Abstract
Objective: To investigate the association of Kruppel-like factor 4 (KLF4) expressions with the prognosis of esophageal squamous cell carcinoma (SCC) patients. Methods: Ninety-eight cases of esophageal carcinoma patients were enrolled. The expression of KLF4 in the esophageal SCC and normal esophageal mucosa tissues were examined by immunohistochemistry. The correlations between the expression of KLF4 protein and patients’ clinical characteristics and prognosis were analyzed. Results: We observed higher expressed KLF4 in normal esophageal mucosa tissues than esophageal SCC tissues, with positive rate of 82.7% (81/98) and 43.8% (43/98) respectively. In patients with lymphatic metastasis, the positive rate of KLF4 was 24.4% (10/41), whereas it was 57.9% (33/57) in patients without lymphatic metastasis, and the difference was significant (x2 = 10.871, P = 0.001). The positive rates of KLF4 were 62.5% (5/8), 53.1% (26/49) and 29.3% (12/41) in stage I, II and III patients, respectively. There were no correlations between the expression of KLF4 and gender, age, tumor size, location, differentiation grade and infiltration depth. The 5-year survival rates and median survival times were 48.8% and 25.5%, and 55 and 26 months for the patients with KLF4 positive and negative expression, respectively. There were significant differences between the patients with KLF4 positive expression and negative expression in the 5-year survival rates and median survival times (x2 = 5.747 and 4.493, P = 0.017 and 0.034). Conclusion: KLF4 might act as a tumor suppressor in esophageal SCC and the expression status of KLF4 could be considered as a prognosis predictor for esophageal SCC patients.
Keywords: Esophageal squamous cell carcinoma, Kruppel-like factor 4, prognosis
Introduction
Kruppel-like factor 4 (KLF4), a zinc-finger-type transcriptional factor that is widely expressed in many human tissues, plays an important role in regulating cell proliferation, differentiation, apoptosis and maintaining the activity of telomerase [1,2]. Studies over the past several decades have also identified key physiologic and pathologic phenotypic modulators of KLF4, it could act as either a cell cycle suppressor or an oncogene, depending on the tissue type [3,4]. KLF4 is able to arrest the G1/S cell cycle and thereby inhibits DNA synthesis, cell proliferation and differentiation. Knockout of KLF4 in gastrointestinal epithelial cells results in the abnormal proliferation, differentiation and cell infiltration [5].
It has been demonstrated that the down regulation of KLF4 in gastric cancer, colorectal cancer, bladder cancer and cervical cancer inhibits the occurrence and development of tumors, whereas KLF4 is up regulated in breast cancer, head and neck cancer, oral cancer and skin cancer, and subsequently promotes the survival and progression of tumors [6-8]. These results suggest that the expression of KLF4 plays a very important role in the occurrence, progression and metastasis of tumors. Research performed by Ghaleb et al indicated that under physiological conditions, KLF4 inhibits cell proliferation. Conversely, KLF4 mediates proinflammatory signaling in macrophages and its overexpression in the esophageal epithelium activates cytokines, leading to inflammation-mediated esophageal squamous cell cancer formation in mice [9]. However, the expression and the role of KLF4 in esophageal squamous cell carcinoma patients have not been clearly investigated.
In this study, we invested the expression of KLF4 in the esophageal squamous cell carcinoma tissues and the corresponding normal esophageal mucosa tissues, and analyzed the correlation with clinical characteristics and prognosis of these patients.
Patients and methods
Patients and ethnic consideration
This study was approved by an Institutional Review Board of Tianjin Medical University Cancer Institute and Hospital and was conducted in accordance with good clinical practice, all applicable regulatory requirements and the guiding principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to admission to the study.
A total of 98 cases of esophageal carcinoma patients (64 men and 34 women; median age: 60 years old) were enrolled successfully from June 2006 to June 2007 in Tianjin Medical University Cancer Institute and Hospital.
Recruited patients were confirmed having no history of chemotherapy, radiotherapy and immunotherapy before the surgery. All the samples used in the present study were confirmed by pathological examination. All the 98 cases were squamous cell carcinoma, including 41 cases with lymph node metastasis and 57 cases without lymph node metastasis. There were 8 cases in I stage, 43 cases in IIa stage, 6 cases in IIb stage and 41 cases in III stage according to the 6th edition of esophageal cancer TNM staging criteria drafted by American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) [10]. Their follow-up ended at August 2012 with a median follow-up period of 45 (3-72) months.
