Abstract
Detection of human epidermal growth factor receptor 2 gene (HER2, also known as erbB2) expression is a preparatory process to decide a treatment strategy for breast cancer patients. 20-30% of breast cancer patients have HER2 overexpression, and they usually show poor recovery rate. For detection of HER2 expression, immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) methods are conventionally used. Although these methods are accurate and reliable, their time-consuming process and high cost need a concise method with high sensitivity and accuracy. As a complementary method to the current IHC/FISH standard techniques, PCR-based methods have been developed. Here we employed a quantitative PCR method to detect HER2 expression in one hundred ninety nine formalin-fixed and paraffin-embedded (FFPE) breast cancer tissue samples from the patients treated over two years at the Yonsei University Severance Hospital, Republic of Korea. Relative expression of HER2 mRNA in the FFPE samples was analyzed using a quantitative RT-PCR (RT-qPCR) method and the obtained HER2 expression levels were compared with those from IHC/FISH methods. Our results show that the RT-qPCR method was highly concordant with IHC/FISH methods for detecting HER2 expression. Overall sensitivity and specificity of the BrightGen HER2 RT-qDx assay kit (Syantra, Calgary, Canada), which is a kit we used for RT-qPCR analyses, were 93.0% and 89.8% (P < 0.0001), respectively. The diagnostic cut-off value of HER2 RT-qDx for the clinical samples was determined by likelihood ratio, among which the highest likelihood ratio of relative HER2 mRNA levels was over 105.5 (AUC = 0.9466) with the highest sensitivity and specificity. Our study indicates that quantification of HER2 mRNA expression with the RT-qPCR could be an alternative method of conventional IHC/FISH methods.
Keywords: Breast cancer, HER2, FFPE, RT-qPCR, molecular diagnosis
Introduction
Breast cancer remains the most common and second deadliest cancer in women. In spite of the high incidence of breast cancer, the survival rate of patients who have been diagnosed and treated in the initial stages is increasing [1]. The World Health Organization (WHO) estimates that worldwide, more than 508,000 women died in 2011 due to breast cancer. In the Republic of Korea, 13,460 new incidence of breast cancer were reported for a year of 2010 [2].
The human epidermal growth factor receptor 2 gene (HER2, also known as erbB2) has been regarded a critical factor for diagnosis and effective treatment of breast cancer patients [3]. It is known that approximately 20~30% of breast cancer patients have over-expression of the HER2 gene, and these patients are known to show poor prognosis [4]. Genomic alterations of proto-oncogene HER2 are associated with poor prognosis and a more aggressive tumor phenotype. Thus, a HER2-positive status indicates a poor prognosis and shorter overall survival time [5]. Patients with HER2 amplification or over-expression are susceptible for treatment with trastuzumab (Herceptin, Roche, Penzberg, Upper Bavaria, Germany), which is a humanized monoclonal antibody raised against the extracellular domain of the HER2 protein. Herceptin is currently the standard first-line treatment toward metastatic breast cancer, and it is also indicated that therapeutic activity of Herceptin was enhanced when it applied as an adjuvant setting with other chemotherapeutics [6,7]. Up to date, the HER2 status might be sole factor indicating that patients are likely to respond to Herceptin. Based on the clinical results, Herceptin was approved by the Food and Drug Administration (FDA) of U.S.A. as a therapeutic drug in 1998 for treatment of advanced breast cancer. Actually, Herceptin prolonged survival time of the patients having operable HER2-positive cancer [8].
