Abstract
Objective: To investigate different protective effects of recombinant H. pylori multi-epitope antigen (rIB) with cholera toxin subunit B (rCTB) as the intramolecular/extramolecular adjuvant though different immunization routes in a Helicobacter pylori infected mouse model. Methods: By using rCTB as the intramolecular/extramolecular adjuvant of rIB, BALB/c mice were immunized through oral administration or intramuscular injection, on day 0, 14, 28. Every 14 days, ELISA was used to detect serum specific IgG and IgA titers after immunization. After the last immunization, H. pylori SS1 challenge was performed, and urease test, Gram staining after smearing of mouse gastric tissue, PCR, pathology and immunohistochemistry were used to evaluate preventive effect of the recombinant protein vaccine. Results: After immunization three times, intramolecular injection could induce high titers of serum specific IgG antibody, and the antibody titer in rIB group, rCTB+rIB and rBIB group was 2000, 5000 and 7500, respectively (P < 0.05). Specific IgA antibody was only detected in rBIB oral administration group. The immune protection rate in rBIB oral administration group was significantly higher than that in rBIB intramolecular injection group (33.3% vs. 83%), indicating significant difference. Conclusion: rCTB has good intramolecular/extramolecular immune adjuvant effects, and its intramolecular immune adjuvant effect is better. Both intramolecular injection and oral administration of rBIB have immune protective effect against H. pylori challenge, and oral administration of rBIB exerts better immune protective effect.
Keywords: Helicobacter pylori, recombinant vaccine, cholera toxin subunit B, adjuvant, immunological response
Introduction
Helicobacter pylori (H. pylori) is an important pathogenic bacterium discovered by Australian scholars Warren and Marshall in 1982. A large amount of research on molecular biology, epidemiology and related diseases have confirmed that H. pylori is an important pathogenic factor of gastritis, peptic ulcer, gastric precancerous lesion, gastric cancer, gastric MALT lymphoma, etc. Helicobacter pylori is the pathogenic bacterium with the highest infection rate in the world, and half of the population in the world is infected by Helicobacter pylori [1]. In recent years, the overuse of antibiotics results in increasingly prominent problems, including low H. pylori eradication rate, reduced drug choice after failure of eradication and high recurrence rate. Therefore, the development of safe and effective vaccine becomes particularly important [2-5].
UreB is the urease activity unit, which is relatively conservative. It has very strong antigenicity and is the key for bacterial colonization in stomach. UreI is a H. pylori urea channel protein. Mollenhauer et al. have found that UreI gene deletion mutants of H. pylori cannot colonize in stomach. Cholera toxin B (CTB) is a non-toxic receptor binding subunit produced by Vibrio cholerae. CTB can be used as a stable carrier of some exogenous polypeptide to increase the immunogenicity of the epitope of a fused antigen so that the human body can produce a relatively strong immune response to increase the immune protection [6-9].
In earlier stage, genetic engineering recombinant technology was used to construct rCT. UreI and UreB were used as core antigen components to construct rIB, and rBIB was constructed by using rCTB as the intramolecular adjuvant. It’s a basic study on vaccine components and vaccine immune routes, and we evaluated the induced immune response and immune protective effect differences through different combinations and different immunization routes by using recombinant H. pylori multi-epitope antigen (rIB) and using cholera toxin subunit B as the adjuvant molecule, so as to provide experimental evidence for the research of H. pylori vaccine.
Materials and methods
Strain, reagents and animals
H. pylori Strain SS1 (Sydney strain 1), to which animal model had adapted, was purchased from Hebei Medical University. rBIB, rIB and rCTB were all prepared by our project group. SPF level BALB/c mice of 6 weeks old (males:females = 1:1) were provided by Huaxi Experimental Animal Center of Sichuan University.
BALB/c mice grouping and immunization
BALB/c mice were randomly divided into nine groups and each group included six mice. rIB, rCTB, rBIB antigens were diluted into 1 mg/ml. All animals received fasting and water deprivation for 12 h before intramuscular injection or oral administration. Then, according to the immunization treatment requirements in Table 1, in intramuscular injection immunization groups (IM groups), mice underwent intramuscular injection immunization from left and right legs; in oral administration immunization groups (oral groups), mice received 0.2 ml by intragastric administration. After initial immunization, booster immunization once was performed on day 14 and 28, respectively, and the dose and method were the same as the initial immunization.
