Abstract
We studied images and histopathological features of primary esophageal malignant melanoma to explore the clinical pathological features, diagnosis, differential diagnoses, and treatment. Immunolabelling was conducted on six cases of esophageal malignant melanoma using histological and immunohistochemical techniques. Combined with the related literature, the clinical manifestations, imaging, histopathological and immunohistochemical features, treatment, and prognosis of primary esophageal malignant melanoma were observed and analyzed. The six patients with primary esophageal malignant melanoma were all male with an average age of 63.4 years. Poor food intake was observed in all patients, and the symptoms showed progressive aggravation. Endoscopic feed tube revealed dark brown and black nodular and polypoid lesions, 1/4-1/2 loop cavity. Tumor histopathology revealed the following characteristics: tumor cells arranged in nests, sheets and cords, round or polygonal, abundant and red-stained cytoplasm, melanin granules in the cytoplasm, heterogeneous nucleus sizes, centered or deviated nuclei, clearly identifiable nucleoli, and apparent pathological mitosis. The immune phenotype was as follows: tumor cells had diffuse expression of HMB45, Melan A, and S100. The cells were CK negative, and the Ki67-positive cell number was 40%-45%. Primary esophageal malignant melanoma is rare with high malignancy and poor prognosis. Immunohistochemical staining is helpful for diagnosing this tumor. The differential diagnosis includes low differentiated carcinoma, primitive neuroectodermal tumor, esophageal sarcomatoid carcinoma, esophageal lymphoma, and other tumors.
Keywords: Esophagus, malignant melanoma, tumor, immunohistochemistry, differential diagnosis
Introduction
Primary malignant melanoma of the esophagus (PMME) is a rare malignant tumor, accounting for only 0.1%-0.5% of the primary malignant tumors of esophagus [1]. The internal walls of the esophagus harbor melanocytes, which are the likely cause of the disease. However, to date, no disease model has been constructed. In the present study, imaging, histopathological, and immunohistochemical observations and analyses were conducted on six cases of PMME. Combined with the related literature, the imaging characteristics, clinical and pathological features, diagnosis and differential diagnosis, and treatment and prognosis are discussed.
Materials and methods
Clinical data
Six male patients with PMME were reviewed from General Hospital of Jinan Military Area (four cases, 2005-2013) and the 456 Hospital of PLA (two cases, 2001-2013) respectively. The patients were 56-72 years old, and the average age was 63.4 years. The tumors of three cases were located in the middle part of the esophagus (with a distance of 25-35 cm from the incisors), while the tumors of two cases were located in the middle and lower segments of the esophagus (35-40 cm from the incisors). All patients showed poor food intake without significant inducing factors. The symptoms demonstrated progressive aggravation with occasional belching. Electronic gastroscopy was conducted on six patients, in whom there were irregular nodular protrusions and polypoid lesions at the front wall and the left side wall of the esophagus 25-35 cm from the incisors in three cases, and there were irregular ulcerative lesions on the right side wall and the right back wall of the esophagus 35-40 cm from the incisors in two cases. Biopsy and pathological examination were conducted for five cases. The routine pathological report results revealed that two cases were malignant melanoma, and three cases were malignant tumors, which were considered low differentiation cancers, not excluding malignant melanoma. Chest, abdomen, and traumatic brain enhancement computed tomography (CT) scanning were conducted on five cases, and the results indicated that other lesions were not present in these patients. Positron emission tomography (PET)-CT scan examination was performed on one patient, and the results showed that there were no foci on the skin or other tissues and organs, and mediastinal and bilateral hilar regions showed no obvious enlargement or β-2-[18F]-Fluoro-2-dexy-D-glucose (FDG) metabolism- increased lymph nodes. Six cases were treated by surgical operation, and the postoperative specimens were sent for pathological examination.
Histology and immunohistochemistry
Specimens sent for examination were fixed in 4% formaldehyde. Conventional dehydration, paraffin embedding, sectioning, hematoxylin and eosin (HE) staining, and light microscopy observation were subsequently conducted. EnVison two-step method was used for immunohistochemical staining. The antibodies used included anti-HMB45, anti-Melan A, anti-S100, anti-CK, and anti-Ki67 were purchased from Beijing Zhongshan Jinqiao Biotech Corp (Beijing, People’s Republic of China). Diaminobenzidine (DAB) was used to develop the staining. Positive and negative controls were also used. HMB45 and Melan A positive expression was found in the cytoplasm and S-100 was localized in nucleus. CK positive expression was found in the cell membrane, and either brown tumor cell cytoplasm, nucleus, or cell membrane staining was considered positive for all staining.