Sample preparation
The tumor tissue was taken from the tumor center with size about of 1 cm * 1 cm * 1 cm, normal esophageal mucosa was taken from operation resection specimens. The resected tissue pieces were put into the freezing tube and rapidly frozen in liquid nitrogen for analysis.
Immunohistochemistry
The expression of KLF4 was examined by immunohistochemical (IHC) staining methods. Esophageal carcinoma tissues were fixed with 4% paraformaldehyde and embedded with paraffin using standard methods. IHC was carried out using a specific mouse monoclonal anti-KLF4 antibody (1:50, Santa Cruz, CA, USA). Counterstaining was performed with hematoxylin. Control staining was conducted by omitting the primary antibody. The images were obtained with an Olympus DP70 optical microscope (Tokyo, JPN) and analyzed by the image analysis system (Beihang University, CM-2000B, Beijing, China).
The image results were scored by two independent pathologists. Briefly, the images were first scored according to the staining depth: negative staining, score 0; faint yellow, score 1; brown madder, score 2; dark brown, score 3. Then the images were scored according to the percentage of KLF4 positive cells in the total tumor cells: ≤ 10%, score 0; 11%-25%, score 1; 26%-50%, score 2; 51%-75%, score 3; > 75%, score 4. The scores of the same slide were summed to produce a final score (Final score = staining depth score × positive cell percentage score): 0-3 was considered as negative (-); 4-6, weakly positive (+); > 6, strong positive (++).Weakly positive and strong positive were considered as high expression.
Western blot
Western blot was used to determine the protein level in the cancer tissues. Briefly, frozen tissues were homogenated with lysis buffer, then centrifuged at 4°C for 30 min (12000 r/min). The supernatant was collected; BCA method was used to determine protein concentration. A 10% polyacrylamide gel was prepared to load protein samples, 5% nonfat dry milk was added to block the non-specific antigen. The primary antibodies (anti-KLF4 (catalog no.ab56542, Abcam, Cambridge, UK) and secondary antibodies were applied, β-actin served as control. Bio-Rad Gel Doc 2000 image processing system (Hercules, CA, USA) was used for image analysis.
Statistical analysis
SPSS 17.0 (SPSS Inc., Chicago, IL) was used to perform the statistical analysis. The Chi-square test was used to assess the association between patients’ clinical features and the expression of KLF4. OS and the 95% confidence intervals (CIs) were evaluated by the Kaplan-Meier method comparing the different groups by log-rank test. P-values were considered statistically significant if less than 0.05.
Results
Expression of KLF4 in the esophageal squamous cell carcinoma and normal esophageal mucosa tissues
Immunohistochemistry showed the KLF4 protein was mainly expressed in cytoplasm and nucleus as yellow or brown-color staining. As shown in Figure 1, we observed KLF4 was expressed in both the cytoplasm and the nucleus. KLF4 was overexpressed in the normal esophageal mucosa tissues (Figure 1A), with a positive expression rate of 82.7% (81/98). But in the esophageal squamous cell carcinoma tissues (Figure 1B), the expression of KLF4 was very low (43.8%, 43/98), at the same time, there was almost no positive brown staining of the negative expression of KLF4 protein in esophageal squamous cell carcinoma (Figure 1C). There was a significant difference between the esophageal squamous cell carcinoma and normal esophageal mucosa tissues (x2 = 31.701, P < 0.001). The immunohistochemical staining scores (Figure 2) were 8.27 ± 0.35 and 3.41 ± 0.33 in the normal esophageal mucosa and esophageal squamous cell carcinoma tissues, respectively. The difference between the two groups was also significant (P < 0.01).
Figure 1.
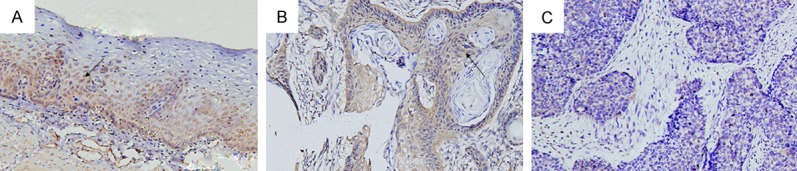
Immunohistochemistry of KLF4 protein expression; it was mainly expressed in cytoplasm and nucleus as yellow or brown-color staining. A. Normal esophageal mucosa tissues; B. Esophageal squamous cell carcinoma tissues; C. Negative expression of KLF4 protein in esophageal squamous cell carcinoma.
