Abstract
Objective: To investigate the mRNA and protein levels of B7-H4, a B7 family molecule, in human urothelial cell carcinoma (UCC), to analyze the relationship between B7-H4 protein expression level and pathological stage of UCC, and to examine the potential of B7-H4 as a prognostic factor in UCC. Methods: mRNA and protein levels of B7-H4 were measured in pairs of tumor tissues and matched adjacent nontumor tissue obtained from patients with UCC by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and immunohistochemical staining, respectively. Association of the protein level of B7-H4 with pathological tumor stage and the overall survival of UCC patients were also analyzed. Results: B7-H4 mRNA and protein level were significantly higher in UCC tumor tissues compared with adjacent nontumor tissues as assessed by qRT-PCR and immunohistochemical staining, respectively. Higher B7-H4 protein levels were observed in patients with more advanced pathological stage of UCC and were also associated with decreased overall survival of patients with UCC. Conclusions: The findings from this study indicate that B7-H4 has the potential to be an independent prognostic indicator for UCC.
Keywords: Bladder cancer, B7 family, B7-H4, prognosis, gene expression
Introduction
Bladder cancer, with 383,000 new cases diagnosed worldwide in 2008 [World Cancer Research Fund International (WCRF Inter national)], is the ninth most common cancer in the world and the second most common genitourinary malignancy [1,2]. About 95% of bladder cancers are urothelial cell carcinoma [UCC, also known as transitional cell carcinoma (TCC)] [3]. UCC is a type of malignant tumour originating from the urothelium lining the urinary tract from the renal calyces to the ureteral orifice. UCC is a clinically heterogeneous disease, with 70% of total cases presenting non-muscle-invasive tumors and 30% presenting muscle-invasive tumors [4-6]. Muscle invasive tumors usually implies metastases and poor prognosis [4-6]. Surgery is the option for most people with UCC. However, a significant number of patients suffer from disease recurrence and progression after radical cystectomy [6]. For example, a group from the University of Texas MD Anderson Cancer Center found that metastases developed in 97 of the 382 patients (25%) with transitional cell carcinoma of the bladder a median of 12 months after cystectomy [7]. Therefore, there is an urgent need to identify prognositc biomarkers with high specificity and sensitivity for UCC in order to distinguish tumors with the potential to progress and metastasize.
T cell-mediated immunity depends on specific recognition of antigen-major histocompatibility complex (MHC) by T cell receptor (TCR) and co-regulatory signals [8]. The co-regulatory signals come from the B7 family molecules, which are a group of structurally related, peripheral membrane proteins mainly located on activated antigen presenting cells (APCs) [8]. B7 molecules function as co-regulatory ligands by binding to corresponding receptors on T cell surfaces, producing co-regulatory signals to either enhance or decrease T cell-mediated, antigen-specific immune responses [9]. The newly identified B7 family member, B7-H4 (also known as B7x or B7S1), negatively regulate in T cell-mediated immunity by arresting T cell cell-cycle progression and thus inhibiting T cell proliferation, cytokine secretion, and cytotoxic activity [10,11]. B7-H4 has been reported to be highly expressed in different tumors, including ovarian, breast, non-small-cell lung cancers, etc, however, there is a little or no B7-H4 expression in normal tissues [12,13]. It has also been reported that B7-H4 promotes malignant transformation and lymph node metastasis [12,14,15].
Up to date, however, no reports have investigated the clinical significance of B7-H4 expression in patients with bladder cancer. In this study, we analyzed B7-H4 expression in UCC tissues and normal urothelium tissues using both immunohistochemical method and real time RT-PCR. Additionally, we investigated the relationship between B7-H4 expression level and clinicopathological variables and evaluated the prognostic values of B7-H4 using log-rank survival analysis.
