Abstract
Chemokine receptors are now known to play an important role in cancer growth and metastasis. However, there is little information regarding chemokine expression in breast cancer. The aim of this study was to evaluate CXCL12 expression in breast cancer and to investigate the question of whether reduced expression of CXCL12 may have any pathological significance in breast cancer development or progression. In this study, we performed western blotting and immunohistochemistry to evaluate the expression of CXCL12 and relevance with clinicopathological factors in the invasive ductal carcinoma. Reduction of CXCL12 was significantly correlated with tumor size, lymph node metastasis, TNM stage and Her-2 expression in breast cancer. Patients with negative CXCL12 expression had significantly lower cumulative postoperative 5 year survival rate than those with positive CXCL12 expression. In addition, we demonstrated that upregulation of CXCL12 expression by infection with an adenovirus containing a CXCL12 vector significantly inhibited cell growth and reduced the migration of breast cancer cells. Furthermore, animal studies revealed that nude mice injected with the Ad-CXCL12 cell lines featured a lighter weight than the control cell lines. These data suggest that CXCL12 plays an important role in cell growth and invasion in human breast cancer and it appears to be a potential prognostic marker for patients with breast cancer.
Keywords: Breast carcinoma, CXCL12, growth, metastasis, nude mice
Introduction
Breast cancer survival has improved significantly over the last 30 years; however, it still ranks second among cancer deaths in women [1]. Therapeutic failure and distant metastasis have been a major challenge in the treatment of breast cancer. Thus, exploring more markers to predict responsiveness of treatment, tumor progression, and potential target therapies is becoming more and more important [2,3].
Chemokines have multiple roles in many kinds of physiologic processes, such as hematopoiesis, lymphocyte development, and wound healing [4,5]. Recent findings demonstrated that there is a close relationship between tumor cells and chemokines [6-8]. Cancer cell invasion and metastasis shares many similarities with the process by which leukocytes enter inflamed tissues [9]. Numerous studies indicate that chemokine receptors are expressed by tumor cells, while chemokines are expressed at organs that turn into metastatic targets [10,11]. To date, the CXCL12-CXCR4 pair was found to be involved in almost all malignancies that were studied, including many solid cancers and tumors of a hematopoietic origin. In most cases, the CXCL12-CXCR4 pair was found to be associated and/or involved with increased malignancy and metastasis, acting at many different levels [12,13], and another CXCL12 receptor, CXCR7, was shown to promote the survival of tumor cells by preventing apoptosis, increased adhesion properties and dissemination, but did not mediate chemotaxis towards CXCL12 [14,15].
Although CXCL12 has been implicated in several types of cancers, its exact role remains largely unknown [16]. In this study, we found that CXCL12 was reduced in breast cancer, and its low-expression was associated with aggressive behavior of breast cancer. We then used adenovirus vector technology to increase endogenous CXCL12 expression in breast cancer cells. We demonstrated that upregulation of CXCL12 inhibited the growth and invasion of breast cancer in vitro and in vivo. Our study strongly highlights the significance of CXCL12 in the growth and invasion of breast cancer, and may provide a therapeutic target in breast cancer.
Materials and methods
Patient samples
The study included 52 patients with histologically confirmed invasive ductal carcinoma (IDC) of the breast who underwent lumpectomy or modified radical mastectomy at the Affiliated Hospital of Medical College, Qingdao University from 2006 to 2010. None of the patients had undergone preoperative chemotherapy or radiation. Research protocols for the use of human tissue were approved by and conducted in accordance with the policies of the Institutional Review Boards at Qingdao University. The histological subtype was determined according to the World Health Organization classification. The TNM stage was determined postoperatively according to the American Joint Committee on Cancer, and the histological grade was determined according to the Scarff-Bloom-Richardson grading system.
Cell line and culture
Six human breast cancer cell lines, MDA-MB-468, HCC-1937, MCF-7, Bcap-37, MDA-MB-231 and MX-1, were obtained from the Cancer Research Institute of Beijing, China. These cells were cultivated in T75 tissue culture flasks in DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 20 mM hydroxyethyl piperazine ethanesulfonic acid, and incubated in a humidified incubator containing 5% CO2 at 37°C.
