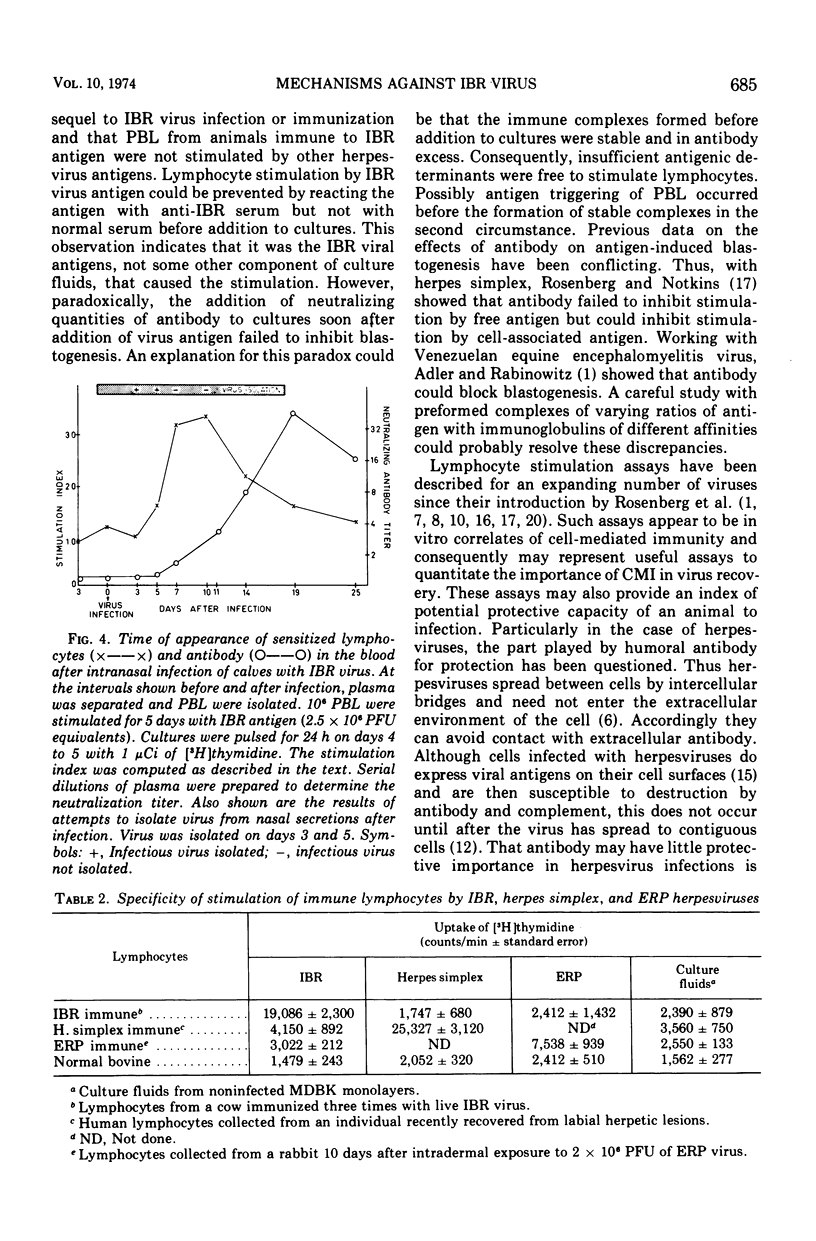
Isolated peripheral blood lymphocytes (PBL) from cattle immunized or infected with infectious bovine rhinotracheitis (IBR) virus were cultured in vitro with ultraviolet light-inactivated IBR virus, and the degree of lymphocyte blastogenesis was quantitated by measurement of the uptake of [3H]thymidine into acid-insoluble material. Lymphocyte blastogenesis only occurred with PBL from immunized or infected animals. The optimal conditions for lymphocyte blastogenesis were defined. Blastogenesis was specific since cells from animals immunized against IBR failed to react with two other herpesvirus antigens tested, herpes simplex and equine rhinopneumonitis viruses. Blastogenesis could be prevented by reacting IBR antigen with IBR-specific antibody before adding to cultures, but incorporating IBR-specific antibody in the culture medium after adding free antigen failed to inhibit blastogenesis. With intranasally infected animals, lymphocyte blastogenesis was detectable after 5 days, reached peak levels between days 7 and 10, and then declined to low levels by day 19. In contrast, levels of neutralizing antibody were barely detectable on day 7 and reached maximal concentrations on day 19. The lymphocyte blastogenesis assay was emphasized as a convenient and useful in vitro correlate of cell-mediated immunity that should help define the role of cell-mediated immunity in immunity to herpesviruses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Allison A. C. Immunity and immunopathology in virus infections. Ann Inst Pasteur (Paris) 1972 Oct;123(4):585–608. [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario T. C., Poland J. D., Wulff H., Chin T. D., Wenner H. A. Six years experience with herpes simplex virus in a children's home. Am J Epidemiol. 1969 Nov;90(5):416–422. doi: 10.1093/oxfordjournals.aje.a121087. [DOI] [PubMed] [Google Scholar]
- Christian R. T., Ludovici P. P., Jeter W. S. Cell-to-cell transmission of herpes simplex virus in primary human amnion cells. Proc Soc Exp Biol Med. 1971 Dec;138(3):1109–1115. doi: 10.3181/00379727-138-36061. [DOI] [PubMed] [Google Scholar]
- Davies D. H., Carmichael L. E. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infect Immun. 1973 Oct;8(4):510–518. doi: 10.1128/iai.8.4.510-518.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein G. J., Rosenberg G. L. In vitro proliferation of rabbit bone marrow-derived and thymus-derived lymphocytes in response to vaccinia virus. Cell Immunol. 1973 Jun;7(3):516–521. doi: 10.1016/0008-8749(73)90216-5. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Cellular immune response to viral infection: in vitro studies of lymphocytes from mice infected with Sindbis virus. Cell Immunol. 1973 Dec;9(3):426–434. doi: 10.1016/0008-8749(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Stevens D. A. Viral infections in man associated with acquired immunological deficiency states. Fed Proc. 1971 Nov-Dec;30(6):1858–1864. [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Notkins A. L. Induction of cellular immunity to herpes simplex virus: relationship to the humoral immune response. J Immunol. 1974 Mar;112(3):1019–1025. [PubMed] [Google Scholar]
- Rouse B. T., Ditchfield W. J. The response of ponies to Myxovirus influenzae A-equi 2. II. Immunoglobulin classes of antibody to the virus in serum and nasal secretions. Can J Comp Med. 1970 Jan;34(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T. Equine influenza immunisation--the role of nasal antibody--a review. Aust Vet J. 1971 Apr;47(4):146–148. doi: 10.1111/j.1751-0813.1971.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Bellanti J. A., Chanock R. M. Immunoglobulins in serum and nasal secretions following infection with type 1 parainfluenza virus and injection of inactivated vaccines. J Immunol. 1967 Jul;99(1):133–141. [PubMed] [Google Scholar]