Materials and methods
Patient identification
The study protocol was approved by the ethics committee of the Third Affiliated Hospital of Soochow University, and all tissue samples were collected from patients and donors with appropriate informed consent. The criteria for study enrollment were histopathological diagnosis of UCC of the bladder, no history of other tumour, no chemotherapy before surgery, availability of sufficient tumour sample, and the potential to follow-up. By applying these criteria, sixty two patients who underwent surgeries for bladder cancer (between July 2006 and July 2012) at the Department of Urology, the Third Affiliated Hospital of Soochow, Changzhou, Jiangsu, China, were included in this study. The patients were followed for 3-6 months after surgery with cystoscopic examination at the outpatient clinic. The tumors were classified according to the 2010 Union for International Cancer Control (UICC) TNM classification for pathologic staging and the 2004 World Health Organization classification for the pathological grading based on the findings of clinical, radiological, or histological examinations [16,17]. The hematoxylin and eosin staining was evaluated by two independent pathologists or urologists without knowledge of patient outcome.
Immunohistochemical staining
The bladder cancer specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, cut into 5 μm serial sections, and then mounted on glass slides. The slides were deparaffinized with xylene and rehydrated in graded alcohol. Antigen retrieval was performed tissue sections in a citrate buffer (10 mmol/l, pH 6.0) at 100°C for 30 min. After cooling, slides were incubated in 0.3% H2O2 solution for 30 min to block endogenous peroxidase. After washing three times with PBS (pH 7.4) for 5 min each, slides were incubated with the primary antibodies (mouse anti-B7-H4 polyclonal antibody, USCNLIFE, USA) diluted 1:400 in PBS with 1.0% bovine serum albumin (BSA, Sigma) in a humid chamber at 4°C overnight followed by three times 5 min washes in PBS. After washing three times with PBS (pH 7.4) for 5 min each, sections were incubated with the secondary antibody (mouse/rabbit general second antibody, Maixin Biotechnology Co. Ltd, Fuzhou) at room temperature for 30 min. After washing again with PBS, the sections were visualized by incubation with diaminobenzidine (Dako Cytomation) substrate for 8 min. Finally, slides were counterstained with for 1 min with hematocylin, and coverslips were applied. Stained sections were photographed using the BX50 microscope (Olympus America, Center Valley, PA) with an attached QImaging Retiga 2000R Digital Camera (Quantitative Imaging, Surrey, BC, Canada).
Evaluation of B7-H4 staining
The slides were examined by two pathologists, and the sections were evaluated according to the immunohistochemical scores (IHS) [18,19]. The staining intensity the proportion of positive cells was semiquantitatively evaluated. The staining intensity was scored as 0, no staining; 1, weak staining; 2, moderate staining; and 3, intense staining. The proportion of positive cells was scored as 0 (< 5% positive cells), 1 (6-25% positive cells), 2 (26-50% positive cells), 3 (51-75% positive cells), and 4 (> 75% positive cells). The final B7-H4 staining score was calculated using the percent of positive cell score × staining intensity score ranging 0-12. In this study, the B7-H4 expression is defined as weak positive (lower expression) when score is less than 4, and positive (higher expression) when score is equal to or more than 4.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Tumor tissues were frozen in liquid nitrogen immediately until RNA extraction. Total RNA was extracted from tissues using a total RNA purification kit (Shenergy Biocolor BioScience and Technology Co., Shanghai, China) according to the manufacturer’s instructions. One microgram of total RNA was reversely transcribed to cDNA with 100 units of Moloney murine leukemia virus (M-MLV) reverse transcriptase (USB, Cleveland, OH, USA) according to the manufacturer’s protocol. TaqMan® gene expression assays (Applied Biosystems, Foster City, CA, USA) were used to quantify mRNA expression of human B7-H4 and β-actin (internal control) genes. Primers and probes in the TaqMan assay are presented in Table 1. PCR reactions were performed on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) in duplicate in a 10 μl volume containing 5 μl Universal PCR Master Mix (Applied Biosystems), 0.5 μl TaqMan® assay and 4.5 μl diluted cDNA (50 ng reverse-transcribed RNA). PCR cycling conditions were 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. PCR products were visualized on 1.2% agarose, purified and then verified by sequencing. The relative expression level of B7-H4 mRNA was normalized with β-actin expression level and calculated using the 2-ΔΔCt method [20].
Table 1.