Western blot analysis
Tissues or cells were lysed in RIPA buffer supplemented with protease inhibitor mixture for 30 min at 4°C. The cell lysates were then sonicated briefly and centrifuged (14,000 g at 4°C) for 15 min to remove insoluble materials. Equal amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with 5% nonfat dry milk and then incubated with first antibody, followed by horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized by ECL chemiluminescence method.
Construction of Tet-off adenoviral-mediated system
The recombinant adenovirus was assembled and produced using the Adeno-X Tet-Off Expression System 1 (Clontech, Mountain View, CA, USA) according to the manufacturer’s instructions. First, a CXCL12 construct (consisting of a full-length CXCL12 cDNA fused to a C-terminal GFP tag) was cloned into a pTREShuttle2 vector (Clontech) containing a tetracycline-responsive element upstream of the cytomegalovirus minimal promoter. Next, the resultant TRE-GFP-CXCL12 expression unit was excised from the pTRE-CXCL12 vector using the I-CeuI and PI-SceI restriction enzymes and then ligated to Adeno-X System 1 Viral DNA (Clontech). The resultant recombinant Adeno-X-GFP-CXCL12 vector (Ad-CXCL12) was packaged into infectious adenoviral particles by transfecting HEK293 cells, and the recombinant adenoviruses were subsequently harvested by lysing the transfected cells. For transient expression of CXCL12, cells were coinfected with a recombinant adenovirus (Ad-CXCL12) and a regulator virus (Adeno-X Tet-off virus) in serum-free media for 12 h, followed by incubation in complete medium.
Invasion assay
Invasion assays were performed using the Chemicon Cell Invasion Assay Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. Briefly, cells (1 × 104) were plated onto a Matrigel-coated transwell invasion chamber and incubated at 37°C for 24 h. Non-invading cells were removed by wiping the upper side of the transwell. Invading cells were fixed with methanol and stained with hematoxylin. Three independent invasion assays were performed in triplicate. Six random fields on average were counted using a light microscope.
MTT proliferation assay
The capability of cellular proliferation was measured by the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] MTT assay. Approximately 5 × 103 cells were seeded into 96-well culture plates with or without CXCL12 knockdown at 24, 48, 72, 96 and 120 h, respectively. Then cells were incubated with 20 μL MTT (10 mg/ml) for 4 hr at 37°C and 200 μL DMSO was pipetted to solubilize the formazan product for 20 min at room temperature. The optical density (OD) was determined using a spectrophotometer (Bio-Rad) at a wavelength of 570 nm. The experiment was repeated 3 times in triplicate.
Growth curve
Approximately 3 × 105 cells from each cell line were seeded in 60-mm plates. Cells were harvested and counted every day after the seeding. Each point on the curve is the average of triplicates.
In vivo tumor model
Six-week-old female athymic nude mice were subcutaneously injected with 5 × 106 cells in 0.2 ml PBS into right scapular region. Three groups (7 each) of mice were tested. Group 1 was injected with MDA-MB-231 cells alone; group 2 was injected with MDA-MB-231 cells stably transfected with Ad-CXCL12; and group 3 was injected with cells stably transfected with Ad-Control. Tumor size was measured every 2 days with calipers. After the mice were killed at 3 weeks, the weight of the tumors was measured.
Statistical analysis
All values in the text and figures are presented as mean ± SD. Overall survival rates were determined using Kaplan-Meier estimator, an event being defined as death for cancer correlated cause. The log-rank test was used to identify differences between the survival curves of different patients’ groups. In univariate analysis, 2-tailed χ 2 tests for categorical variables and 2-tailed t test for continuous variables were used for statistical comparisons. Values of P < 0.05 were taken to show a significant difference between means.
Results
Down-regulation of CXCL12 in breast cancers and correlated with clinicopathological features of breast cancer patients
We determined the CXCL12 expression in breast cancer tissues and matched distal normal tissues. Figure 1A illustrated CXCL12 expression in four randomly picked patients. Low levels of CXCL12 protein were found in human breast cancer tissues compared with the paired normal tissues from the patients. This was also confirmed by immunohistochemical staining (Figure 1B). Moreover, in order to further investigate the correlation between expression of CXCL12 and clinicopathological features, 52 samples were used for examination with immunohistochemical staining. Statistical analysis revealed negative CXCL12 expression was significantly associated with tumor size, lymph node metastasis, TNM stage and Her-2 expression compared with those patients with positive CXCL12 expression (Table 1). Calculation of the survival duration of the 52 involved patients by the Kaplan-Meier method revealed that the patients who featured CXCL12-negative tumors demonstrated a shorter survival when compared with those patients who suffered from CXCL12-positive tumors (Figure 1C, P < 0.01).
