Abstract
We herein present a case of a left cervical cystic mass, for which the initial pathological diagnosis was branchial cleft cyst carcinoma (following complete mass excision). Thorough postoperative examinations, including with FDG positron emission tomography/computed tomography (PET/CT), revealed a primary tumor in the retromolar region of the left mandible. A 52-year-old female presented with a 2-month history of a painless, progressively enlarged left-sided neck mass. Fine-needle aspiration biopsy suggested a branchial cleft cyst. Physical examination revealed a 3 × 3-cm smooth, tender mass in the upper-left neck and anterior border of the sternocleidomastoid muscle. Examination using nasendoscopy and a strobolaryngoscope revealed no abnormalities of the nasal cavity, nasopharynx, oropharynx, hypopharynx or larynx. MRI of the neck revealed a solitary, round, cystic mass under the left parotid gland. The mass was excised completely. Pathologic results indicated a branchial cleft cyst carcinoma. According to the diagnostic criteria for a branchial cleft cystic carcinoma, PET/CT was performed to detect the occult primary site. PET/CT revealed high FDG uptake in the tooth root of the left mandible. Frozen sections of the mass were indicative of moderate, differentiated squamous cell carcinoma. The carcinoma in the retromolar region of the left mandible was locally excised under general anesthesia. A partial left maxillectomy, partial mandibulectomy, and left radical neck dissection were performed. The patient received postoperative concurrent chemoradiotherapy, and was disease-free at the 8-month follow-up. True branchial cleft cyst carcinoma is rare: once diagnosed, it should be distinguished from metastatic cystic cervical lymph and occult primary carcinoma. FDG PET/CT is useful in the identification of occult primary tumor.
Keywords: Branchial cleft cyst carcinoma, metastatic cystic cervical lymph node, carcinoma of unknown primary site, positron emission tomography/computed tomography
Introduction
The majority of metastatic cervical lymph nodes in head-and-neck squamous cell carcinomas (HNSCCs) are solid masses. However, between 33% and 62% of cases are cystic metastatic squamous cell carcinomas (SCC) [1-4]. Diagnosis and management for both clinicians and pathologists is problematic if lesions are characterized by a solitary cystic appearance [5].
The most-common variety of solitary cervical cystic lesion is the branchial cleft cyst (BCC), which can become malignant. According to the strict diagnostic criteria for branchial cleft cystic carcinoma (BCCC), proposed by Martin et al. [6] and modified by Khafif et al. [7], fewer than 40 cases have been reported [1]. The majority of previously suspected BCCCs were actually metastatic cystic lymph nodes; misdiagnosis can occur due to the absence of long-term follow-up [6,8]. Thus, solitary metastatic cystic lymph nodes, from carcinomas of the upper aerodigestive tract, are difficult to differentiate from BCCCs, especially in patients more than 40 years of age [9]. The proportion of metastatic cystic SCCs initially diagnosed as BCCs, or BCCCs, ranges between 11% and 21% [2,10,11]. Metastatic cystic SCCs in the upper lateral neck are easily mistaken for BCCCs if the primary site of the carcinoma is not detected [5].
The challenge, therefore, is to distinguish BCCC from occult metastatic cystic SCC, and to identify the primary site of metastatic cystic SCC in the head and neck. Routine work-ups, with ultrasound (US), computed tomography (CT), magnetic resonance image (MRI), and panendoscopy, can aid in diagnosis and differentiation. Several studies have emphasized the role of fine-needle aspiration cytology (FNAC), or fine needle aspiration biopsy (FNAB), under US guidance. [1,5] [18F] 2-Fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/CT (PET/CT) imaging has been widely used to detect unknown primary tumors [12]. However, the utility of FDG PET/CT for the diagnosis and differentiation of cervical cystic masses is limited, and its role disputed [13-15], especially in adults in whom the primary tumor is very small [15]. Therefore, the utility of PET/CT requires further validation.
In the present study, we report a case of a left cervical cystic mass for which the initial pathological diagnosis was of branchial cleft cyst carcinoma (following complete excision of the mass). Thorough postoperative examination, including with FDG PET/CT, revealed a primary tumor in the retromolar region of the left mandible.