Table 1.
Treatments in different vaccination group
| Groups (n = 6) | 0 D | 14 D | 28 D | |
|---|---|---|---|---|
| Bland group (PBS) (0.2 ml/20 g) | - | - | - | |
| Intramuscular injection (2.5 mg/kg) | rCTB | 2.5 mg/kg | 2.5 mg/kg | 2.5 mg/kg |
| rIB | 2.5 mg/kg | 2.5 mg/kg | 2.5 mg/kg | |
| rCTB+rIB | 2.5 mg/kg | 2.5 mg/kg | 2.5 mg/kg | |
| rBIB | 2.5 mg/kg | 2.5 mg/kg | 2.5 mg/kg | |
| Oral group (10 mg/kg) | rCTB | 10 mg/kg | 10 mg/kg | 10 mg/kg |
| rIB | 10 mg/kg | 10 mg/kg | 10 mg/kg | |
| rBIB | 10 mg/kg | 10 mg/kg | 10 mg/kg | |
| rCTB+rIB | 10 mg/kg | 10 mg/kg | 10 mg/kg |
Note: “-” does not contain the components in the mixed solution.
H. pylori challenge
14 days after the last immunization, 6 mice in each group were all challenged by freshly cultured H. pylori. Before the challenge, fasting and water deprivation lasted for 12 h. Each mouse received gastric gavage of H. pylori bacterial liquid 200 μl with about 1 × 108 cFu of bacterial bodies by oral route; the infection challenge was repeated two more times by once every other day. Four hours after each challenge, food and water supplies were restored.
Serum specific IgG and IgA antibodies detection
After mice were immunized, samples were collected once every 14 days. Indirect ELISA assay was used to detect serum specific IgG and IgA.
Evaluation for colonization of Helicobacter pylori in gastric tissue
On day 28 after the last challenge, the mice were sacrificed, and stomach tissue in the mice was sampled under sterile condition. Along the greater curvature the stomach was longitudinally cut open and along the lesser curvature the stomach was cut into two halves. One half was placed into 4% neutral formalin for fixation so as to prepare for HE staining and immunohistochemistry, and another half was further longitudinally divided into three equal parts, which would be used for urease test, smearing and Gram stain, PCR detection, respectively.
Results
Detection of serum specific IgG antibody
In rIB oral group, rBIB IM group, rBIB oral group, rIB+rCTB IM group and rIB+rCTB oral group, low level of serum anti-H. pylori specific IgG antibody could be detected from day 14 after the mice were immunized, while in rIB IM group no specific IgG antibody was detected. On day 28 after immunization, the titers of every IM group and every oral group increased to some degree, but there was significant difference (P = 0.000) in serum IgG antibody titer between IM groups and oral groups. On day 42 after immunization, titer in each IM group was significantly higher than the second immunization, while the difference between different IM groups was significant; specific IgG antibody titers were in order by rBIB IM group > rIB+rCTB IM group > rIB IM group. On day 42 after immunization, the titer in each oral group increased, but there was no significant difference between different oral groups, detailed in Table 2; Figure 1.
Table 2.
Anti-SS1 IgG antibody titer after immunization with different sample (x̅ ± s, n = 6)
| Groups | Serum IgG titers | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intramuscular injection | Oral group | |||||
|
| ||||||
| 14 D | 28 D | 42 D | 14 D | 28 D | 42 D | |
| Bland group (PBS) | 0 | 0 | 0 | 0 | 0 | 0 |
| CTB | 0 | 0 | 0 | 0 | 0 | 0 |
| IB | 0 | 1200 ± 400 | 2000 ± 0 | 100 ± 0 | 120 ± 51 | 200 ± 0 |
| IB+CTB | 50 ± 0 | 1200 ± 900 | 5000 ± 0 | 100 ± 0 | 150 ± 50 | 200 ± 100 |
| BIB | 100 ± 0 | 2800 ± 900 | 7500 ± 3763 | 100 ± 0 | 200 ± 0 | 300 ± 100 |
Figure 1.
