Abstract
Background: The predictors for the involvement of lymph node (LN) have been widely studied. But the implication of the molecular type has not been well studied. Using the database of our institution, we investigated this relation. Methods: Patients with T1 and T2 primary breast cancer without distant metastasis were included in our study from 2012 Jan to 2013 Dec. All patients undertook the resection of the primary and the axillary lymph nodes (ALNs). We collected the clinical data including age at diagnosis, the status of ER, PR and HER2, tumor size, nodal status, and histological type. The relationship between demographic, tumor characteristics and lymph node status was evaluated. Results: 814 patients were included in our study. The number and the percentage (in parentheses) of each type of breast cancer is as follows: Luminal A 230 (28.3%), Luminal Her2- 284 (34.9%), Luminal Her2+ 104 (12.8%), HER2+ 72 (8.8%), TNBC 124 (15.2%). On univariate and multivariate analysis, tumor size and tumor subtype show statistical significance with LN involvement. Using TNBC as a reference, both Luminal B type (Luminal HER2-, Luminal HER2+) shows significant higher probability of LN involvement. Conclusions: LN involvement is an intrinsic characteristic for molecular subtype of breast cancer. Triple positive and triple negative breast cancer accounts the most and least possibility of LN involvement.
Keywords: Breast cancer subtypes, axillary lymph node involvement
Introduction
Breast cancer is highly heterogeneous, conferring different progression, treatment and prognosis [1,2]. Molecular type based on the high throughput technology has revolutionized the opinions and the treatment of breast cancer [3]. In addition, the traditional IHC based molecular type showed the highly consistency with the genetic expression, conferring the similar prognostic values [4]. So in our study, IHC based classification were taken.
Lymph node status is critical for the treatment of breast cancer. Axillary Lymph Node Dissection (ALND) is based on the sequential metastasis in lymphatic vessels and has been proven a success by the Halsted radical mastectomy compared with local excision. The use of Sentinel Node Biopsy (SNB) has shown perfect predictability, thus avoiding unnecessary ALND and the following morbidity. Novel clinical trials have challenged the recent opinions in axillary treating and two recent systemic reviews [5,6] reach the new recommendations. ALND should not be appropriate for patients undertaking breast conserving surgery and whole-breast radiation with less than 3 metastatic axillary lymph nodes [5].
Despite of some relevant studies [7-10], the data of the relation between ALN status and molecular type is insufficient. And there are controversies about the role of LN involvement as an intrinsic characteristic. The aim of this study is to identify the relation between ALN status and molecular subtype.
Patients and methods
Inclusion and exclusion criteria of the study
A prospective database between 2012 Jan and 2013 Dec was reviewed for this study. All patients were included consecutively. Inclusion criteria included: 1. Primary tumor without distant metastases; 2. All patients underwent resection of primary cancer and ALNs for definitive LN staging; 3. All patients are female of Han nationality; 4. All patients were at T1 or T2 stage. Exclusion criteria included: 1. patients with recurrent tumor; 2. diagnosis of In situ breast cancer only. Clinicopathological data was collected as follows: age at diagnosis, pathological tumor size, ALN status, histological type and IHC biomarkers for molecular subtyping.
Criteria for the molecular subtype and other clinical data
Five molecular types were determined according to the current guideline [11]. The categorization were made as follows: Luminal A (ER+/PR+, HER2-, Ki67 < 14% or PR ≥ 20%); Luminal Her2- (ER+/PR+, HER2-, Ki67 ≥ 14% or PR < 20%); Luminal HER2+ (ER+/PR+, HER2+); HER2+ (ER-, PR-, HER2+); TNBC (ER-, PR-, HER2-). ER/PR was conceived to be positive, if the percentage of nuclear-staining cancer cells is no less than 1%. Both HER2 FISH (Fluorescence in situ hybridization) and IHC test were used for the confirmation of the status of HER2/neu. IHC 3+ or FISH+ was conceived to be positive of HER2 expression.
Lymph node was considered positive according to the HE staining and IHC test. Tumor size was calculated by the largest diameters pathologically. Categorization for Tumor size, nodal status, and staging were made by the American Joint Committee on Cancer (AJCC) TNM staging system for Breast cancer.