Case report
A 52-year-old female presented with a 2-month history of a painless, progressively enlarged left-sided neck mass. There was no nasal obstruction, nasal bleeding, respiratory distress, hoarseness, cough, odynophagia, weight loss, or dysphagia, and no childhood history of neck swellings. In November, 2013, the patient visited a local hospital. US and CT revealed a cystic mass in the left upper neck. FNAB further demonstrated that the mass contained yellow liquid, and that squamous epithelial cells and a portion of nuclear cells were atypical. The pathological results suggested a branchial cleft cyst accompanied by infection. The patient was admitted to our department for surgery. Physical examinations revealed a 3 × 3-cm smooth, tender mass in the upper-left neck, and in the anterior border of the sternocleidomastoid muscle. Nasendoscopy, and a strobolaryngoscope, revealed no abnormalities in the nasal cavity, nasopharynx, oropharynx, hypopharynx or larynx. MRI of the neck revealed a solitary, round mass under the left parotid gland. The T1- and T2-weighted signals were hyperintense. Diffusion-weighted imaging (DWI) suggested hyperintense lesions (b = 1000 s/mm²): contrast-enhanced T1-weighted images revealed peripheral, but not cystic, enhancement (Figure 1). On November 13, 2013, the mass was excised completely using a left-neck lateral approach under general anesthesia (Figure 2). Pathologic results indicated a BCCC.
Figure 1.
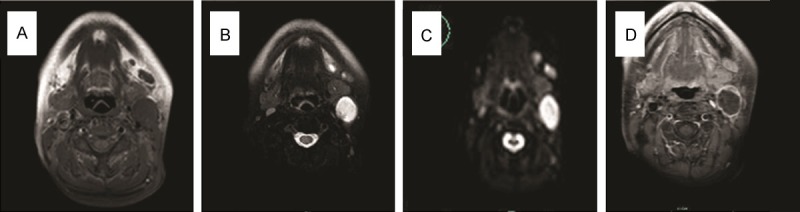
MRI of the neck revealed a solitary, round mass under the left parotid gland: (A) The T1- and (B) T2-weighted signals were hyperintense; (C) DWI suggested hyperintense lesions (b = 1000 s/mm²); (D) Contrast-enhanced T1-weighted images revealed peripheral enhancement, but no enhancement in the cystic region.
Figure 2.
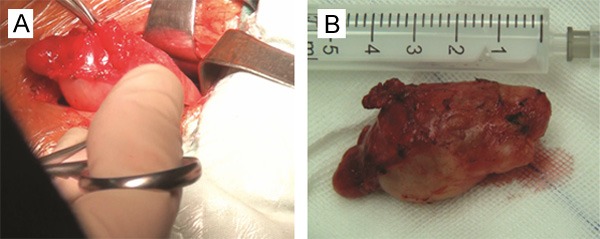
The mass was excised completely using a left-neck lateral approach under general anesthesia (A): a surgical sample (B).
According to the diagnostic criteria for BCCC, proposed by Martin et al. [6] and modified by Khafif et al. [7], PET/CT was performed to detect the occult primary site. The scan revealed high FDG uptake in the surgical regions previously delineated, and in the tooth root of the left mandible. The surrounding mandibular bone was partially degraded, with high FDG uptake (Figure 3). Oral examination revealed a 1 × 1-cm rough mass in the retromolar region of the left mandible. On December 27, 2013, frozen sections of the mass indicated moderately differentiated SCC. Carcinoma in the retromolar region of the left mandible was locally excised under general anesthesia. A partial left maxillectomy, partial mandibulectomy and left radical neck dissection were performed. The tongue flap was used to address the surgical defect. The postoperative period was uneventful: the patient received postoperative concurrent chemoradiotherapy (CCR; 5,000 cGy in 200-cGy fractions delivered over 25 days and chemotherapy using cisplatin, at 37 mg, on days 1-3 [one cycle per 4 weeks, four cycles in total]). The patient was disease-free at 8 months postoperatively.
Figure 3.