There are several reports compared HER2 status between in the primary tumors and in the matched metastatic regions; the results indicated that there was an acceptable level of concordance for HER2 expression between the two tumor regions (concordance rate = 80-94%) [9,10]. For this reason, the HER2 test is important for targeted therapy in breast cancer patients. The determination of HER2 status on primary tumor tissues is routinely performed by immunohistochemistry (IHC) or fluorescence in situ hybridization analysis (FISH). IHC is carried out on formalin-fixed paraffin-embedded (FFPE) breast cancer tissue samples to screen HER2 status in a clinical setting [11]. When the IHC score is 2+, HER2 gene amplification is detected by FISH. These two methods are standard for testing the expression of HER2. Although these are reliable methods, IHC/FISH are time-consuming, expensive, difficult to screening multiple samples in a short time, and require a high-resolution fluorescence microscope. Moreover, these methods are difficult to standardize across laboratories [12,13]. Therefore, it is necessary to develop a precise quantitative method to measure HER2 expression that are complementary to the IHC and FISH test.
Molecular techniques based on the quantitative evaluation of HER2 messenger RNA (mRNA) have been proposed. Especially, a reverse transcription quantitative PCR (RT-qPCR) was successfully used to evaluate the expression levels of HER2 mRNA in FFPE tissue samples. In this study, we employed the RT-qPCR method using a commercial diagnostic kit called ‘BrightGen HER2 RT-qDx’ (Syantra, Calgary, Canada), for quantification of HER2 expression and comparison with standard IHC and FISH methods using a total number of 199 FFPE breast cancer tissue samples.
Materials and methods
Clinical samples
A total of 199 FFPE tissue samples obtained from patients diagnosed with breast cancer at Yonsei University Severance Hospital (Seoul, Republic of Korea) for 2 years were analyzed in this study. With all patient tissues, IHC was already performed to verify expression of HER2, estrogen receptor (ER), and progesterone receptor (PR). All subjects provided written informed consent and the study was approved by the Institutional Ethics Committee of Yonsei University Severance Hospital (approval number 1-2010-0018).
Cell lines and cell culture
Human breast carcinoma cell line SK-BR3, which over-expresses HER2, was obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). Human breast carcinoma cell line MCF7 and MDA-MB-231, both of which weakly expresses HER2, were kindly provided by the Yonsei University Cancer Center (Seoul, Republic of Korea). THP-1 human monocytic cell line, which weakly expresses HER2, was kindly provided by Yonsei University College of Medicine (Seoul, Republic of Korea). The SK-BR3, MCF7, and MDA-MB-231 cancer cells were maintained as monolayer cultures in DMEM at 37°C, 5% CO2. THP-1 cells were cultured in RPMI 1640 supplemented with 2 mM glutamine. All the culture media were supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Gibco-BRL, Carlsbad, California, USA).
Deparaffinization of FFPE tissue and total RNA isolation
In order to remove paraffin from FFPE tissue samples, two pieces of 10-μm-thick FFPE breast cancer tissue sections were put into a 1.5-mL microcentrifuge tube, to which 1 mL of 100% xylene was added. After shaking and vortexing, the tube was heated for 5 min at 50°C to melt the paraffin and centrifuged for 2 min at room temperature at 20,000 x g to precipitate the tissue. After centrifugation, xylene solution was removed, and then 1 mL of 100% EtOH was added to the precipitated tissue. This tube was centrifuged at 20,000 x g for 2 min at room temperature. After that, EtOH was carefully discarded without disturbing the pellet. The EtOH washing was repeated twice. In the final washing, residual EtOH was removed as much as possible without disturbing the pellet, and the pellet was dried in the air for 25 min.
MagNA Pure LC RNA Isolation Kit III-Tissue (Roche Diagnostics, Mannheim and Penzberg, Germany) was used for total RNA extraction. In brief, 140 μL of tissue homogenized buffer (Roche Diagnostics) and 16 μL of 10% SDS solution were added to the deparaffinized tissue, sequentially. Then, the mixed samples were vortexed and incubated overnight at 55°C, after which 220 μL of tissue lysis buffer (Roche Diagnostics) was added to the tissue lysate supernatant. Finally, MagNA Pure LC 2.0 (Roche Diagnostics) machine was used to purify total RNA from the tissue lysates. The purity and concentration of total RNA were determined by measuring the absorbance at 260 nm and 280 nm using the Infinite 200® spectrophotometer (Tecan, Salzburg, Austria). All steps in the preparation and handling of total RNA were conducted in a laminar flow hood under RNase-free conditions. The isolated total RNA was stored at -70°C until used for cDNA synthesis.