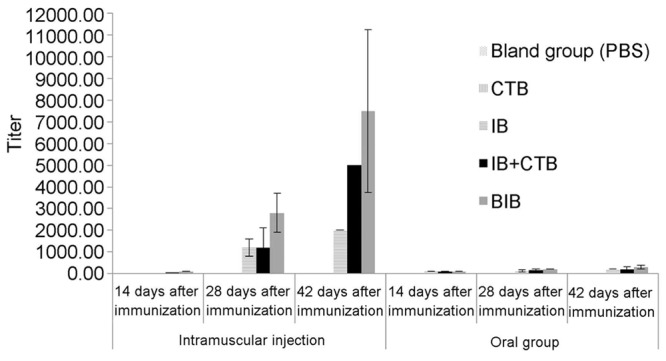
Anti-SS1 IgG titers after immunization with different antigens (x̅ ± s, n = 6).
Detection of serum specific IgA antibody
After oral immunization three times by using rCTB as intramolecular adjuvant plus using rIB protein (i.e. rBIB), serum specific IgA antibody could be detected (P = 0.000) and the titer was up to 1:120. Meanwhile, serum IgA antibody could not be detected in other groups. The results are shown in Table 3.
Table 3.
Anti-SS1 IgA titers after immunization with different antigens (x̅ ± s, n = 6)
| Groups | Serum IgA titers | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intramuscular injection | Oral group | |||||
|
| ||||||
| 14 D | 28 D | 42 D | 14 D | 28 D | 42 D | |
| Bland group (PBS) | 0 | 0 | 0 | 0 | 0 | 0 |
| CTB | 0 | 0 | 0 | 0 | 0 | 0 |
| IB | 0 | 0 | 0 | 0 | 0 | 0 |
| IB+CTB | 0 | 0 | 0 | 0 | 0 | 0 |
| BIB | 0 | 0 | 0 | 100 ± 0 | 120 ± 51 | 120 ± 0 |
Analysis for colonization of Helicobacter pylori in gastric tissue
On day 28 after the challenge, four methods, i.e., PCR, urease test, immunohistochemistry and Gram staining, were used to detect H. pylori colonization in gastric tissue of the mice. If two methods of the four were positive, positive H. pylori infection was judged. The results are shown in Table 4.
Table 4.
Result of protection rates of different groups
| Groups | Positive rate (number of positive/number of test, n = 6) | Infection rate (%) | Protection rate (%) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| PCR | RUT | Immunohistochemistry | Gram stain | ||||
| Bland group (PBS) | 6/6 | 6/6 | 6/6 | 6/6 | 100 | 0 | |
| Intramuscular injection | CTB | 6/6 | 6/6 | 6/6 | 6/6 | 100 | 0 |
| IB | 6/6 | 6/6 | 6/6 | 3/6 | 100 | 0 | |
| CTB+IB | 6/6 | 6/6 | 6/6 | 6/6 | 100 | 0 | |
| BIB | 4/6 | 4/6 | 4/6 | 4/6 | 66.7 | 33.3 | |
| Oral group | rCTB | 6/6 | 6/6 | 6/6 | 6/6 | 100 | 0 |
| rIB | 5/6 | 6/6 | 6/6 | 6/6 | 100 | 0 | |
| rCTB+rIB | 6/6 | 6/6 | 6/6 | 6/6 | 100 | 0 | |
| rBIB | 1/6 | 1/6 | 3/6 | 1/6 | 16.7 | 83.3 | |
By using Fisher exact test for analysis, the results showed that the protection rate against H. pylori challenge in rBIB oral group was higher by 50% than rBIB IM group, and there were significant differences in H. pylori challenge infection rate and protection rate against H. pylori challenge between rBIB oral group and other groups. (P = 0.000). See Figure 2.
Figure 2.
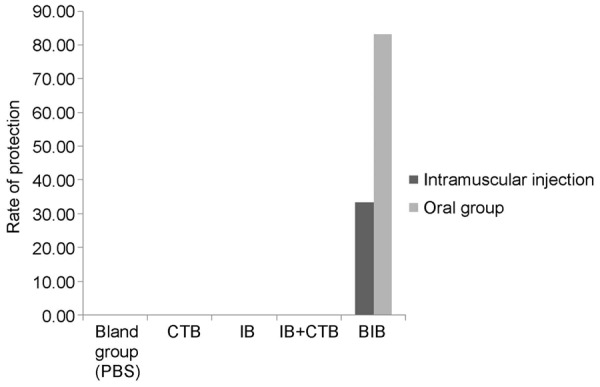
Result of protection rates of different groups.