Figure 1.
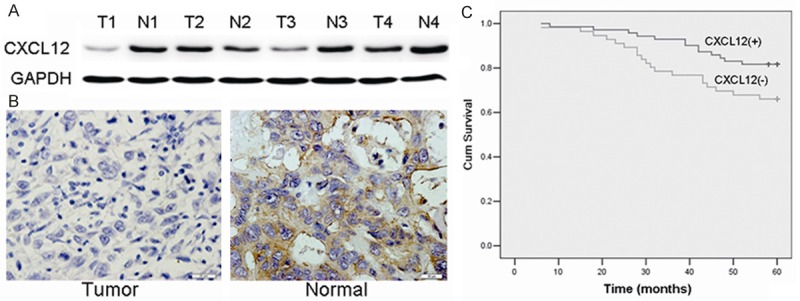
Low-expression of CXCL12 in breast cancer with worse prognosis. A: Western blot analysis demonstrated the CXCL12 expression in breast cancer tissues and matched distal normal tissues from four randomly selected breast cancer patients. B: Immunohistochemistry results of CXCL12 expression in paired breast cancer tissue samples. C: Kaplen-Meir survival curves for 52 patients with breast cancer, grouped according to CXCL12 expression.
Table 1.
Univariate surviva analysis of clinicopathological parameters and CXCL12 expression
| Number (52) | CXCL12 expression | ||||
|---|---|---|---|---|---|
|
|
|||||
| + | - | χ 2 | P | ||
| Age (years) | |||||
| < 50 | 12 | 2 | 10 | 1.31 | > 0.05 |
| ≥ 50 | 40 | 16 | 24 | ||
| Tumor size | |||||
| < 2 cm | 14 | 10 | 4 | 9.35 | < 0.01 |
| ≥ 2 cm | 38 | 8 | 38 | ||
| Lymph node metastasis | |||||
| Negative | 24 | 4 | 20 | 6.34 | < 0.05 |
| Positive | 28 | 14 | 14 | ||
| Tumor grade | |||||
| I-II | 26 | 8 | 18 | 0.34 | > 0.05 |
| III | 26 | 10 | 16 | ||
| TNM Stage | |||||
| I-II | 30 | 7 | 23 | 3.99 | > 0.05 |
| III-IV | 22 | 11 | 11 | ||
| ER | |||||
| Negative | 20 | 6 | 14 | 0.31 | |
| Positive | 32 | 12 | 20 | ||
| PR | |||||
| Negative | 20 | 8 | 12 | 0.42 | > 0.05 |
| Positive | 32 | 10 | 22 | ||
| Her-2 | |||||
| Negative | 37 | 37 | 20 | 7.28 | < 0.01 |
| Positive | 15 | 1 | 14 | ||
Expression of CXCL12 in human breast cancer cell lines and upregulation of CXCL12 by adenovirus vector
First, we examined CXCL12 expression in six breast cancer cell lines (MDA-MB-468, HCC-1937, MCF-7, Bcap-37, MDA-MB-231, MX-1) by western blot. CXCL12 was detected in all cell lines evaluated, and with MDA-MB-231 cells expressing the lowest level (Figure 2A). Therefore, MDA-MB-231 cells were selected as the model for the subsequent function studies. After infected with an adenovirus containing a CXCL12 vector, CXCL12 expression was higher in MDA-MB-231 breast cancer cells (Ad-CXCL12) than in cells infected with a control adenovirus (Ad-cont) (Figure 2B).
Figure 2.

Expression of CXCL12 in breast cancer cell lines and upregulation of CXCL12 in MDA-MB-231. A: Western blot showing the expression of CXCL12 in 6 breast cancer cell lines. GAPDH served as protein loading control. B: Western blot of CXCL12 expression in Ad-cont and Ad-CXCL12 cells after normalization to GAPDH.