cDNA synthesis
Complementary DNA (cDNA) was synthesized using an M-MLV Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) and random hexamers (Invitrogen) according to the manufacturer’s recommendations. Briefly, 10 μL of total RNA was added to the master mix containing 1 μL of 10 mM dNTP mix (10 mM each dATP, dGTP, dCTP, and dTTP at a neutral pH), 0.25 μg of random hexamers, and 5 μL of DEPC-treated water in PCR tubes. The reaction mixture was incubated at 65°C for 5 min, and then quickly chilled on ice. Subsequently, a mixture of 4 μL of 5× first-strand buffer, 2 μL of 0.1 M dithiothreitol (DTT), and 1 μL of M-MLV reverse transcriptase (RT) was added to the reaction mixture in PCR tubes, and the cDNA synthesis reaction was performed at 25°C for 10 min, 37°C for 50 min, and 70°C for 15 min.
HER2 mRNA RT-qPCR assay (BrightGen HER2 RT-qDx assay)
The HER2 mRNA expression level relative to GAPDH mRNA level was measured by RT-qPCR employing TaqMan probes using the CFX-96 real-time PCR system (Bio-Rad, Hercules, CA, USA), which was used for thermo-cycling and fluorescence detection. The RT-qPCR TaqMan assay was carried out with the BrightGen HER2 RT-qDx assay kit (Syantra) according to the manufacturer’s recommendations. Real-time PCR amplification for HER2 mRNA was performed using a total volume of 20 μL that contained 10 μL of 2× Thunderbird probe qPCR mix (Toyobo, Osaka, Japan), 5 μL of primer and TaqMan probe mixture, 2 μL of template cDNA, and D.W. to give a final volume of 20 μL for each sample. Positive and negative controls were included throughout the procedure. No-template controls with sterile D.W. instead of template DNA were incorporated into each run under the following conditions: 95°C for 3 min, followed by 40 cycles of 15 sec at 95°C and 30 sec at 55°C. The mRNA expression level was quantified by determining the cycle threshold (CT), which is the number of PCR cycles required for the fluorescence to exceed a value significantly higher than the background fluorescence. To avoid false negatives due to degradation of mRNA, the reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as a control. Target gene mRNA expression levels relative to GAPDH were automatically calculated using the comparative Ct method by CFX Manager Software v1.6 (Bio-Rad) or Genex Software (Bio-Rad), and the cut-off value for distinguishing between positive and negative results was a relative HER2 mRNA expression level of 100.
Statistical analysis
For statistical data analysis, the software PRISM 5 Software (GraphPad, La Jolla, CA, USA) was used. In order to determine the statistical significance of data, Student’s t-test and one-way ANOVA were carried out for the two-group comparison and multiple-group comparison, respectively. Receiver operating characteristic (ROC) curve analysis between conventional HER2 IHC, FISH and HER2 mRNA RT-qPCR was performed for all data.
Results
Analytical sensitivity of the RT-qPCR for HER2 mRNA detection using reference breast cancer cell lines
In order to test whether the BrightGen HER2 RT-qDxI used in this study was able to detect HER2 mRNA expression quantitatively, cDNA of breast cancer cell SK-BR3, which expresses high levels of HER2, was 10-fold diluted serially from 1×105 cells/mL to 1 cell/mL. HER2 mRNA RT-qPCR was performed three times, and the mean Ct values from serially diluted SK-BR3 cDNA are show in Figure 1. The mean Ct values of RT-qPCR using 105, 104, 103, 102, 10, and 1 cell(s)/mL were 19.45, 23.20, 26.49, 29.79, 33.35, and 36.08, respectively. The Ct value from the blank control reaction did not show any signal in any of the experiments (Figure 1).
Figure 1.