Immunohistochemical results
On day 28 after infection challenge of H. pylori, immunohistochemistry showed that blank control group (PBS group), rCTB IM group, rIB IM group, rCTB+rIB IM group and rCTB oral group, rIB oral group, rCTB+rIB oral group all had cluster of H. pylori colonization in gastric tissue samples; in only 4 of 6 BALB/c mice in rBIB IM group, dispersedly distributed H. pylori were detected; in only 3 of 6 BALB/c mice in rBIB oral group, H. pylori colonization was detected. See Figure 3.
Figure 3.
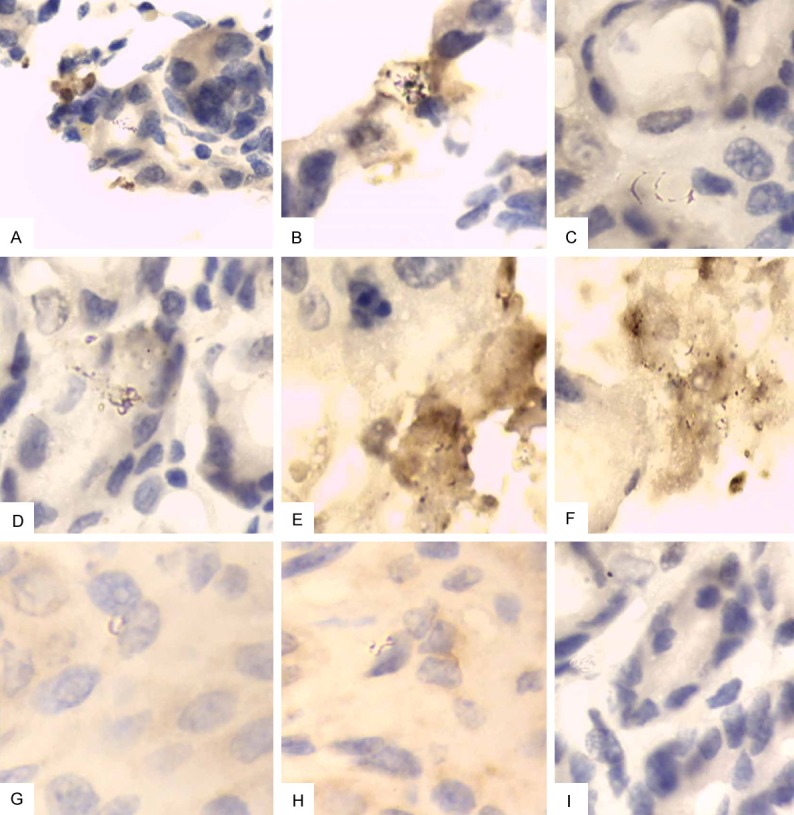
Mouse gastric tissue (immunohistochemistry, × 1000). A: Blank control group; B: rCTB IM group; C: rCTB oral group; D: rIB IM group; E: rIB oral group; F: rIB+rCTB IM group; G: rIB+rCTB oral group; H: rBIB IM group; I: rBIB oral group.
HE staining
On day 28 after infection challenge of H. pylori, pathological HE staining results showed that in non-immunized control group, rCTB IM group, rIB IM group, rCTB+rIB IM group and rCTB oral group, rIB oral group, rCTB+rIB oral group, rBIB IM group, majority of the samples exhibited: necrosis, shedding, inflammatory cell infiltration and other pathological changes; in rBIB oral group, HE staining of the gastric tissues rarely exhibited obvious lesion. See Figure 4.
Figure 4.