Figure 2.
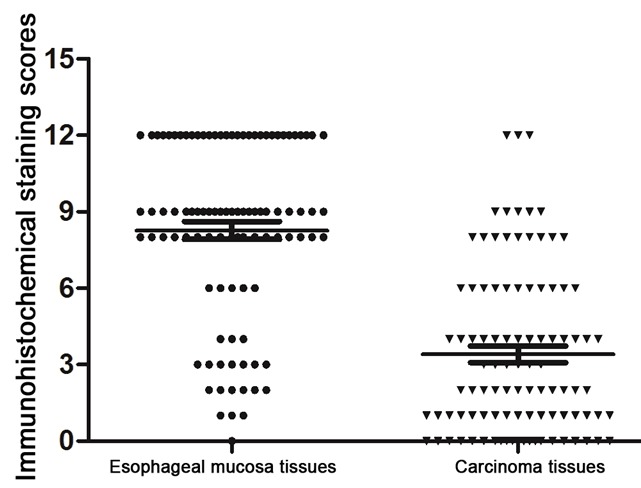
Immunohistochemical staining scores comparison in the normal esophageal mucosa and esophageal squamous cell carcinoma tissues.
Correlation between the expression of KLF4 protein and patients’ clinical characteristics
For esophageal cancer patients with lymphatic metastasis, the positive expression rate of KLF4 was 24.4% (10/41), whereas it was 57.9% (33/57) in patients without lymphatic metastasis. The difference between the two groups was significant (x2 = 10.871, P = 0.001). The positive expression rates of KLF4 were 62.5% (5/8), 53.1% (26/49) and 29.3% (12/41) in stage I, II and III patients, respectively. A significant difference was detected among the 3 groups (x2 = 6.482, P = 0.039). However, there were no correlations between the expression of KLF4 and gender, age, tumor size, location, differentiation grade and infiltration depth (Table 1).
Table 1.
Correlation between the expression of KLF4 protein and patients’ clinical characteristics
| Pathological factors | Case (N) | expression of KLF4 protein | x 2 value | P value | |
|---|---|---|---|---|---|
|
| |||||
| Negative (55) | Positive (43) | ||||
| Gender | |||||
| Male | 64 | 37 (57.8%) | 27 (42.2%) | 0.214 | 0.644 |
| Female | 34 | 18 (52.9%) | 16 (47.1%) | ||
| Age (yr) | |||||
| ≤60 | 50 | 32 (64.0%) | 18 (36.0%) | 3.442 | 0.064 |
| >60 | 48 | 23 (47.9%) | 25 (52.1%) | ||
| The location of the tumor | |||||
| The upper esophagus | 7 | 5 (71.4%) | 2 (28.6%) | 5.748 | 0.056 |
| Esophageal | 65 | 31 (47.7%) | 34 (52.3%) | ||
| The lower esophagus | 26 | 19 (73.1%) | 7 (26.9%) | ||
| Tumor diameter (cm) | |||||
| <5 | 54 | 28 (51.9%) | 26 (48.1%) | 0.891 | 0.345 |
| ≥5 | 44 | 27 (61.4%) | 17 (38.6%) | ||
| Differentiation degree | |||||
| I, I-II, II | 87 | 49 (56.3%) | 38 (43.7%) | 0.013 | 0.911 |
| II-III, III | 11 | 6 (54.5%) | 5 (45.5%) | ||
| T Staging | |||||
| T1 | 8 | 3 (37.5%) | 5 (62.5%) | 1.479 | 0.477 |
| T2 | 19 | 10 (52.6%) | 9 (47.4%) | ||
| T3 | 71 | 42 (59.2%) | 29 (40.8%) | ||
| Lymph node metastsis | |||||
| No | 57 | 24 (42.1%) | 33 (57.9%) | 10.871 | 0.001 |
| Yes | 41 | 31 (75.6%) | 10 (24.4%) | ||
| TNM Staging | |||||
| Stage I | 8 | 3 (37.5%) | 5 (62.5%) | 6.482 | 0.039 |
| Stage IIA-IIB | 49 | 23 (46.9%) | 26 (53.1%) | ||
| Stage III | 41 | 29 (70.7%) | 12 (29.3%) | ||
Western blot
We compared 20 cases of normal esophageal mucosa and esophageal squamous cell carcinomas. Result showed that there were 16 cases of KLF4 protein expression level were lower in esophageal squamous cell carcinomas than that of normal esophageal mucosa (Figure 3A). After image scanning and analysis by using Bio-Rad Gel Doc 2000 imaging system, we measured gray values of KLF4 and β-actin products, the expression level of KLF4 protein was calculated as the ratio of KLF4 protein and β-actin. Results showed the average level of KLF4 expression in esophageal squamous cell carcinoma was 0.576 ± 0.050, and 0.684 ± 0.095 in normal esophageal mucosa tissues, the difference was statistically significant (t = 4.932, P < 0.01) (Figure 3B).