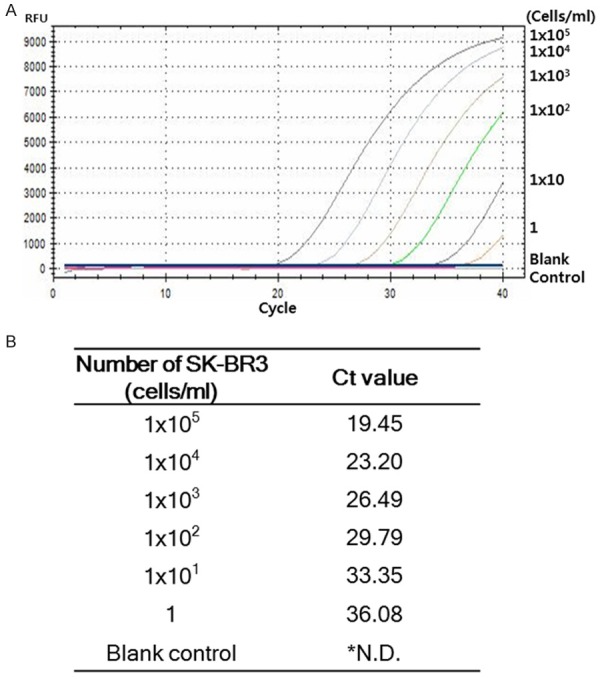
Analytical sensitivity of HER2 mRNA RT-qPCR. A. Analytical sensitivity of HER2 mRNA RT-qPCR was determined using serially diluted breast cancer HER2 over-expressing SK-BR3 cells. B. HER2 mRNA RT-qPCR was performed three times and the mean Ct value was calculated (*N.D.; Not detected).
Data expressed as relative HER2 mRNA levels were calculated based on the basal expression level of HER2 in MDA-MB-231 cell line having low HER2 expression. Thus, fold increase of HER2 expression is relative value to the HER2 expression that is set 1.0 in MDA-MB-231 cells. As we expected, SK-BR3, a HER2 over-expressing cell line, exhibited 57-fold higher HER2 mRNA expression, whereas, monocyte cell line THP-1, a cell line weakly expressing HER2, exhibited 0.7-fold expression of HER2 mRNA (Table 1).
Table 1.
Relative HER2 mRNA expression levels detected by HER2 mRNA RT-qPCR in various cell lines
| Cell line | HER2 status | Origin of cell | Relative HER2 mRNA expression level |
|---|---|---|---|
| SK-BR-3 | Over-expressing | Breast cancer | 56.99 ± 7.34 |
| MCF-7 | Weak-expressing | Breast cancer | 5.43 ± 2.25 |
| MDA-MB-231 | Weak-expressing | Breast cancer | 1 |
| THP-1 | Weak-expressing | Monocyte | 0.7 ± 0.2 |
Expression levels of HER2 mRNA in the clinical specimens
In order to determine whether BrightGen HER2 RT-qDx was useful to detect HER2 expression in FFPE clinical specimens, a total number of 199 FFPE samples from breast cancer patients who had IHC scores were subjected to RT-qPCR analysis of HER2 and GAPDH.
According to the BrightGen HER2 RT-qDxI analyses, the samples having HER2 IHC 0 score showed relative HER2 mRNA expression levels from 4.5 to 395 (average, 83.7). And the samples having HER2 IHC 1+ score showed relative HER2 mRNA expression levels from 0.5 to 401 (average, 86.6). The HER2 IHC score 2+/FISH- samples showed relative HER2 mRNA expression level from 0.01 to 813 (average, 198.1). In the HER2-positive groups, IHC 2+/FISH+ samples, showed relative HER2 mRNA expression level from 13.3 to 2711 (average, 709.4), and HER2 IHC 3+ samples showed relative HER2 mRNA expression levels from 22.5 to 4850 (average, 1936.7) (Figure 2).
Figure 2.