Upregulation of CXCL12 expression inhibits the growth of breast cancer cells
The colony formation assay was used to evaluate the growth of the cells in which infected with an adenovirus containing a CXCL12 vector. As shown in Figure 3A, Ad-CXCL12 cells formed significantly fewer colonies on soft agar compared to MDA-MB-231 and Ad-cont cells. To further test the effect of CXCL12 on breast cancer cell growth, MTT assay was performed and growth curves were generated (Figure 3B). As shown by the curves, Ad-CXCL12 cells proliferated lower than MDA-MB-231 and Ad-cont cells during the first 96 h after the cells were plated. The dramatic reduction of colony formation and growth of Ad-CXCL12 cells suggested CXCL12 expression might negatively regulate breast cancer cell growth. Since CXCL12 confers an inhibitory effect on growth of MDA-MB-231 cells in vitro, its effect in vivo was also investigated. Ad-CXCL12 cells, control cell lines, and MDA-MB-231 cells were injected into 3 separate groups of nude mice. Tumor volume was measured every 2 days until the mice were sacrificed on day 21. Figure 3C showed that Ad-CXCL12 cells formed substantially smaller tumors in nude mice than the control cell groups. At the time of death, the mean tumor weight at the end of the experiment was remarkably lower in the Ad-CXCL12 cells group (0.31 ± 0.06 g) than in the MDA-MB-231 group (0.65 ± 0.07 g, P < 0.05) or control group (0.59 ± 0.08 g, P < 0.05) (Figure 3D).
Figure 3.
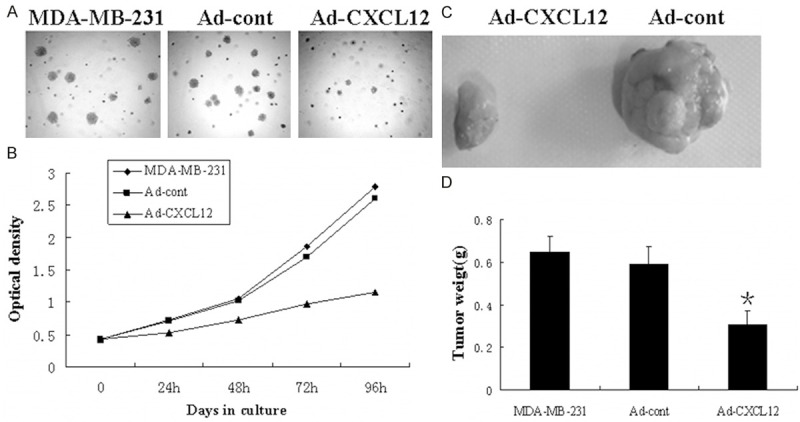
Upregulation of CXCL12 inhibits the growth of breast cancer cells in vivo and in vitro. A: Colony formation assay. B: MTT proliferation assay. MDA-MB-231 and Ad-CXCL12 cell lines were injected into right scapular region of nude mice (each group = 7). 3 weeks later, the mice were sacrificed, photographed, dissected and the weight of tumor were counted. C: The photograph of excised tumors from each group terminated in 3 weeks. D: The mean weight of each group. All results were reproducible in three independent experiments.
Upregulation of CXCL12 suppresses the invasive ability of breast cancer cells
To further examine whether the reactivation of CXCL12 expression can regulate breast cancer invasion, we analyzed the invasion capability of the highly metastatic MDA-MB-231 cells using the methods described above. The number of MDA- MB-231 cells in the untreated group that migrated through the membrane was 125.06 ± 15.42/HP. The number of invading cells was significantly increased when MDA-MB-231 cells infected with an adenovirus containing a CXCL12 vector (57.04 ± 8.83/HP). A significant reduction in the number of invasive cells was observed for 24 h when the cells infected with an adenovirus containing a CXCL12 vector compared to the control (Figure 4).
Figure 4.

Upregulation of CXCL12 decreases the invasive potential of breast cancer cells. A: Ad-cont and Ad-CXCL12 cells were plated (1×104 cells per well) in Matrigel-coated transwell chambers. After 24 h, the invaded cells on the lower side of the chamber were fixed and stained with hematoxylin. B: The number of invaded cells was counted using an inverted light microscope. The data are the results of 3 independent experiments performed in triplicate. An average of 6 fields of cells was counted at 100×magnification. Representative images of the invasion assay are shown in the right panel. Data were analyzed using the Student’s t test. P < 0.01 was considered statistically significant.