Figure 3.
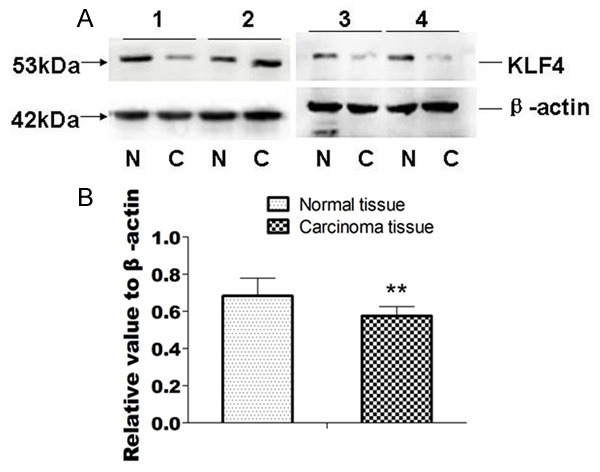
Western blot result of KLF4 protein expression in esophageal squamous cell carcinomas and normal esophageal mucosa tissues. A. Western blot result, N: normal esophageal mucosa tissues; C. Carcinomas tissues. 1, 2, 3, 4 were sample numbers; B. The column comparison result of KLF4 protein expression in normal esophageal mucosa tissues and carcinomas tissues.
Correlation between the expression of KLF4 and the prognosis of patients with esophageal squamous cell carcinoma
All the patients were followed-up till August 2012, with a median follow-up period of 45 months (3~72 months). The 5-year survival rate was 35.7% for all the patients. The 5-year survival rates and median survival times were 48.8% and 25.5%, and 55 and 26 months for the patients with KLF4 positive expression and negative expression, respectively (Figure 4). There were significant differences between the patients with KLF4 positive expression and negative expression in the 5-year survival rates and median survival times (x2 = 5.747 and 4.493, P = 0.017 and 0.034).
Figure 4.
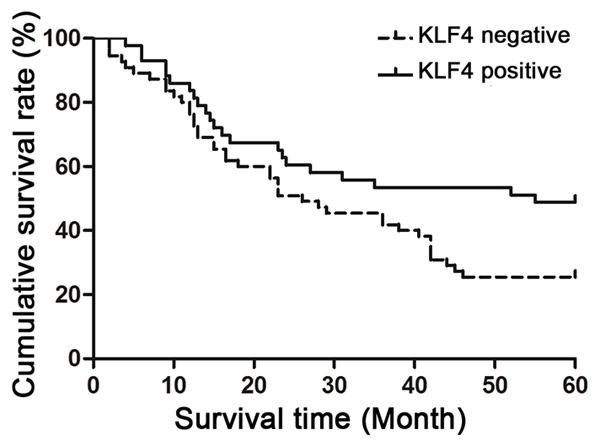
Comparison of survival time of negative and positive KLF4 protein expression in esophageal squamous cell carcinoma patients.
Discussion
KLF4, also known as gut-enriched Kruppel-like factor (GKLF) and epithelial zinc finger (EZF), is a member of the Kruppel-type zinc finger transcription factors [11-13]. The human KLF4 gene located on chromosome 9q31, which contains cDNA encodes a 55kD protein of 470 amino acids. Research indicated that KLF4 is anmportant regulator of cell proliferation and differentiation through regulating the transcription of target genes, blocking the G1/S conversion process by inhibiting DNA synthesis and cell proliferation and differentiation. In addition, knocking out of KLF4in epithelial cells at the gastrointestinal tract could change the abnormal proliferation and differentiation, infiltration [5].