Statistical analysis
Continuous data was shown as the median [interquartile range (IQR)] or mean (SD), and the categorical data as the number (percentage). We categorized lymph node status negative and positive. Demographic and tumor characteristics were compared across categories of lymph node status using one-way ANOVA or rank sum test for continuous variables and Chi-square test for categorical variables. Univariate and multivariate logistic regression were applied to assess the influencing factors of lymph node metastases in breast cancer. Furthermore, a nomogram was depicted to show the result of multivariate logistic regression. A 2-sided P < 0.05 was considered statistically significant. We performed statistical analysis using the software of SAS.
Results
In our cohort, 814 patients are identified, 343 (42.1%) patients with ALNM (Table 1). The mean age of our cohort is 54.1 years old. The most histological type is invasive ductal type. The number and the percentage (in parentheses) of each type of breast cancer is as follows: luminal A 230 (28.3%), Luminal Her2- 284 (34.9%), luminal Her2+ 104 (12.8%), HER2+ 72 (8.8%), TNBC 124 (15.2%).
Table 1.
Demographic and tumor characteristics
| Variable | Population (n = 814) |
|---|---|
| Age | 54.1 ± 9.8 |
| LN positive (%) | 42.1 (343/814) |
| Histology subtype (%) | |
| IDC | 87.1 (709/814) |
| ILC | 2.9 (24/814) |
| Others | 10.0 (81/814) |
| ER positive (%) | 74.4 (606/814) |
| PR positive (%) | 54.2 (441/814) |
| HER2 positive (%) | 23.7 (193/814) |
| Molecular subtype (%) | |
| Luminal A | 28.3 (230/814) |
| Luminal Her2- | 34.9 (284/814) |
| Luminal Her2+ | 12.8 (104/814) |
| Her2+ | 8.8 (72/814) |
| TNBC | 15.2 (124/814) |
| Tumor size | 2.0 (1.4, 2.5) |
| T (%) | |
| T1 | 69.8 (568/814) |
| T2 | 30.2 (246/814) |
| N (%) | |
| N0 | 57.9 (471/814) |
| N1 | 26.4 (215/814) |
| N2 | 9.8 (80/814) |
| N3 | 5.9 (48/814) |
| Stage (%) | |
| 1 | 44.2 (360/814) |
| 2 | 40.0 (326/814) |
| 3 | 15.7 (128/814) |
As shown in Table 2, the tumor size is positively relevant with the LN positivity significantly. Based on the up-to-date AJCC classification, LN positivity in T1 and T2 is 36.6% and 54.9% respectively. The correlation between the frequency of LN metastasis and each T stage is shown in Figure 1. There are differences in LN positivity by molecular types, shown by the chi-square test. Furthermore, luminal Her2+ is deemed with the highest LN positivity (49.0%), followed by luminal Her2- (46.8), HER2+ (44.4%), Luminal A (36.5%) and TNBC (34.7%), which was shown in Figure 2 in the form of frequency.
Table 2.
Demographic and tumor characteristics by lymph node status
| Variable | LN negative (n = 343) | LN positive (n = 471) | P value |
|---|---|---|---|
| Age | 54.4 ± 9.9 | 53.7 ± 9.7 | 0.285 |
| Histology subtype (%) | |||
| IDC | 56.7 (402/709) | 43.3 (307/709) | 0.217 |
| ILC | 66.7 (16/24) | 33.3 (8/24) | |
| Others | 65.4 (53/81) | 34.6 (54/82) | |
| ER positive (%) | 56.4.9 (342/606) | 43.6 (264/606) | 0.167 |
| PR positive (%) | 55.8 (246/441) | 44.2 (195/606) | 0.2 |
| HER2 positive (%) | 52.8 (102/193) | 47.2 (91/196) | 0.113 |
| Molecular subtype (%) | |||
| Luminal A | 63.5 (146/230) | 36.5 (84/234) | 0.032 |
| Luminal Her2- | 53.2 (151/284) | 46.8 (133/284) | |
| Luminal Her2+ | 51.0 (53/104) | 49.0 (51/104) | |
| Her2+ | 55.6 (40/72) | 44.4 (32/72) | |
| TNBC | 65.3 (81/124) | 34.7 (43/124) | |
| Tumor size | 1.5 (1.2, 2.0) | 2.0 (1.5, 2.5) | < 0.001 |
| T (%) | |||
| T1 | 63.4 (360/568) | 36.6 (208/568) | < 0.001 |
| T2 | 45.1 (111/246) | 54.9 (135/246) | |
| Stage (%) | |||
| 1 | 100.0 (360/360) | 0.0 (0/360) | < 0.001 |
| 2 | 34.0 (111/326) | 62.7 (215/326) | |
| 3 | 0.0 (0/128) | 100.0 (128/128) | |
Figure 1.