Primers and probes for human B7-H4 and β-actin
| Gene | Primers and probes | Sequence (5’ to 3’) |
|---|---|---|
| B7-H4 | Forward primer | CACCAGGATAACATCTCTCAGTGAA |
| Reverse primer | TGGCTTGCAGGGTAGAATGA | |
| Probe | FAM-AAGCTGAAGATAATCCCATCAGGCAT-TAMRA | |
| β-Actin | Forward primer | GGAAGGTGAAGGTCGGAGTC |
| Reverse primer | CGTTCTCAGCCTTGACGGT | |
| Probe | FAM-TTTGGTCGTATTGGGCGCCTG-TAMRA |
Statistical analysis
Statistical analyses were performed using the GraphPad Prism version 5.0 software package (GraphPad Software, San Diego, CA, USA). Data are presented as the mean standard error (SE) from at least three independent experiments. Paired samples t-test was performed to analyze significant differences between the UCC and adjacent nontumor tissue. The relationship between B7-H4 expression and clinical parameters was evaluated using Pearson χ2 test. The overall survival rates were calculated by the Kaplan-Meier method, and the difference in survival was compared with the log-rank test. The Cox proportional hazards regression model was used for univariate and multivariate analyses to assess the effects of the clinicopathological variables and B7-H4 expression on overall survival. Two-tailed P values < 0.05 were considered to be statistically significant.
Results
B7-H4 expression in human UCC tissues
B7-H4 expression in 62 tissue specimens obtained from patients with bladder cancer was assessed by immunohistochemical staining. Interobserver agreement in the assessment of immunohistochemical findings was excellent. Positive B7-H4 immunohistochemical staining was predominantly observed on the membrane and in cytoplasm of the urothelial cancer cells (Figure 1), while weak staining was found in normal bladder tissues. Forty seven out of 62 specimens of bladder cancer tissues showed positive B7-H4 staining. Therefore, our result indicated that higher B7- H4 expression was identified in 75.8% bladder cancer specimens. Immunohistochemical analysis demonstrated that B7-H4 was highly expressed in bladder cancer tissues (Figure 1B and 1C), whereas there was no or very weak B7-H4 staining in the or adjacent normal tissues (Figure 1A).
Figure 1.
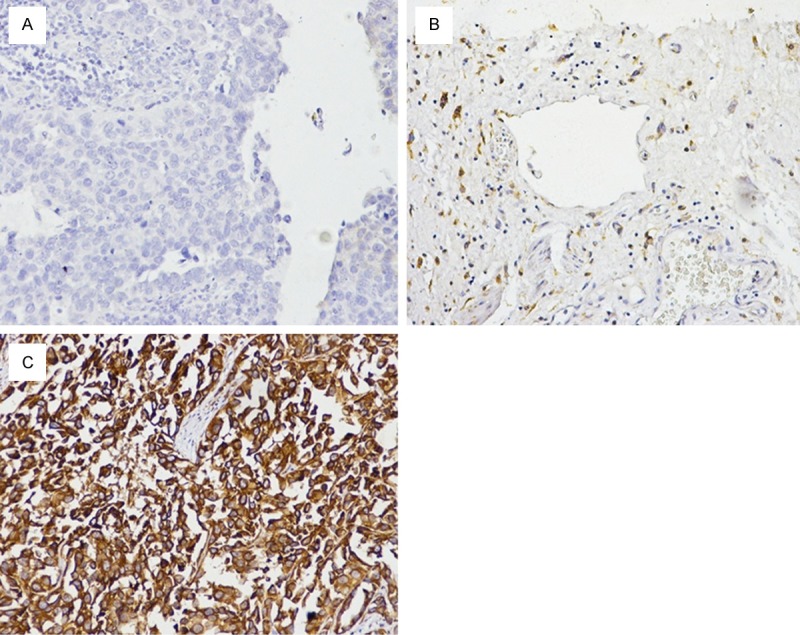
Immunohistochemical staining of B7-H4. The B7-H4 immunohistochemical staining in bladder cancer. A: Adjacent normal tissues with negative Expression. B: Noninvasive bladder cancer with lower expression. C: High-grade invasive bladder cancer with higher expression.