Results
Digestive endoscopy and imaging characteristics
Electronic gastroscopy results revealed that irregular nodules and polypoid masses at the front wall and side wall of esophagus 25-35 cm from the incisors in four cases, which ran 1/4-1/2 the length of the cavity. In general, the polypoid mass bases were wide. The mass protruded into the lumen and exhibited dark brown and black color (Figure 1A). There were irregular ulcerative lesions on the right side wall and the right back wall of the esophagus 35-40 cm from the incisors in two cases. Tumor surfaces were ulcerated and covered with contaminated moss of a dark brown color. The surrounding mucosa was fragile and bled easily when touched. Ultrasonic endoscope revealed nodular and polypoid lesions in the feed tube wall, 1/4-1/2 loop cavities that were dark brown and black in color (Figure 1B). CT examination revealed eccentric tumors in the middle and lower segment of the esophageal lumen. The maximum transverse diameter was 3.5-4.5 cm, and the average transverse diameter was 3.8 cm. Tumor CT values were 27-42 Hounsfield Unit (HU) when plain scanning was used, and the density was uniform. Tumors were enhanced in six cases using enhanced scanning, among which two cases showed homogeneous enhancement, while the other four cases showed heterogneous enhancement. The CT values were 32-75 HU. There was no mediastinal lymph node enlargement or mediastinal invasion in any of the six cases. PET-CT systemic examination results of one patient revealed the following aspects: the anterior wall was obviously thickened at the middle thoracic esophageal 5-8 vertebral level, involving a length of about 6.2 cm. The metabolism of FDG was increased, and the maximum value was standardized uptake value (SUV) 8.3. The maximum value of the delayed scanning was SUV9.1, and there was luminal stenosis at the corresponding level, which is in accordance with esophageal malignant tumor PET-CT characteristics. There was no obvious swelling and FDG metabolism-increased lymph nodes at the mediastinal or bilateral hilar region, and there was no increased FDG metabolism in other organs or tissues (Figure 1C).
Figure 1.
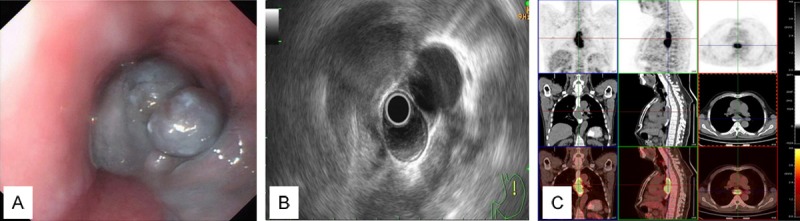
A. Endoscopic characteristics of esophageal malignant melanoma. B. Ultrasonic endoscope of the feed tube wall shows 1/4-1/2 loop cavity, dark brown and black nodular and polypoid lesions. C. Positron emission tomography-computed tomography (PET-CT) features of esophageal malignant melanoma.
Pathological characteristics
Visual observations were as follows: the sizes of nodular and polypoid tumors in four cases were: 3.5 cm × 3.0 cm × 2.5 cm, 4.5 cm × 3.5 cm × 2.0 cm, 6.5 cm × 3.5 cm × 2.5 cm, and 3.8 cm × 3.6 cm × 2.2 cm. The color of the tumors was grayish black. The cut surface was solid and soft, and the boundary with the surrounding mucosa was clear. The tumors invaded into the shallow muscle layer of the esophageal wall (Figure 2). The sizes of the ulcerative lesions in the two cases were: 3.5 cm × 2.5 cm × 1.0 cm and 3.3 cm × 2.0 cm × 1.5 cm. The surrounding ulcer was raised and dark brown. It had no clear boundaries with the surrounding mucosa and invaded into the deep muscle layer of the esophageal wall. Microscopic examination results were as follows: tumor cells exhibited nest shapes and were arranged in cords or diffuse sheet. The cell sizes were heterogenous. The shapes of the tumor cells were round, polygonal, and irregular, and the nucleus size differed. The chromatin granules were thick and large, and the nucleoli were large, located in the center of the nucleus, and were slightly eosinophilic. Mitotic figures were clearly visible. The cytoplasm of the tumor cells was abundant and melanin granules were identified in the cytoplasm (Figure 3A). Immunohistochemical staining results showed that tumor cells were positive for HMB45 (Figure 3B), Melan A (Figure 3C), and S100 (Figure 3D), but negative for CK (Figure 3E). The number of Ki67 positive cells was 40%-45% (Figure 3F).