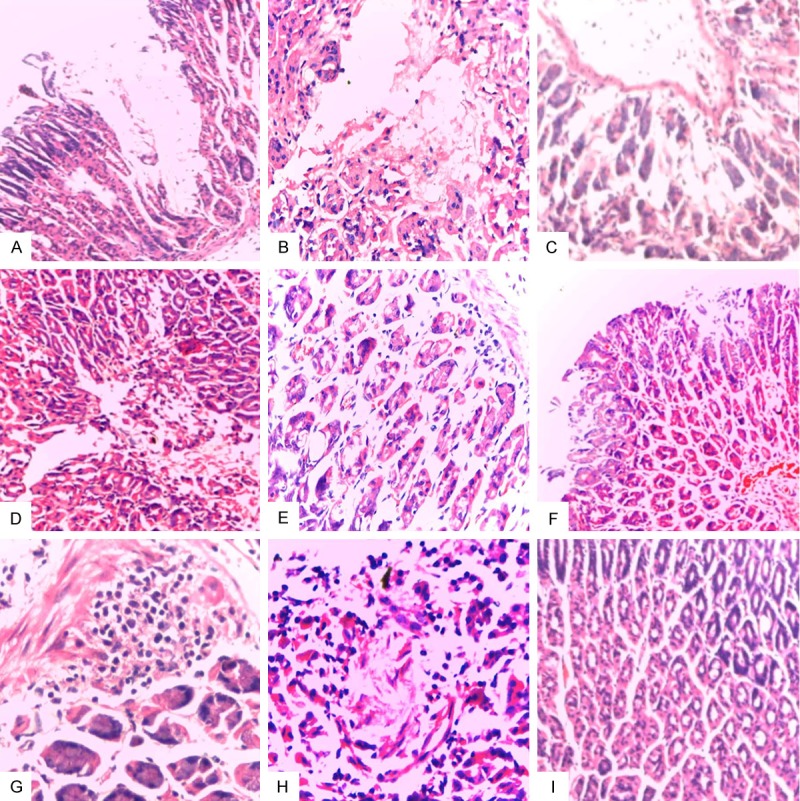
Mouse gastric tissue (HE, × 400). A: Bland control group: within gastric intrinsic membrane, glands reduced by 2/3, and severe atrophy existed; B: rCTB IM group: on the gastric surface, there were epithelial focal necrosis with inflammatory cell infiltration; C: rCTB oral group: mild shedding, necrosis with inflammatory cell infiltration; D: rIB IM group: epithelial hemorrhagic necrosis on the surface; E: rIB oral group: obvious inflammatory cell infiltration within the lamina propria; F: rIB+rCTB IM group: superficial ulcer with bleeding; G: rIB+rCTB oral group: lymphocytic infiltration in deep part of the lamina propria; H: rBIB IM group: focal necrosis; I: rBIB oral group: normal.
Pathological comprehensive score of each immunization group
Referring to Arlin B. Rogers scoring criteria [10], each of inflammation, hemorrhage, edema, H. pylori bacterial body colonization, necrosis, atrophy was scored into 1, 2, 3, 4 points according to the lesion severity, and the total points were 4 (theoretical total of 6 items was 24 points); in one group, the accumulative total score of all mice with 6 items was 24 × 6 = 144 points, and in each group, the actual pathological accumulative score was divided by theoretical total score (144 points) and was multiplied by 100%, and then the gastric pathological injury degree (%) was obtained. The 1-gastric pathological injury degree (%) was the gastric pathological injury decline degree (%).
Pathological score statistics showed that after the mice received infection challenge of H. pylori, the gastric pathological injury degree in rBIB oral group was the lowest (only 14.6%), and was lower by 64.6% than blank group; compared with oral adjuvant group, the gastric pathological injury decline degree in rBIB oral group increased by 47.9%; compared with rBIB IM group, the gastric pathological injury decline degree in rBIB oral group increased by 27.1%; compared with rIB recombinant group and rCTB+rIB group, the gastric pathological injury decline degree in rBIB oral group increased by 47.9% and 45.8%, respectively, and there were significantly differences between the groups (P = 0.000). The results are shown in Table 5.
Table 5.