B7-H4 mRNA expression in tumour tissues and adjacent non-tumour tissues from 20 UCC patients was assessed by real-time RT-PCR. As shown in Figure 2, the mRNA levels of B7-H4 in UCC tumour samples was significantly higher than those in non-tumour tissue samples (P = 0.012).
Figure 2.
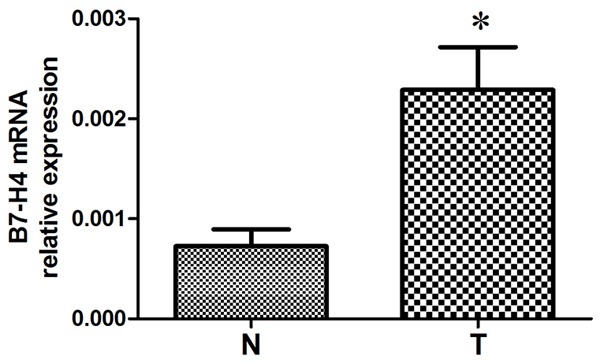
B7-H4 mRNA levels in tumour tissues (T) and adjacent nontumour tissues (N) from 62 patients with urothelial cell carcinoma as assessed by real-time RT-PCR reaction. P = 0.012 as determined by paired sample t-test.
Correlations of B7-H4 expression with clinicopathological parameters for UCC patients
The relationship between B7-H4 protein expression (immunohistochemical staining) and clinicopathologic features is summarized in Table 2. Gender was not found to be significantly associated with B7-H4 expression (P = 0.4299). There was a significant association between B7-H4 expression and the cancer grade: [low grade (G1/2: 8 out of 19: 42.1%) and high grade (G3/4: 39 out of 43: 90.7%)]. In addition, B7-H4 expression was significantly higher in patients with muscle invasive tumors (43 out of 51: 84.3%) than in those with superficial tumors (4 out of 11: 36.4%). The rate of B7-H4 higher expressing specimens in patients with recurrence (41 out of 43: 95.4%) was also significantly higher than that for B7-H4 lower expressing patients without recurrence (6 out of 19: 31.6%).
Table 2.
Correlation between B7-H4 protein expression and clinicopathologic parameters of the patients with urothelial cell carcinoma (UCC) (n = 62)
| Parameter | Number of patients | B7-H4 expression Negative (%) | B7-H4 expression Positive (%) | P value (χ2 test) |
|---|---|---|---|---|
| Sex | 0.4299 | |||
| Male | 48 | 10 (20.8) | 38 (79.2) | |
| Female | 14 | 5 (35.7) | 9 (64.3) | |
| TNM stage | 0.0029 | |||
| Superficial | 11 | 7 (63.6) | 4 (36.4) | |
| Invasive | 51 | 8 (15.7) | 43 (84.3) | |
| Grade | 0.0001 | |||
| Low | 19 | 11 (57.9) | 8 (42.1) | |
| High | 43 | 4 (9.3) | 39 (90.7) | |
| Recurrence | < 0.0001 | |||
| Negative | 19 | 13 (68.4) | 6 (31.6) | |
| Positive | 43 | 2 (4.6) | 41 (95.4) |
Correlation between B7-H4 expression and bladder cancer recurrence-free rate
The recurrence-free rate of UCC was analyzed by the Kaplan-Meier method. A time period of 60 months was defined to investigate the recurrence-free rate. The recurrence-free rate was determined from the date of the operation to the time of the detection of bladder cancer recurrence or the last follow-up. The impacts of B7-H4 staining, tumour stage, and cancer grade on the recurrence free rate were investigated. A log-rank test revealed that positive B7-H4 expression was significantly associated with an increased incidence of the cancer recurrence (Figure 3A). Additionally, the higher tumour stage (Figure 3B) and the higher cancer grade (Figure 3C) showed significant association the poor recurrence free rate.
Figure 3.