Discussion
Various types of cancer cells express chemokine receptors and the chemokines may play a role in cancer progression and/or organ-selective metastasis. It is assumed that disseminated tumor cells expressing chemokine receptors invade the circulation and are then attracted and arrested by the corresponding ligand. The specific metastatic sites to which tumor cells preferred to metastasize expressed more chemokines, and these chemokines are then able to induce the migration of tumor cells [17-19]. The ability of a specific chemokine to act on chemokine receptor-expressing tumor cells and to support their directionality requires that chemokine-induced cellular changes occurring in the tumor cells would culminate into motility in response to chemokine gradients. Solid evidence to such a mechanism was provided by the study of Li et al demonstrating that a highly potent functional axis exists between CXCL12 and its CXCR4 receptor in breast cancer metastasis [20]. In addition to this role, CXCR4 signaling is also a key regulator of organogenesis as well as lymphopoiesis and myelopoiesis [21,22]. Previous studies have defined the co-expression of both CXCR4 and CXCL12 by the cells of the human intestinal epithelium [23], and, also, many studies reported the different expression levels of CXCL12 in the cancer cells and tissues [24,25].
In this study, we compared CXCL12 protein expression with clinicopathological values in 52 patients with IDC breast cancer. In the present study, positive cytosol staining of CXCL12 was found in normal ductal and glandular epithelium of the breast and in 34.6% of IDC cases, suggesting that CXCL12 is required for a normal physiological function. However, 34 cases (65.4%) of IDC exhibited reduced expression of CXCL12, which was significantly correlated with tumor size, lymph node metastasis, TNM stage and Her-2 expression in breast cancer. Furthermore, patients with negative CXCL12 expression had significantly lower cumulative postoperative 5 year survival rate than those with positive CXCL12 expression. In agreement with our findings, other researchers found the same phenomenon in colon cancer, gastric carcinoma, and non-small lung cancer [23,25]. Collectively, these data suggest that reduced CXCL12 expression is positively correlated with breast cancer development and progression.
When we detected the expression of CXCL12 in breast cancer cells, there was a difference in MCF-7, Bcap-37 and MX-1 vs MDA-MB-468, HCC-1937 and MDA-MB-231 cell lines. The difference may be caused by the different histological type of the six breast cancer cells. MDA-MB-468, HCC-1937 and MDA-MB-231 cell lines are poorly differentiated adenocarcinoma, MDA-MB-468 cell line is inflammatory breast carcinoma, HCC-1937 cell line is triple negative breast adenocarcinoma, and MDA-MB-231 is a highly invasive human breast cancer cell line. In this study we explored that the expression of CXCL12 in MDA-MB-231 cells was the lowest level. Therefore, MDA-MB-231 cells were selected as the model for the subsequent function studies. Our subsequent studies verified that upregulation of CXCL12 expression could inhibit the proliferation and growth of MDA-MB-231 cells in vitro and in vivo.
To explore the relationship between CXCL12 and the biological behavior of breast cancer cells, we upregulation CXCL12 function by using gene transfection technology in the MDA-MB-231 cell line which has low endogenous CXCL12 expression. Cancer metastasis is a major cause of morbidity in cancer patients. Cancer metastasis consists of multiple sequential steps and invasion is one of the most characteristic steps during the cascade of metastasis [26]. Many studies have demonstrated the importance of invasion in the early stages of metastasis [27]. To investigate the pathological role of CXCL12 in breast cancer, we carried out invasion assays using CXCL12 overexpressing cells. Upregulation of CXCL12 decreased the invasiveness of breast cancer cells. Since invasive growth of tumors is one of the important hallmarks of malignancy, these results suggest that CXCL12 may be a key protein responsible for the breast cancer development.