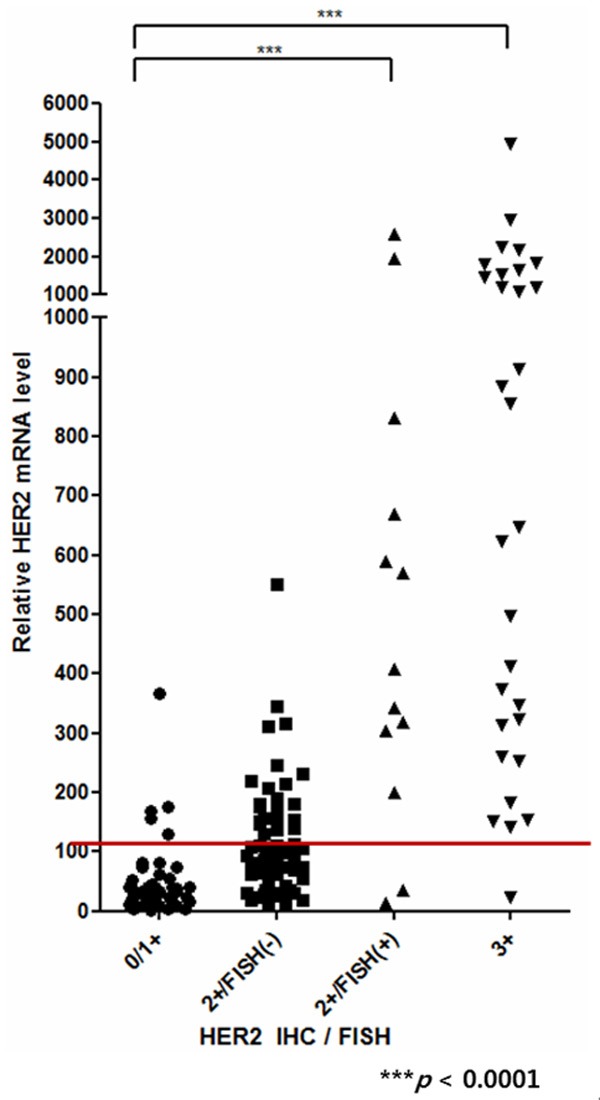
Evaluation of HER2 mRNA RT-qPCR according to the HER2 IHC and FISH score. FFPE breast cancer tissue samples were grouped by HER2 IHC and FISH results. The P-values between HER2 IHC negative and positive samples were calculated using one-way ANOVA (P < 0.0001).
Diagnostic cut-off determination of HER2 mRNA RT-qPCR assay
ROC analysis was performed to determine the optimal diagnostic cut-off value for clinical use. For ROC analysis, both of the HER2 mRNA levels obtained with the BrightGen HER2 RT-qDx analyses and the HER2 IHC score were used. The 199 FFPE samples were divided into two groups: HER2 IHC-positive and HER2 IHC-negative. Samples having IHC scores greater than 2+ and FISH+ were included in the HER2 IHC-positive group. Samples having IHC scores of 0 and 1+ were included in the HER2 IHC-negative group. IHC 2+ and FISH- samples were excluded from the analysis for its inconsistent results between the two assays.
Sensitivity and specificity of the ROC analysis were expressed by the area under curve (AUC) value. Among the 199 FFPE breast cancer tissue samples, 153 samples had GAPDH Ct value below 30. The highest AUC value was obtained from the samples having GAPDH Ct values below 30 indicating the highest sensitivity and specificity (AUC = 0.9466). According to the ROC analysis, the cut-off value of HER2 RT-qPCR was determined by likelihood ratio (Table 3). The highest likelihood ratio was the relative HER2 mRNA levels over 105.5 fold, indicating highest sensitivity and specificity. Based on this result, samples with a relative HER2 mRNA expression level greater than 105.5 by HER2 RT-qPCR assay were considered positive in this study (Table 3).
Table 3.