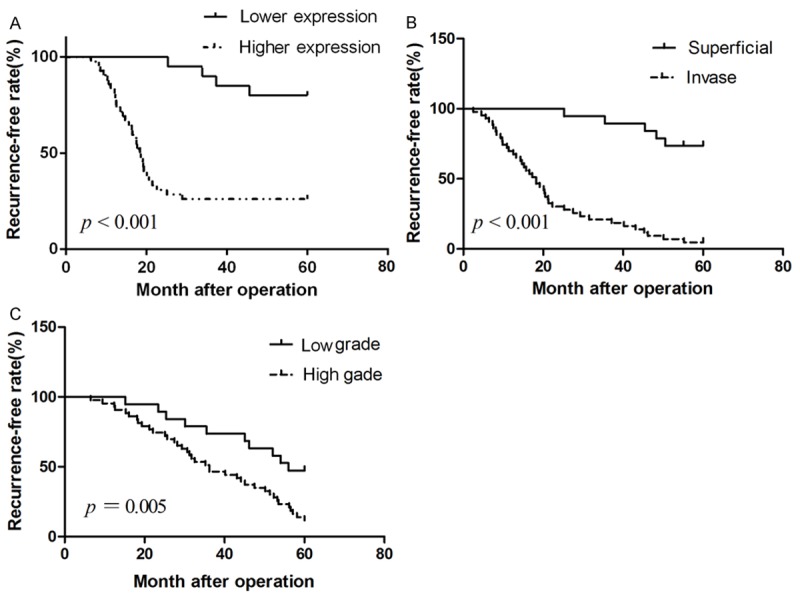
Kaplan-Meir curves demonstrating overall survival of 62 patients with urothelial cell carcinoma following surgery according to mRNA levels of B7-H4 (A), tumour stage (B), and tumour grade (C). P-value was determined by paired samples t-test.
Cox proportional hazards model univariate and multivariate analyses of individual parameters for correlations with overall survival
Univariate analysis using a Cox proportional hazards model to evaluate the potential of using B7-H4 mRNA expression level as a prognostic marker for UCC patients after surgery showed that B7-H4 overexpression (P = 0.006), tumour grade (P = 0.013), and primary tumour stage (P < 0.001) were the prime variables for UCC prognosis (Table 3). After adjusting for clinicopathologic variables, B7-H4 overexpression (P = 0.011), tumour state (P = 0.013), and primary tumour stage (P = 0.023) remained significantly correlated with the prognosis of UCC patients (Table 3).
Table 3.
Univariate and multivariate analyses of different clinicopathological variables and B7-H4 expression status as predictors for overall survival of urothelial cell carcinoma (UCC)
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex (male vs. female) | 1.341 (0.689-2.678) | 0.354 | 1.571 (0.759-3.210) | 0.215 |
| TNM stage (high vs. low) | 2.818 (1.545-5.032) | < 0.001 | 2.033 (0.122-3.688) | 0.013 |
| Grade (G3/4 vs. G1/2) | 2.215 (1.310-4.925) | 0.013 | 1.523 (1.183-4.125) | 0.023 |
| B7-H4 expression (high vs. low) | 1.561 (1.125-2.358) | 0.006 | 1.365 (1.115-2.521) | 0.011 |
CI, confidence interval; HR, hazard ratio.
Discussion
The co-regulatory B7 family members are cell-surface protein ligands, binding to receptors on lymphocytes to regulate immune responses [9]. They can provide either positive or negative signal to stimulate or inhibit T-cell activation [9]. B7-H4 is a recently identified member of the B7 family [10]. In this study, we investigated the mRNA and protein levels of B7-H4 in human UCC and analyzed the relationship between B7-H4 protein expression level and clinicopathological parameters of UCC.
An association between tumour-associated B7-H4 expression and clinicopathological features has been recently found in prostate, renal cell, and esophageal cancers. In prostate cancer, strong expression of B7-H4 is positively correlated with extra capsular extension, seminal vesicle invasion, and distant metastasis [21]. In clear-cell renal cell cancer, patients with B7-H4-positive tumors showed a poorer survival rate than those with B7-H4-negative tumors [22]. Higher B7-H4 expression was found to be significantly associated with poor prognosis of the patients suffering from gastric cancer [23]. B7-H4 expression in human esophageal squamous cell cancer was shown to be associated with cancer progression, reduced tumour immune surveillance and worse patient outcomes [24]. In this study, B7-H4 mRNA and protein level were found to be significantly higher in UCC tumour tissues compared with adjacent nontumor tissues as assessed by qRT-PCR and immunohistochemical staining, respectively. Higher B7-H4 protein levels were observed in patients with more advanced pathological stage of UCC and associated with decreased overall survival of patients with UCC. Thus, these previous studies along with our findings suggest that B7-H4 expression may serve as a universal prognostic indicator for various cancers.