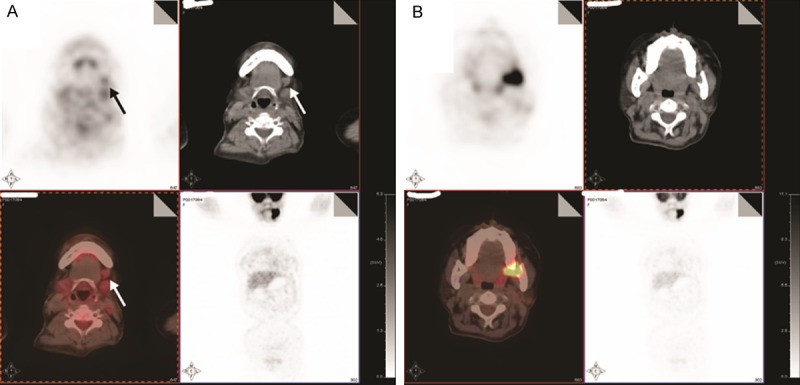
PET/CT revealed high FDG uptake in the above-mentioned surgical regions (A; SUVmax = 2.27) and at the site of the tooth root of the left mandible (B; SUVmax = 20.77); the surrounding, the mandibular bone was partially degraded.
Discussion
BCCC is currently regarded as an uncommon clinicopathological entity [1]. In 1950, Martin et al. reviewed 250 cases of BCCC, of which the majority represented metastatic head and neck primary cancers resulting from an absence of long-term follow-up [6]. Strict diagnostic criteria for BCCC were subsequently established, as follows: a) tumor located along the anterior border of the sternocleidomastoid muscle; b) histological findings consistent with tissue originating from a branchial cleft; c) histological evidence of carcinoma arising in the wall of an epithelial-lined cyst; and d) no evidence of a primary source during a minimum 5-year follow-up period [6]. In 1989, Khafif et al. modified the above criteria as follows: a) tumor located in the anatomic region of a branchial cleft cyst; b) histological appearance consistent with a branchial vestige origin; c) for a squamous cell carcinoma, presence of the carcinoma within the lining of an identifiable epithelial cyst; d) evidence of transition from a normal squamous epithelium of the cyst to carcinoma; and e) absence of any identifiable primary malignant tumor following exhaustive evaluation of the patient [7]. According to these criteria, fewer than 40 cases were reported [1].
A solitary cystic metastatic cervical lymph node, from a small occult primary tumor, is easily misdiagnosed as BCCC, especially during initial presentation. Hardee et al. reported two cases of solitary cystic metastatic cervical lymph nodes initially presenting as branchial cysts [16], one of which was not located at the primary site. In the other case, primary carcinoma was discovered in the right tonsil [16]. Solitary cystic masses located in level II of the neck are problematic for clinicians. Briggs et al. suggested the following reasons for this diagnostic difficulty: a) the two diseases always manifest in similar locations; b) cervical cystic metastases might represent the initial presentation of an occult primary carcinoma in the upper aerodigestive tract; and c) histological features usually confer difficulty with respect to differentiation of a branchial cleft carcinoma from solitary cystic degeneration of a metastatic cervical lymph node [9]. In the present case, the patient also presented with a painless solitary cystic mass in the left side of level II of the neck. FNAB suggested a branchial cleft cyst. A primary lesion was not discovered during thorough work-up investigations. Subsequently, the mass was completely excised. The postoperative histological results revealed that the lesion was a BCCC, and should therefore not be considered an occult primary carcinoma according to the criteria of Martin et al. [6] and Khafif et al. [7].
Routine work-ups usually employ US, CT, MRI and panendoscopy, and can aid in the detection of the primary site. Between 72% and 90% of primary tumors of cervical metastatic SCC are located within Waldeyer’s ring [1,2,4]. Directed biopsy of this area using panendoscopy or tonsillectomy is recommended if these routine work-ups do not detect any primary lesions. Several researchers have recommended that bilateral tonsillectomy represent the standard approach to detect the primary site of a carcinoma of unknown primary treatment (CUP) in the head and neck [17]. Metastatic cervical lymph nodes in thyroid carcinoma are also a common cause of cystic masses in the neck [18,19]. Recently, PET/CT has been shown to confer advantages in the detection of CUPs. Researchers within our center have assessed the role of PET/CT in the diagnosis of CUPs [20-22]. In a previous study, we reported that the success rates of FDG PET and CT in the detection of CUPs were 73.3% and 52.4%, respectively [22]. We initially reported a case of tonsillar metastasis of small-cell neuroendocrine carcinoma of the lung using FDG PET/CT following tonsillectomy [20]. Wong et al. reported use of FDG PET/CT to detect 30 primary cancers in 75 patients in whom no primary tumor was detected using CT/MRI. Furthermore, PET/CT was still useful even in patients subjected to tonsillectomy [23]. However, Ferris et al. reported that PET/CT might not confer diagnostic advantages in adults with suspicious cystic neck masses if the primary tumor is very small [24]. However, with technical improvements, contrast-enhanced FDG PET/CT now possesses advantages compared with non-enhanced FDG PET/CT in the detection of cystic lymph nodes [25]. In the present case, FDG PET/CT revealed that the primary site was the retromolar region of the left mandible. Findings during surgery and postoperative pathologic results confirmed the PET/CT findings. The patient received timely and appropriate treatment.