Clinical cut-off values of HER2 mRNA RT-qPCR obtained by ROC curve analysis with GAPDH Ct value below 30
| Relative HER2 mRNA expression level | Sensitivity % | 95% CI | Specificity % | 95% CI | Likelihood ratio |
|---|---|---|---|---|---|
| > 73.9 | 93.0 | 80.9% to 98.5% | 83.7 | 70.3% to 92.7% | 6.5 |
| > 78.1 | 93.0 | 80.9% to 98.5% | 85.7 | 72.8% to 94.1% | 9.1 |
| > 105.5 | 93.0 | 80.9% to 98.5% | 89.8 | 80.4% to 97.7% | 11.4 |
| > 135.1 | 90.7 | 77.9% to 97.4% | 91.8 | 80.4% to 97.7% | 11.1 |
Abbreviations: ROC, Receiver operating characteristic; CI, Confidence interval.
Comparison of the RT-qPCR results with IHC and FISH results for HER2 expression
Among the HER2-positive samples decided by IHC or IHC/FISH (HER2 IHC 3+ and IHC 2+/FISH+), all the samples were positive with HER2 RT-qPCR except 3 samples. Among the HER2-negative samples (HER2 IHC 0 and 1+), 44 samples were negative and 5 samples were detected positive with the RT-qPCR. Therefore, the overall sensitivity and specificity of the BrightGen HER2 RT-qDx were 93.0% and 89.8%, respectively (Table 2). The P-values between HER2 IHC negative and positive were statically significant (P < 0.0001) via one-way ANOVA test when it was decided by the results from the RT-qPCR analyses.
Table 2.
Comparison of HER2 mRNA RT-qPCR results with HER2 IHC and FISH results
| HER2 IHC/FISH score | HER2 mRNA RT-qPCR No. of samples (%) | Total | |
|---|---|---|---|
|
| |||
| Positive | Negative | ||
| 3+ | 29 (96.7) | 1 (3.3) | 30 (100) |
| 2+/FISH+ | 11 (84.6) | 2 (15.4) | 13 (100) |
| 2+/FISH- | 28 (45.9) | 33 (54.1) | 61 (100) |
| 0/1+ | 5 (10.2) | 44 (89.8) | 49 (100) |
| Total | 73 (47.7) | 80 (52.3) | 153 (100) |
Correlation level between the RT-qPCR and HER2 IHC/FISH score for detection of HER2 expression
In order to verify the correlation between relative HER2 mRNA expression level and HER2 IHC/FISH score, correlation coefficient analysis was performed using clinical samples having GAPDH Ct value below 30 (Figure 3). For the analysis of the correlation coefficient, the HER2 IHC test results were given as scores: a HER2 IHC score of 0 for 0, a HER2 IHC score of 1+ for 25, a HER2 IHC score 2+/FISH- for 50, a HER2 IHC score 2+/FISH+ for 75, and a HER2 IHC score of 3+ for 100. Then, HER2 mRNA RT-qPCR results of FFPE breast cancer tissue samples were compared with the HER2 IHC/FISH test results. The data showed that there was a correlation between relative HER2 mRNA expression levels and standard HER2 IHC/FISH test results (Pearson r = 0.5408, r2 = 0.2936, P < 0.0001) (Figure 3).
Figure 3.