In conclusion, CXCL12 expression plays an important role in progression, metastasis and prognosis of breast cancer. With gene transfection technology, we showed that upregulation of CXCL12 expression could suppress breast cancer cell growth and invasion in vitro and in vivo. These data provide a sound scientific rationale for further investigation into targeting CXCL12 in breast cancer.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81302290 and 81101932) and Youth Science Fund project of the Affiliated Hospital of Qingdao University.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kabbage M, Trimeche M, Ben Nasr H, Hammann P, Kuhn L, Hamrita B. Expression of the molecular chaperone alphaBcrystallin in infiltrating ductal breast carcinomas and the significance there of: an immunohistochemical and proteomics-based strategy. Tumour Biol. 2012;33:2279–2288. doi: 10.1007/s13277-012-0490-4. [DOI] [PubMed] [Google Scholar]
- 3.Elfagieh M, Abdalla F, Gliwan A, Boder J, Nichols W, Buhmeida A. Serum tumour markers as a diagnostic and prognostic tool in Libyan breast cancer. Tumour Biol. 2012;33:2371–2377. doi: 10.1007/s13277-012-0500-6. [DOI] [PubMed] [Google Scholar]
- 4.Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M, Ishii S, Sato S, Shimizu T. Interplay between CXCR2 and BLT1 Facilitates Neutrophil Infiltration and Resultant Keratinocyte Activation in a Murine Model of Imiquimod-Induced Psoriasis. J Immunol. 2014;192:4361–4369. doi: 10.4049/jimmunol.1302959. [DOI] [PubMed] [Google Scholar]
- 5.Chiba F, Soda K, Yamada S, Tokutake Y, Chohnan S, Konishi F, Rikiyama T. The importance of tissue environment surrounding the tumor on the development of cancer cachexia. Int J Oncol. 2014;44:177–186. doi: 10.3892/ijo.2013.2180. [DOI] [PubMed] [Google Scholar]
- 6.Zhao DX, Li ZJ, Zhang Y, Zhang XN, Zhao KC, Li YG, Zhang MM, Yu XW, Liu MY, Li Y. Enhanced antitumor immunity is elicited by adenovirus-mediated gene transfer of CCL21 and IL-15 in murine colon carcinomas. Cell Immunol. 2014;289:155–161. doi: 10.1016/j.cellimm.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014;20:154–165. doi: 10.1016/j.molmed.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Sui P, Hu P, Zhang T, Zhang X, Liu Q, Du J. High expression of CXCR-2 correlates with lymph node metastasis and predicts unfavorable prognosis in resected esophageal carcinoma. Med Oncol. 2014;31:809. doi: 10.1007/s12032-013-0809-z. [DOI] [PubMed] [Google Scholar]
- 9.Gunzer M. Migration, cell-cell interaction and adhesion in the immune system. Ernst Schering Found Symp Proc. 2007;3:97–137. doi: 10.1007/2789_2007_062. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhao T, Yang Z, Li Q. CX3CR1 RNAi inhibits hypoxia-induced microglia activation via p38MAPK/PKC pathway. Int J Exp Pathol. 2014;95:153–157. doi: 10.1111/iep.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itakura M, Terashima Y, Shingyoji M, Yokoi S, Ohira M, Kageyama H, Matui Y, Yoshida Y, Ashinuma H, Moriya Y, Tamura H, Harigaya K, Matushima K, Iizasa T, Nakagawara A, Kimura H. High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br J Cancer. 2013;109:1100–1108. doi: 10.1038/bjc.2013.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Liu W, Wei D, Hu K, Wu X, Yao Y. Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor proliferation, migration and invasion of ovarian cancer cell lines. Oncol Lett. 2014;7:1581–1585. doi: 10.3892/ol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamamis P, Floudas CA. Elucidating a Key Component of Cancer Metastasis: CXCL12 (SDF-1α) Binding to CXCR4. J Chem Inf Model. 2014;54:1174–1188. doi: 10.1021/ci500069y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uto-Konomi A, McKibben B, Wirtz J, Sato Y, Takano A, Nanki T, Suzuki S. CXCR7 agonists inhibit the function of CXCL12 by down-regulation of CXCR4. Biochem Biophys Res Commun. 2013;431:772–776. doi: 10.1016/j.bbrc.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsumoto K, Kume S. The role of CXCL12-CXCR4 signaling pathway in pancreatic development. Theranostics. 2013;3:7–11. doi: 10.7150/thno.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238:30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25:573–587. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 19.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 21.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 23.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 24.Yoshitake N, Fukui H, Yamagishi H, Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H, Fujimori T. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–1689. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Göke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29:219–225. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 27.Lv ZD, Na D, Liu FN, Du ZM, Sun Z, Li Z, Ma XY, Wang ZN, Xu HM. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res. 2010;29:139. doi: 10.1186/1756-9966-29-139. [DOI] [PMC free article] [PubMed] [Google Scholar]