In conclusion, true branchial cleft cyst carcinoma is rare. Once diagnosed, it should be distinguished from metastatic cystic cervical lymph and occult primary carcinoma. FDG PET/CT is useful for the identification of an occult primary tumor.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant nos. 81172562 and 81372903).
Disclosure of conflict of interest
None.
References
- 1.Bradley PT, Bradley PJ. Branchial cleft cyst carcinoma: fact or fiction? Curr Opin Otolaryngol Head Neck Surg. 2013;21:118–23. doi: 10.1097/MOO.0b013e32835cebde. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg D, Sciubba J, Koch WM. Cystic metastasis from head and neck squamous cell cancer: a distinct disease variant? Head Neck. 2006;28:633–8. doi: 10.1002/hed.20381. [DOI] [PubMed] [Google Scholar]
- 3.Mallet Y, Lallemant B, Robin YM, Lefebvre JL. Cystic lymph node metastases of head and neck squamous cell carcinoma: pitfalls and controversies. Oral Oncol. 2005;41:429–34. doi: 10.1016/j.oraloncology.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Regauer S, Mannweiler S, Anderhuber W, Gotschuli A, Berghold A, Schachenreiter J, Jakse R, Beham A. Cystic lymph node metastases of squamous cell carcinoma of Waldeyer’s ring origin. Br J Cancer. 1999;79:1437–42. doi: 10.1038/sj.bjc.6690229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietarinen-Runtti P, Apajalahti S, Robinson S, Passador-Santos F, Leivo I, Mäkitie AA. Cystic neck lesions: clinical, radiological and differential diagnostic considerations. Acta Otolaryngol. 2010;130:300–4. doi: 10.3109/00016480903127450. [DOI] [PubMed] [Google Scholar]
- 6.Martin H, Morfit HM, Ehrlich H. The case for branchiogenic cancer. Ann Surg. 1950;132:867–887. doi: 10.1097/00000658-195011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khafif RA, Prichep R, Minkowitz S. Primary branchiogenic carcinoma. Head Neck. 1989;11:153–163. doi: 10.1002/hed.2880110209. [DOI] [PubMed] [Google Scholar]
- 8.Thompson DR, Heffner DK. The clinical importance of cystic squamous cell carcinoma in the neck. Cancer. 1998;82:944–956. doi: 10.1002/(sici)1097-0142(19980301)82:5<944::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Briggs RD, Pou AM, Schnadig VJ. Cystic metastasis versus branchial cleft carcinoma: a diagnostic challenge. Laryngoscope. 2002;112:1010–4. doi: 10.1097/00005537-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja A, Ng CF, King W, Metreweli C. Solitary cystic nodal metastasis from occult papillary carcinoma of the thyroid mimicking a branchial cyst: a potential pitfall. Clin Radiol. 1998;53:61–3. doi: 10.1016/s0009-9260(98)80037-8. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan PM, Roland NJ, Jones AS. Cervical node metastases presenting with features of branchial cysts. J Laryngol Otol. 1994;108:1068–1071. doi: 10.1017/s0022215100128907. [DOI] [PubMed] [Google Scholar]
- 12.Zhao K, Luo XM, Zhou SH, Liu JH, Yan SX, Lu ZJ, Yang SY, Lin LL, Dong MJ. 18F-fluoro-deoxyglucose positron emission tomography/computed tomography as an effective diagnostic workup in cervical metastasis of carcinoma from an unknown primary tumor. Cancer Biother Radiopharm. 2012;27:685–693. doi: 10.1089/cbr.2011.1134. [DOI] [PubMed] [Google Scholar]