Results of pathological scores of different groups
| Groups | Pathological scores of gastric in each group (n = 6) | Degree of gastric mucosa injury | Declined degree of injury | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Inflammation | Hemorrhage | Edema | H. pylori | Necrosis | Atrophy | ||||
| PBS | 18 | 18 | 18 | 18 | 24 | 18 | 79.2% | 20.8% | |
| Intramuscular injection | CTB | 18 | 9 | 9 | 24 | 18 | 18 | 66.7% | 33.3% |
| IB | 18 | 12 | 9 | 24 | 18 | 12 | 64.6% | 35.4% | |
| CTB+IB | 18 | 12 | 6 | 18 | 18 | 18 | 62.5% | 37.5% | |
| BIB | 12 | 6 | 6 | 12 | 12 | 12 | 41.7% | 58.3% | |
| Oral group | CTB | 18 | 9 | 9 | 24 | 18 | 12 | 62.5% | 37.5% |
| IB | 12 | 12 | 12 | 18 | 24 | 12 | 60.4% | 39.6% | |
| CTB+IB | 18 | 12 | 9 | 24 | 12 | 12 | 62.5% | 37.5% | |
| BIB | 9 | 0 | 6 | 3 | 3 | 0 | 14.6% | 85.4% | |
Pathological score statistics showed that after challenge of H. pylori, the gastric pathological injury degree in rBIB oral group was the lowest (only 14.6%) and was lower by 54.6% than non-treated control group (blank control) group; compared with rBIB IM group, the gastric pathological injury degree in oral rBIB group decreased by 27.1%; compared with oral adjuvant group, the gastric pathological injury decline degree in oral rBIB group increased by 47.9%; compared with rCTB adjuvant group, rIB recombinant group and rCTB+rIB group, the gastric pathological injury decline degree in oral rBIB group was higher by 47.9%, 47.9% and 45.8%, respectively. There was significantly difference between the groups (P = 0.000). The results are shown in Figure 5.
Figure 5.
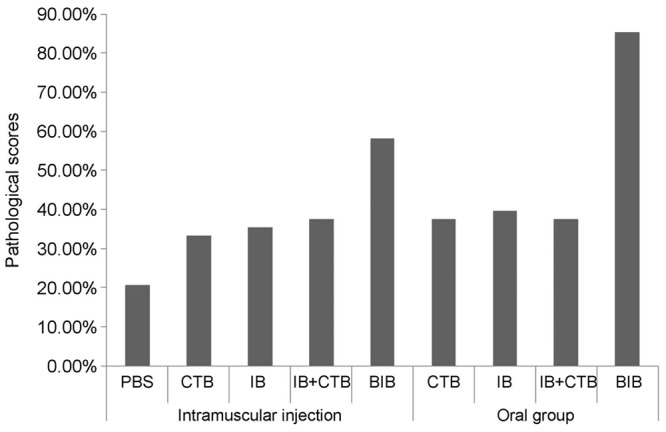
Pathological injury score of the infected stomach in each group of mice.
Discussion
Our study compared differences of immune responses produced by three combination ways, i.e., rIB, rIB+rCTB and rBIB, with two medication routes, i.e., intramuscular injection and oral administration, and then evaluated immune protective effects generated by different immunizations through challenge of H. pylori. Detection results of serum specific IgG antibody showed: among IM groups, on day 14 after immunization, the titers of specific IgG antibody in rIB+rCTB group and rBIB group were 50 and 100, respectively, and in group with rIB alone, group with rCTB alone and non-immunized control group, specific IgG antibody had not been produced; after immunization twice, rIB and rIB+rCTB group titers were all 1200 as well as rBIB group titer reached 2800; after immunization three times, rIB group antibody titer was 2000, rCTB+rIB group antibody titer was 5000 and rBIB group antibody titer was 7500, significantly higher than other groups. The above results have indicated that as the extramolecular adjuvant and intramolecular adjuvant, while rCTB enhances the humoral immune response, it accelerates the humoral immunity response and the intramolecular adjuvant effect is stronger. Detection results of serum specific IgA antibody showed: every IM group had not produced serum specific IgA antibody; among oral groups, only rBIB oral group with rCTB as intramolecular adjuvant had produced specific IgA antibody significantly higher than other groups, indicating that rCTB can enhance mucosal immunity and the intramolecular adjuvant effect is strong. These results have demonstrated that rBIB containing intramolecular adjuvant is the best vaccine antigen component.