Although B7-H4 overexpression has been found various human tumors, the exact role of B7-H4 in tumourigenesis is still under active investigations. Currently, it is believed that B7-H4 may protect cancer cells by inhibiting tumour-targeted T cell-mediated immune surveillance. In vitro studies suggest that B7-H4 may deliver an inhibitory signal to T cells, thereby inhibiting CD4+ and CD8+ T cell proliferation, progression, and cytokine production [25-27]. Blockade of B7-H4 has also been shown to enhance the activity of cytotoxic T lymphocytes [28]. Thus, these results imply that B7-H4 may be involved in the evasion mechanism of cancer cells from tumour-targeted T cell-mediated immune surveillance. In addition, B7-H4 may promote tumourigenesis by rendering tumour cells refractory to apoptosis. For example, knockdown of B7-H4 mRNA and protein expression in the SKBR3 breast cancer cell line enhanced intracellular caspase activity, leading to acceleration of tumour cell apoptosis [29].
In conclusion, the present study has shown for the first time that B7-H4 mRNA and protein are increased in UCC tissues and that higher B7-H4 levels are associated with advanced clinical tumour stage and shorter overall survival. The precise role of B7-H4 in UCC development and progression, however, remains to be elucidated and further investigations in cell and animal models are in progress.
Acknowledgements
Preparation of this manuscript was supported by Changzhou Health Research Program, grant no. CE20125025.
Disclosure of conflict of interest
None.
References
- 1.Badar F, Sattar A, Meerza F, Irfan N, Siddiqui N. Carcinoma of the urinary bladder in a tertiary care setting in a developing country. Asian Pac J Cancer Prev. 2009;10:449–452. [PubMed] [Google Scholar]
- 2.Rogers CG, Palapattu GS, Shariat SF, Karakiewicz PI, Bastian PJ, Lotan Y, Gupta A, Vazina A, Gilad A, Sagalowsky AI, Lerner SP, Schoenberg MP. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol. 2006;175:2048–2053. doi: 10.1016/S0022-5347(06)00317-X. [DOI] [PubMed] [Google Scholar]
- 3.Abel PD. Prognostic indices in transitional cell carcinoma of the bladder. Brit J Urol. 1988;62:103–109. doi: 10.1111/j.1464-410x.1988.tb04286.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 5.Catto JW, Yates DR, Rehman I, Azzouzi AR, Patterson J, Sibony M, Cussenot O, Hamdy FC. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177:1715–1720. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 7.Slaton JW, Swanson DA, Grossman HB, Dinney CP. A stage specific approach to tumor surveillance after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 1999;162:710–714. doi: 10.1097/00005392-199909010-00021. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 11.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 12.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7:925–934. doi: 10.1016/S1470-2045(06)70939-1. [DOI] [PubMed] [Google Scholar]
- 15.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Gospodarowicz MK, Wittekind CH, editors. TNM classification of malignant tumors. 7th edition. Oxford: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 17.Seitz M, Zaak D, Knüchel-Clarke R, Stief C. [Urinary bladder tumors. The new 2004 WHO classification] . Urologe A. 2005;44:1073–1086. doi: 10.1007/s00120-005-0901-x. [DOI] [PubMed] [Google Scholar]
- 18.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin M, Zhang Y, Guo TB, Matsushima K, Zhang Y. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett. 2007;253:34–42. doi: 10.1016/j.canlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaspy JA. Therapeutic options in the management of renal cell carcinoma. Semin Oncol. 2002;29:41–46. doi: 10.1053/sonc.2002.33083. [DOI] [PubMed] [Google Scholar]
- 26.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 28.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 29.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]