- 13.Alhilali L, Reynolds AR, Fakhran S. Osteoradionecrosis after radiation therapy for head and neck cancer: differentiation from recurrent disease with CT and PET/CT imaging. AJNR Am J Neuroradiol. 2014;35:1405–11. doi: 10.3174/ajnr.A3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CP, Shiau YC, Yen RF. F-18 FDG uptake in papillary carcinoma arising from thyroglossal duct cyst. Clin Nucl Med. 2011;36:141–2. doi: 10.1097/RLU.0b013e318203bc9c. [DOI] [PubMed] [Google Scholar]
- 15.Ferris RL, Branstetter BF, Nayak JV. Diagnostic utility of positron emission tomography-computed tomography for predicting malignancy in cystic neck masses in adults. Laryngoscope. 2005;115:1979–82. doi: 10.1097/01.mlg.0000178328.70288.55. [DOI] [PubMed] [Google Scholar]
- 16.Hardee PS, Hutchison IL. Solitary nodal metastases presenting as branchial cysts: a diagnostic pitfall. Ann R Coll Surg Engl. 1999;81:296–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Kothari P, Randhawa PS, Farrell R. Role of tonsillectomy in the search for a squamous cell carcinoma from an unknown primary in the head and neck. Br J Oral Maxillofac Surg. 2008;46:283–7. doi: 10.1016/j.bjoms.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Hwang CF, Wu CM, Su CY, Cheng L. A long-standing cystic lymph node metastasis from occult thyroid carcinoma-report of a case. J Laryngol Otol. 1992;106:932–4. doi: 10.1017/s0022215100121322. [DOI] [PubMed] [Google Scholar]
- 19.Chi HS, Wang LF, Chiang FY, Kuo WR, Lee KW. Branchial cleft cyst as the initial impression of a metastatic thyroid papillary carcinoma: two case reports. Kaohsiung J Med Sci. 2007;23:634–8. doi: 10.1016/S1607-551X(08)70063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen XH, Bao YY, Zhou SH, Wang QY, Zhao K. Palatine Tonsillar Metastasis of Small-Cell Neuroendocrine Carcinoma from the Lung Detected by FDG-PET/CT After Tonsillectomy: A Case Report. Iran J Radiol. 2013;10:148–51. doi: 10.5812/iranjradiol.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao K, Luo XM, Zhou SH, Liu JH, Yan SX, Lu ZJ, Yang SY, Lin LL, Dong MJ. 18F-fluorodeoxyglucose positron emission tomography/computed tomography as an effective diagnostic workup in cervical metastasis of carcinoma from an unknown primary tumor. Cancer Biother Radiopharm. 2012;27:685–93. doi: 10.1089/cbr.2011.1134. [DOI] [PubMed] [Google Scholar]
- 22.Zhao K, Dong MJ, Sheng ZK, Liu KF, Yang SY, Liu ZF, Sheng JF. Elevated uptake of 18F-FDG in PET/CT imaging of a nocardial pleural nodule. Clin Imaging. 2012;36:383–5. doi: 10.1016/j.clinimag.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Wong WL, Sonoda LI, Gharpurhy A, Gollub F, Wellsted D, Goodchild K, Lemon C, Farrell R, Saunders M. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers-an audit and review of published studies. Clin Oncol (R Coll Radiol) 2012;24:190–5. doi: 10.1016/j.clon.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Ferris RL, Branstetter BF, Nayak JV. Diagnostic utility of positron emission tomography-computed tomography for predicting malignancy in cystic neck masses in adults. Laryngoscope. 2005;115:1979–1982. doi: 10.1097/01.mlg.0000178328.70288.55. [DOI] [PubMed] [Google Scholar]
- 25.Haerle SK, Strobel K, Ahmad N, Soltermann A, Schmid DT, Stoeckli SJ. Contrast-enhanced 18F-FDG-PET/CT for the assessment of necrotic lymph node metastases. Head Neck. 2011;33:324–9. doi: 10.1002/hed.21447. [DOI] [PubMed] [Google Scholar]