At present most studies believe that specific IgA is the key to clear H. pylori [11,12], similar to the results of this study. In this study, each of IM groups (rIB, rCTB, rBIB, rCTB+rIB) had produced high level of IgG antibody by induction, but could not produce serum IgA antibody by induction; rBIB oral group not only produced high level of IgG antibody by inducing blood, but also produced specific IgA by induction. In the experiment for evaluating protective effect, rBIB oral group protection rate was as high as up to 83.3%, and the gastric pathological injury degree was the lowest (only 14.6%), and its pathological injury decreased by 54.6% than non-treated control group (blank control); compared with rBIB IM group, its pathological injury degree decreased by 27.1%. Compared with rCTB adjuvant group, rIB recombinant group and rCTB+rIB group, the pathology injury decline degree in rBIB oral group increased by 47.9%, 47.9%, 45.8%, respectively. rBIB oral immunization had the highest infection protection rate against H. pylori and the highest pathological injury decline rate, demonstrating that oral immunization is the best immunization route of rBIB vaccine. This study has provided solid research data for new H. pylori vaccine components and immune routes and lays the foundation for further evaluating rBIB as a new vaccine.
Disclosure of conflict of interest
None.
References
- 1.Zhang WD, Hu FL, Xiao SD, Xu ZM. Prevalence of Helicobacter pylori infection in China. Mode Dige & Inte. 2010;15:265–270. [Google Scholar]
- 2.Meng LM, Zhou LY, Lin SR, Yan XE, Ding SG, Huang YH, Gu F, Zhang L, Li Y, Cui RL, Zhang DH, Zhang J. The relationship between Helicobacter pylori and peptic ulcer: A 10-year follow-up study. Chin J Dige. 2009;29:361–364. [Google Scholar]
- 3.Multiple Center Study Group In Beijing Area, China. [Effects of different triple therapies on duodenal ulcer-associated Helicobacter pylori infection and a one-year follow-up study] Zhonghua Yi Xue Za Zhi. 2004;84:1161–1165. [PubMed] [Google Scholar]
- 4.Del Giudice G, Malfertheiner P, Rappuoli R. Development of vaccines against Helicobacter pylori. Expert Rev Vaccines. 2009;8:1037–1049. doi: 10.1586/erv.09.62. [DOI] [PubMed] [Google Scholar]
- 5.Velin D, Michetti P. Advances in vaccination against Helicobacter pylori. Expert Rev Gastroenterol Hepatol. 2010;4:157–166. doi: 10.1586/egh.10.6. [DOI] [PubMed] [Google Scholar]
- 6.Ihan A, Pinchuk IV, Beswick EJ. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter. 2012;17(Suppl 1):16–21. doi: 10.1111/j.1523-5378.2012.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabir S. The current status of Helicobacter pylori vaccines: a review. Helicobacter. 2007;12:89–102. doi: 10.1111/j.1523-5378.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 8.Moise L, Moss SF, De Groot AS. Moving Helicobacter pylori vaccine development forward with bioinformatics and immunomics. Expert Rev Vaccines. 2012;11:1031–1033. doi: 10.1586/erv.12.80. [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Liu K, Zhao W, Li X, Li T, Tang F, Zhang R, Wu W, Xi T. Immunological features and efficacy of the reconstructed epitope vaccine CtUBE against Helicobacter pylori infection in BALB/c mice model. Appl Microbiol Biotechnol. 2013;97:2367–2378. doi: 10.1007/s00253-012-4486-1. [DOI] [PubMed] [Google Scholar]
- 10.Rogers AB. Histologic scoring of gastritis and gastric cancer in mouse models. Methods Mol Biol. 2012;921:189–203. doi: 10.1007/978-1-62703-005-2_22. [DOI] [PubMed] [Google Scholar]
- 11.Guo TS, Zou QM, Guo G, Xie QH, Liu KY, Zeng WK, Gao ZG. Experimental study of BALB/c mice orally immunized by recombinant Hp vaccine. Chin J Microbio and Immuno. 2005;25:239–242. [Google Scholar]
- 12.Liu CJ, Zhang ZS. Host immune responses to Helicobacter pylori infection and implications for vaccine development. Letters in Biotechnology. 2003;14:48–50. [Google Scholar]