Compelling reports have indicated the transcriptional role of KLF4 in regulating inflammatory stimuli [14,15], breast cancer [16,17], stem cells [2] and esophageal cancer cell lines [18]. KLF4 has been found to be overexpressed in oral and skin squamous carcinoma cells as well [19], reduced expression of KLF4 decreased the capacities of cancer stem cells to resist the chemicals, migrate, invade, and generate tumors in vitro and in vivo [20]. Although these results are not consistent, they suggest that KLF4 plays an important role in the development and progression of these tumors [21].
Our results showed that the expression of KLF4 was lower than that in the normal esophageal mucosa tissues. The expression of KLF4 was negatively correlated with the lymphatic metastasis and the disease stage of esophageal SCC patients. In addition, our results also showed that positive KLF4 expression in the esophageal SCC tissues correlated with higher 5-year survival rate and longer median survival time compared with negative KLF4 expression.
In the current study, a large number of esophageal SCC patients (98 cases), who were followed-up for a long period (a mean follow-up duration of 45 months), were enrolled. The immunohistochemical staining showed the expression features of KLF4 expression and its clinical-pathological relationships. Our results showed that the expression of KLF4 protein in esophageal SCC was obviously lower than that of normal esophageal mucosa, indicating that KLF4 protein participates in the terminal differentiation of epithelial cells and plays an important role in regulating and maintaining the esophageal mucosal homeostasis and in the progression of esophageal SCC. Some studies have suggested that the downregulation or inactivation of KLF4 in many tumors may be due to the point mutation, loss of heterozygosityor hypermethylation in the 5’ non-transcribed region of KLF4 gene.
Noti’s study also found that KLF4 could recruit histone deacetylases to the promoter region of the CD11d, and thereby inhibit gene expression, suggesting that KLF4 may also promote the occurrence of tumor through chromatin remodeling [22]. However, the mechanisms of the down regulation of KLF4 in esophageal SCC need to be further studied in the future. We also found that KLF4 was closely correlated with the lymphatic metastasis and the clinical stage of esophageal SCC patients.
The expression of KLF4 is low in the esophageal SCC patients with lymphatic metastasis or late stage disease, consistence with what was found in gastric and colorectal cancer [23,24]. However, there was no difference in the KLF4 expression among tumor tissues with different differentiation degree, which was in consistence with the results observed in other gastrointestinal cancers [25]. Furthermore, we found that esophageal SCC patients with negative KLF4 expression showed a lower 5-year survival rate that patients with positive KLF4 expression, suggesting KLF4 may be used as a prognosis predictor of the esophageal SCC after radical resection.
In conclusion, our data demonstrated that KLF4 was highly expressed in the esophageal mucosa compared with esophageal SCC tissues. KLF4 expression is correlated with the lymphatic metastasis, the pathologic stage and prognosis of esophageal SCC patients. Our data indicate KLF4 may act as a tumor suppressor in esophageal SCC and the expression status of KLF4 may be considered as a prognosis predictor of the esophageal SCC patients.
Disclosure of conflict of interest
None.
References
- 1.Li Z, Zhao J, Li Q, Yang W, Song Q, Li W, Liu J. KLF4 promotes hydrogen-peroxide-induced apoptosis of chronic myeloid leukemia cells involving the bcl-2/bax pathway. Cell Stress Chaperones. 2010;15:905–912. doi: 10.1007/s12192-010-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CW, Hou PS, Tseng SF, Chien CL, Wu KJ, Chen HF, Ho HN, Kyo S, Teng SC. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells. 2010;28:1510–1517. doi: 10.1002/stem.477. [DOI] [PubMed] [Google Scholar]
- 3.Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 4.El-Karim EA, Hagos EG, Ghaleb AM, Yu B, Yang VW. Kruppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol Cancer. 2013;12:89. doi: 10.1186/1476-4598-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu T, Chen X, Zhang W, Li J, Xu R, Wang TC, Ai W, Liu C. Kruppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One. 2012;7:e32492. doi: 10.1371/journal.pone.0032492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YJ, Wu CY, Chang CC, Ma CJ, Li MC, Chen CM. Nuclear Kruppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7:777–782. doi: 10.4161/cbt.7.5.5768. [DOI] [PubMed] [Google Scholar]