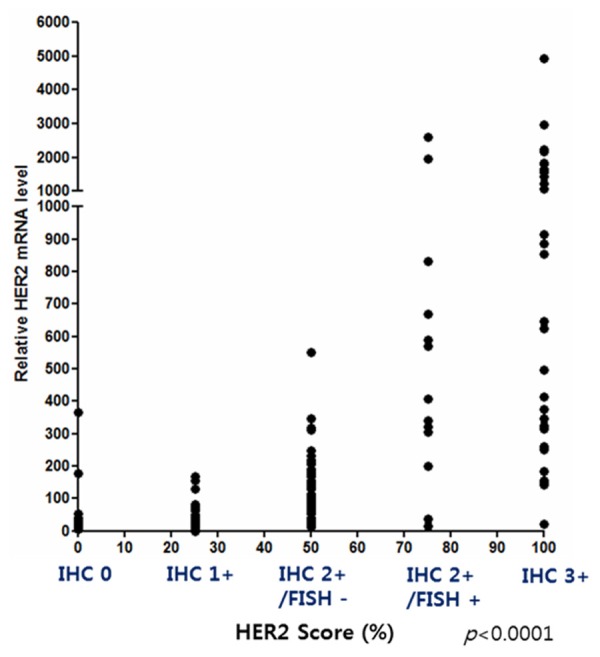
Correlation coefficient analysis between the HER2 mRNA RT-qPCR results and HER2 IHC and FISH results. The correlation between HER2 mRNA RT-qPCR and HER2 IHC and FISH results was good (Pearson r = 0.5418, r2 = 0.2936, P < 0.0001).
Discussion
IHC and FISH are regarded as standard assays for detection of HER2 expression in breast cancer samples. Determination of HER2 expression is essential for application of Herceptin-mediated therapy to breast cancer patients. In particular, IHC has been widely used for its multiple application to screening, diagnosis, and cancer stage determination. Nevertheless, IHC has fundamental weakness: it uses antibody to detect target protein in cell and tissue samples, so that IHC requires a long process time and there are variations between each laboratories [14]. And the intensity of IHC is decided depending on the enzyme activity of horseradish peroxidase (HRP) that is conjugated on antibodies. Therefore, the staining intensity may be influenced by reaction time, temperature, and HRP substrate concentrations [16]. For HER2 detection, IHC provides reliable results with 0/1+ indicates HER2-negative status and 3+ indicates HER2-positive status in the samples. In case of 2+ score by HER2 IHC, the samples should be further tested by FISH to confirm HER2 status [15]. FISH assay also has weaknesses: since it uses fluorescent dyes, the stained slides cannot be preserved a long time, and FISH assay need a high-resolution fluorescent microscope that is not equipped in every hospital laboratory [17].
To complement the weaknesses of IHC/FISH methods, we evaluated a RT-qPCR method using a new RT-qPCR assay kit, BrightGen HER2 RT-qDx assay, with which accuracy and sensitivity of the RT-qPCR method were compared with IHC/FISH for HER2 detection. We expected that RT-qPCR method could be standardized and reduce variances between laboratories for quantification of HER2 expression in clinical samples because RT-qPCR assay has strength for quantitating target gene mRNA expression level. RT-qPCR is also recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines, and it is highly sensitive and specific because primers and probes are target gene sequence-specific [18]. In this study, HER2 mRNA expression levels were expressed as a fold induction in reference to expression of a housekeeping gene, GAPDH. Therefore, obtaining the minimum Ct value of the GAPDH gene expression level from specimens influenced the sensitivity and specificity of the assay. In this study, only the Ct values below 30 were used for GAPDH mRNA RT-qPCR (Table 3).
We compared the RT-qPCR results for HER2 expression with that from the current standard methods, IHC and FISH. The overall sensitivity of the RT-qPCR was 93.0% and the specificity was 89.8% (Figure 3 and Table 3). Some of the samples showing HER2 IHC 2+/FISH- were positive by the RT-qPCR. We don’t know the reason for the inconsistency clearly, but there might be lost of HER2 mRNA in the samples while the HER2 protein remained. In the previous studies [19,20], it has been reported that Herceptin had an effect on the patients with HER2 IHC 2+/FISH-. In our study, there were 61 (39.9%) out of 153 samples from the breast cancer patients had HER2 IHC 2+/FISH-. Although, all of the 61 patients are not subjected to Herceptin treatment, 28 patients (45.9%) were detected positive by the current RT-qPCR analyses. Those 28 patients might have high possibility to be susceptible to Herceptin treatment.