Figure 2.
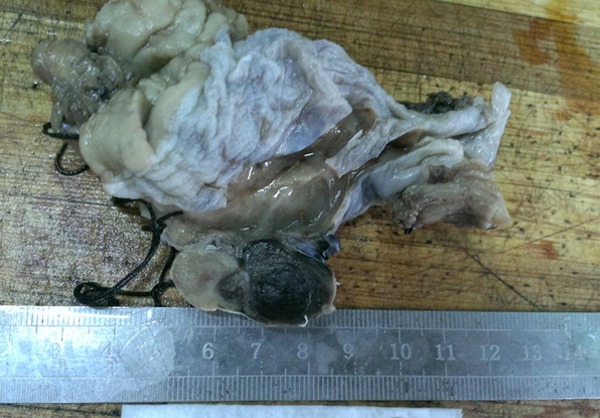
Gross characteristics of malignant melanoma in the locus medialis.
Figure 3.
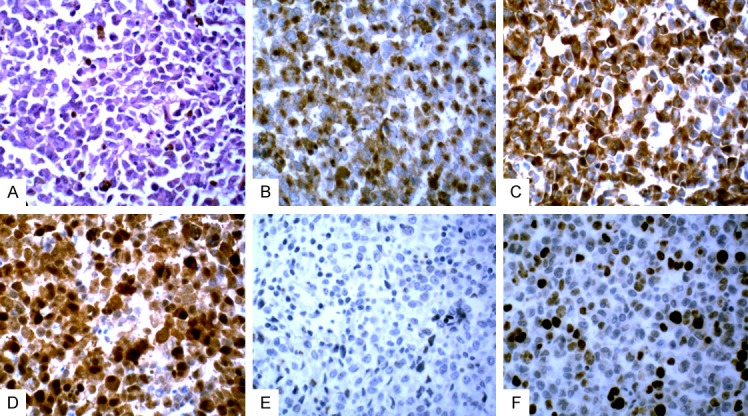
A. Histopathological features of esophageal malignant melanoma. B. Malignant melanoma immunohistochemistry staining of positive HMB45 in the locus medialis. C. Malignant melanoma staining positive for Melan A in the locus medialis. D. Malignant melanoma staining positive for S-100 in the locus medialis. E. Malignant melanoma negative for CK in the locus medialis. F. Positive Ki67 cells (40%-50%) of malignant melanoma staining in the locus medialis.
Pathological diagnosis
The six cases were diagnosed as PMME with tumor invasion into the shallow and deep muscle layers of the esophageal wall. Lymph node metastasis was not found in any patient.
Discussion
Clinical features
Malignant melanoma is a highly malignant tumor originating from melanoma cells. It occurs in the skin, eyes, oral cavity, rectum, and anus, from which melanoma can spread to the digestive tube, but it rarely spreads to the esophagus. Chest, abdomen, and brain enhanced CT examinations were conducted on all our six cases. PET-CT whole body scan was used in one case. Except for the esophagus, foci were not found on the skin or other tissues or organs. Clinical evidence supported a diagnosis of PMME.
Malignant melanoma originating from esophagus accounts for only 0.1%-0.5% of malignant tumors of esophagus [1]. Previously, it was believed that the melanoma did not occur in the esophagus, but rather spread there. Until 1906, Baur et al. first described PMME, but it was not generally accepted by clinicians. In 1963 DeLa Pava et al. conducted an autopsy study of 100 cases of normal esophageal mucosa, and they found that there were dendritic melanoblasts and melanocytes in the basal layer of the mucous epithelium in four cases, proving, for the first time that there were melanocytes in the esophagus. With the development and application of immunohistochemical techniques, pathological diagnoses are becoming increasingly more accurate. As a result, the numbers of case reports of PMME have increased. According to statistics, since the first report on the disease by Baur et al. in 1906, there have been about 250 cases reported worldwide [2].