- 7.Hu D, Zhou Z, Davidson NE, Huang Y, Wan Y. Novel insight into KLF4 proteolytic regulation in estrogen receptor signaling and breast carcinogenesis. J Biol Chem. 2012;287:13584–13597. doi: 10.1074/jbc.M112.343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CJ, Lin SE, Lin YM, Lin SH, Chen DR, Chen CL. Association of expression of Kruppel-like factor 4 and Kruppel-like factor 5 with the clinical manifestations of breast cancer. Pathol Oncol Res. 2012;18:161–168. doi: 10.1007/s12253-011-9422-7. [DOI] [PubMed] [Google Scholar]
- 9.Ghaleb AM, Laroui H, Merlin D, Yang VW. Genetic deletion of Klf4 in the mouse intestinal epithelium ameliorates dextran sodium sulfate-induced colitis by modulating the NF-kappaB pathway inflammatory response. Inflamm Bowel Dis. 2014;20:811–820. doi: 10.1097/MIB.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, Wijnhoven BP. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol. 2012;19:2142–2148. doi: 10.1245/s10434-012-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KA, Eklund EA, Peddinghaus ML, Cao Z, Fernandes N, Turk PW, Thimmapaya B, Weitzman SA. Kruppel-like factor 4 regulates laminin alpha 3A expression in mammary epithelial cells. J Biol Chem. 2001;276:42863–42868. doi: 10.1074/jbc.M108130200. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, Jain MK. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122:4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SH, Kwon YW, Heo SC, Jeong GO, Kim BR, Seo EJ, Kim JH. Kruppel-like factor 4 mediates lysophosphatidic acid-stimulated migration and proliferation of PC3M prostate cancer cells. Exp Mol Med. 2014;46:e104. doi: 10.1038/emm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik DK, Gupta M, Das S, Basu A. Kruppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J Neuroinflammation. 2010;7:68. doi: 10.1186/1742-2094-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon JS, Kim HE, Koh E, Park SH, Jin WJ, Park BW, Park SW, Kim KS. Kruppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J Biol Chem. 2011;286:23808–23816. doi: 10.1074/jbc.M111.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, Chhieng DC, Grizzle WE, Engler JA, Krontiras H, Bland KI, LoBuglio AF, Lobo-Ruppert SM, Ruppert JM. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Zhu H, Wang Y, Yang S, Liu M, Zhang W, Quan L, Bai J, Liu Z, Xu N. Kruppel-like factor 4 represses transcription of the survivin gene in esophageal cancer cell lines. Biol Chem. 2009;390:463–469. doi: 10.1515/BC.2009.060. [DOI] [PubMed] [Google Scholar]
- 19.Abrigo M, Alvarez R, Paparella ML, Calb DE, Bal de Kier Joffe E, Gutkind JS, Raimondi AR. Impairing squamous differentiation by Klf4 deletion is sufficient to initiate tongue carcinoma development upon K-Ras activation in mice. Carcinogenesis. 2014;35:662–669. doi: 10.1093/carcin/bgt349. [DOI] [PubMed] [Google Scholar]
- 20.Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen J, Xi H, Li J, Zheng H. Kruppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells. PLoS One. 2013;8:e56082. doi: 10.1371/journal.pone.0056082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27:23–31. doi: 10.1093/carcin/bgi243. [DOI] [PubMed] [Google Scholar]
- 22.Noti JD, Johnson AK, Dillon JD. The leukocyte integrin gene CD11d is repressed by gut-enriched Kruppel-like factor 4 in myeloid cells. J Biol Chem. 2005;280:3449–3457. doi: 10.1074/jbc.M412627200. [DOI] [PubMed] [Google Scholar]
- 23.Choi BJ, Cho YG, Song JW, Kim CJ, Kim SY, Nam SW, Yoo NJ, Lee JY, Park WS. Altered expression of the KLF4 in colorectal cancers. Pathol Res Pract. 2006;202:585–589. doi: 10.1016/j.prp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Zhang J, Wang ZW, Zha L, Huang Z. Altered expression of Kruppel-like factor 4 and beta-catenin in human gastric cancer. Oncol Lett. 2012;3:1017–1022. doi: 10.3892/ol.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu R, Zuo Y, Zuo L, Liu C, Zhang S, Wu Q, Zhou Q, Gui S, Wei W, Wang Y. KLF4 Expression Correlates with the Degree of Differentiation in Colorectal Cancer. Gut Liver. 2011;5:154–159. doi: 10.5009/gnl.2011.5.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]