In conclusion, this study shows that the RT-qPCR method produced quite correlated results with that from the conventional IHC/FISH methods with some exception of the IHC2+/FISH- samples. Because PCR-based analyses employed sequence-specific primers and probes for target DNA, we expect that there is much room for improvement of sensitivity and specificity of the RT-qPCR method by developing new primers and probes. As long as the quality is guaranteed, RT-qPCR method could be the first choice for determining HER2 expression in clinical samples of breast cancer patients.
Acknowledgements
This study was supported by The Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant HI12C18370100 [A121986] to H.L.).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.WHO Cancer factsheet N°297 updated February 2009. Last accessed April 2011 at http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
- 3.Dinh P, de Azambuja E, Piccart-Gebhart MJ. Trastuzumab for early breast cancer: current status and future directions. Clin Adv Hematol Oncol. 2007;5:707–717. [PubMed] [Google Scholar]
- 4.Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F. HER2/neu role in breast cancer: from a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62. doi: 10.1097/GCO.0b013e328012980a. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire W. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ. Adjuvant trastuzumab therapy for HER2-overexpressing breast cancer: what we know and what we still need to learn. Eur J Cancer. 2006;42:1715–1719. doi: 10.1016/j.ejca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura R, Okumura Y, Arima N. Trastuzumab monotherapy versus combination therapy for treating recurrent breast cancer: time to progression and survival. Breast Cancer. 2008;15:57–64. doi: 10.1007/s12282-007-0014-z. [DOI] [PubMed] [Google Scholar]
- 8.Hillner BE, Smith TJ. Do the large benefits justify the large costs of adjuvant breast cancer trastuzumab? J. Clin. Oncol. 2007;25:611–613. doi: 10.1200/JCO.2006.09.3542. [DOI] [PubMed] [Google Scholar]
- 9.Edgerton SM, Moore D 2nd, Merkel D, Thor AD. erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol. 2003;11:214–221. doi: 10.1097/00129039-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauter G, Lee J, Bartlett JMS, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J. Clin. Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 12.Moerland E, van Hezik RL, van der Aa TC, van Beek MW, van den Brule AJ. Detection of HER2 amplification in breast carcinomas: comparison of Multiplex Ligation-dependent Probe Amplification (MLPA) and Fluorescence In Situ Hybridization (FISH) combined with automated spot counting. Cell Oncol. 2006;28:151–159. doi: 10.1155/2006/741586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis CM, Dyson MJ, Stephenson TJ, Maltby EL. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol. 2005;58:710–714. doi: 10.1136/jcp.2004.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gjerdrum LM, Sorensen BS, Kjeldsen E, Sorensen FB, Nexo E, Hamilton-Dutoit S. Real-time quantitative PCR of microdissected paraffin-embedded breast carcinoma: an alternative method for HER-2/neu analysis. J Mol Diagn. 2004;6:42–51. doi: 10.1016/S1525-1578(10)60490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks DG, Tubbs RR. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum Pathol. 2005;36:250–261. doi: 10.1016/j.humpath.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Ellis CM, Dyson MJ, Stephenson TJ, Maltby EL. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol. 2005;58:710–714. doi: 10.1136/jcp.2004.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onody P, Bertrand F, Muzeau F, Bieche I, Lidereau R. Fluorescence in situ hybridization and immunohistochemical assays for HER-2/neu status determination: application to node-negative breast cancer. Arch Pathol Lab Med. 2001;125:746–750. doi: 10.5858/2001-125-0746-FISHAI. [DOI] [PubMed] [Google Scholar]
- 18.Barberis M, Pellegrini C, Cannone M, Arizzi C, Coggi G, Bosari S. Quantitative PCR and HER2 testing in breast cancer: a technical and cost-effectiveness analysis. Am J Clin Pathol. 2008;129:563–570. doi: 10.1309/1AKQDQ057PQT9AKX. [DOI] [PubMed] [Google Scholar]
- 19.Horton J. Her2 and trastuzumab in breast cancer. Cancer Control. 2001;8:103–110. doi: 10.1177/107327480100800113. [DOI] [PubMed] [Google Scholar]
- 20.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]