The pathogenesis of PMME is not clear; some scholars believe that the disease is most common in the middle and lower segments of the esophagus. In esophagitis and hyperplastic esophageal, the abnormal and increase in the number of melanocytes may be precursor lesions of PMME [3]. The disease is more common in the elderly over the age of 50, of whom those aged 60-70 years are most susceptible. The incidence of PMME in men and women is approximately 1.6-2:1. There are no obvious symptoms in the early clinical stages. With disease progression, symptoms of gradual progressive dysphagia and even belching manifest. Clinical symptoms are not specific, and thus they are easily ignored, thereby delaying diagnosis.
In gastrointestinal barium meal imaging, the disease mainly appears as esophageal intraluminal polypoid and nodositas swelling. Swelling with smooth surfaces may also be affected because they arise from the submucosa. It forms an obtuse angle with the esophageal interface [4]. Chest CT examination can verify the scope of the esophageal tumor. More impotantly, the relationship between the tumor mass and the surrounding tissues can be observed to confirm whether there is mediastinal invasion and it is also helpful for finding enlarged lymph nodes. Enhanced CT scanning results reveal obvious lesion enhancement, suggesting a rich tumor blood supply [5], but CT scanning is not helpful in identifying properties of esophageal tumors. PET-CT examinations reveal increased FDG metabolism in the main lesion areas. Moreover, PET-CT can not only judge the scope of the esophageal tumor and whether there are lymph node metastases in the surrounding tissues, but it can also verify whether there are lesions or metastases in other body tissues and organs, which has important significance for the development of treatment programs. However, to differentiate this disease from esophageal cancer, carcinosarcoma of the esophagus, leiomyoma, leiomyosarcoma, and fibrovascular polyps, the value of imaging examinations for diagnosis is limited [6].
PMWE mostly occurs in the middle and lower segment of esophagus. Single or multiple polypoid are commonly seen with a pedicle or a wide base without pedicle. Pigmentation is most common, but occasionally there are cases that lack pigmentation. Microscopically, the tumor cells vary in size and are round, polygonal, or irregularly shaped, and the cytoplasm contains melanin granules. Tumor cells show diffuse growth, which can invade into the submucosa and even the muscular layer.
Most of the tumor cells were HMB45 positive, Melan A positive, S100 positive, and CK negative, and the number of Ki67 positive cells ranged between 40%-50%.
Because PMME cells have various phenotypes (the cells can be small, oval, or spindle shaped) they should be differentiated from poorly differentiated carcinoma, carcinosarcoma of the esophagus, esophageal lymphoma, neuroendocrine carcinoma, and esophageal leiomyosarcoma.
Low differentiated esophageal cancers are typically squamous cell carcinoma, while some are adenocarcinoma and small cell neuroendcrine carcinoma. In general, they are polypoid in nature. Microscopically, tumor cells are round and oval. Due to their poorly differentiated nature, it is difficult to differentiate these tumors from PMME by histomorphologic analysis alone. However, they can be differentiated by immunohistochemistry staining: low differentiated carcinomas are CK positive and HMB45 and Melan A negative, while PMME are CK negative and HMB45 and Melan A positive.
The vast majority of esophageal carcinosarcomas are comprised of polypoid masses. Microscopically, tumor cells exhibit epithelioid and spindle cell differentiation, the transition between the two types of cells can be identified by immunohistochemistry. When these tumors are sarcomas, they are CK positive and vimentin positive. Conversely, when they are malignant, they are CK negative and HMB45 positive.
Some PMME tumor cells are small and show diffuse distribution, which are very similar to lymphomas in cell morphology. However, immunohistochemistry reveals that esophageal lymphomas are LCA positive, B cells or T cells label positive for CD20 or CD3, and are HMB45 and S100 negative. Conversely, PMMEs are LCA negative, CD20/CD3 negative, and HMB45 and Melan A positive, which can be used for differentiation.
Microscopically, neuroendocrine carcinoma of the esophagus manifests as small or large cell carcinoma. Immunohistochemical staining is helpful for differentiation purposes; neuroendocrine carcinomas are CK, CD56, and syn/cgA positive, and HMB45 and Melan A negative, while PMME expression is the opposite.
Fusiform cell melanoma should be differentiated from leiomyosarcoma. Immunohistochemical staining revealed that leiomyosarcomas show myogenic expression, and are desmin, actin, and SMA positive, but HMB45, Melan A and S-100 negative, and melanoma myogenic expression is negative.
The present research results suggest that resection is an effective treatment for esophageal melanoma. According to statistics, the postoperative 5 years survival rate is 37% [7]. Early-stage PMME can have local lymph node metastases, and radical resection combined with esophageal lymphadenectomy is the main treatment approach [8,9]. Ho et al [10] showed that postoperative adjuvant chemotherapy does not increase survival. Recently, a randomized phase III trial of 502 melanoma patients treated with a combination of drug therapy and the monoclonal antibody ipilimumab was undertaken. The patients were divided into the ipilimumab group and the ipilimumab and dacarbazine combination group. The results illustrated that the overall survival in the combination group was 11.2 months, while the overall survival in the ipilimumab monotherapy group was 9.1 months, indicating that the combined application of ipilimumab with dacarbazine can improve the prognosis of metastatic melanoma patients [11].
In recent years, immunotherapy for PMME has made great progress. For example, vaccines, cytokines (IL-2), and immune regulatory antibodies have been applied for the clinical treatment of melanoma and have achieved some degree of efficacy [12]. In the present investigation, there was one patient that died six months after operation without taking any treatment, two patients that underwent postoperative radiotherapy and chemotherapy relapsed after 7 months, two cases with postoperative radiotherapy and chemotherapy and immunotherapy, one of whom recurred in 13 months while the other did not recur 16 months after surgery.
In short, PMME is a highly malignant tumor with a poor prognosis. A PMME diagnosis should be made by combining clinical imaging and relying on histopathological and immunohistochemical markers. Diagnosis in the early stages and differential diagnosis, timely operation therapy, and postoperative appropriate chemotherapy and immune therapy combined with the initial patient condition may prolong survival time.
Acknowledgements
This study was supported by the President Fund from the General Hospital, Jinan Military Command, China (Grant No. 2011M02).
Disclosure of conflict of interest
None.
References
- 1.Machado J, Ministro P, Araujo R, Cancela E, Castanheira A, Silva A. Primary malignant melanoma of the esophagus: a case report. World J Gastroenterol. 2011;17:4734–4738. doi: 10.3748/wjg.v17.i42.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly J, Leader M, Broe P. Primary malignant melanoma of the esophagus: a case report. J Med Case Reports. 2007;1:50. doi: 10.1186/1752-1947-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oshiro T, Shimoji H, Mstsuura F, Uchima N, Kinjo F, Nakayama T, Nishimaki T. Primary malignant melanoma of the esophagus arising from a melanotic lesion: report of a case. Surg Today. 2007;37:671–675. doi: 10.1007/s00595-006-3444-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin PW, Lee RC, Chern MS, Chiang JH, Chang CY. Primary malignant melanoma of the esophagus. J Chin Med Assoc. 2006;69:334–337. doi: 10.1016/S1726-4901(09)70269-2. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Lei W, Shao K, Zhang C, Chen Z, Shi S, He J. Characteristics and prognosis of primary malignant melanoma of the esophagus. Melanoma Res. 2007;17:239–242. doi: 10.1097/CMR.0b013e3281c4a079. [DOI] [PubMed] [Google Scholar]
- 6.Machado J, Ministro P, Araújo R, Cancela E, Castanheira A, Silva A. Primary malignant melanoma of the esophagus: a case report. World J Gastroenterol. 2011;17:4734–4738. doi: 10.3748/wjg.v17.i42.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpin E, Sauvanet A, Couvelard A, Belghiti J. Primary malignant melanoma of the esophagus: a case report and review of the literature. Dis Esophagus. 2002;15:244–249. doi: 10.1046/j.1442-2050.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- 8.Cardena R, Yep-Gamarra V, Donet-Mostacero J, Rodas J. Primary melanoma of the esophagus. Rev Gastroenterol Peru. 2012;32:303–308. [PubMed] [Google Scholar]
- 9.Li B, Lei W, Shao K, Zhang C, Chen Z, Shi S, He J. Characteristics and prognosis of primary malignant melanoma of the esophagus. Melanoma Res. 2007;17:239–242. doi: 10.1097/CMR.0b013e3281c4a079. [DOI] [PubMed] [Google Scholar]
- 10.Ho KY, Cheng J, Wee A, Soo KC. Malignant melanoma of the esophagus with multiple esophageal lesions. Nat Clin Pract Gastroenterol Hepatol. 2007;4:171–174. doi: 10.1038/ncpgasthep0761. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 12.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]