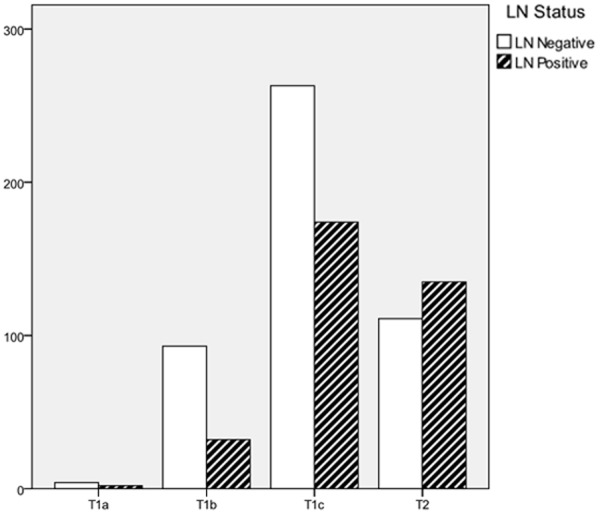
The frequency of lymph node positivity according to the T categorization.
Figure 2.
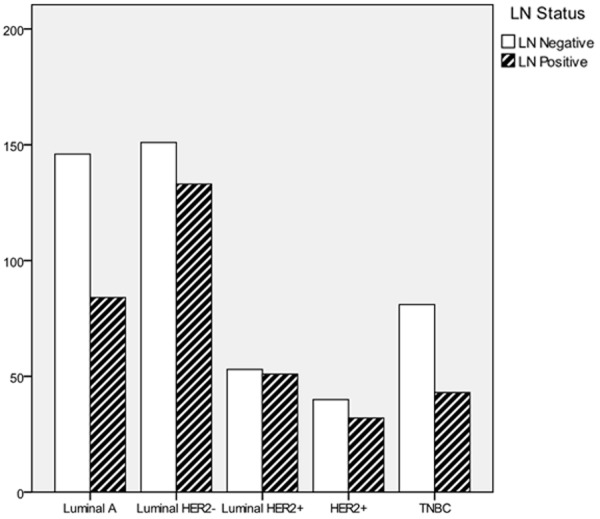
The frequency of lymph node positivity according to the molecular subtype.
For further prediction of the lymph nodal status, univariate and multivariate logistic regression models were used (Table 3). Tumor size shows a significant correlation with the ALN status. Compared with TNBC, both luminal B types show higher relevance with ALN status. Compared with the TNBC, the adjusted OR value is 1.993, 1.954, 1.666, 1.653, and 1.388 for luminal Her2+, luminal Her2-, HER2+ and luminal A respectively. A nomogram (Figure 3) calculates the risk factors of ALNM by tumor size and molecular subtype. The logistic regression model shows tumor size is the stronger indicator for LN positivity than molecular type.
Table 3.
Univariate and multivariate logistic regression models for lymph node positive breast cancer
| Covariate | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age | 0.992 (0.978-1.006) | 0.285 | ||
| Histology subtype (versus IDC) | ||||
| ILC | 0.655 (0.277-1.550) | 0.335 | ||
| Others | 0.692 (0.427-1.120) | 0.134 | ||
| ER positive | 1.260 (0.913-1.741) | 0.16 | ||
| PR positive | 1.205 (0.911-1.594) | 0.191 | ||
| HER2 positive | 1.306 (0.944-1.808) | 0.107 | ||
| Molecular subtype (versus TNBC) | ||||
| Luminal A | 1.084 (0.686-1.712) | 0.73 | 1.388 (0.862-2.237) | 0.178 |
| Luminal Her2- | 1.659 (1.072-2.569) | 0.023 | 1.954 (1.241-3.075) | 0.004 |
| Luminal Her2+ | 1.813 (1.063-3.090) | 0.029 | 1.993 (1.152-3.447) | 0.014 |
| Her2+ | 1.507 (0.832-2.729) | 0.176 | 1.666 (0.906-3.064) | 0.101 |
| Tumor size | 1.629 (1.372-1.933) | < 0.001 | 1.653 (1.387-1.970) | < 0.001 |
| T (versus T1) | ||||
| T2 | 2.105 (1.554-2.852) | < 0.001 | ||
Figure 3.
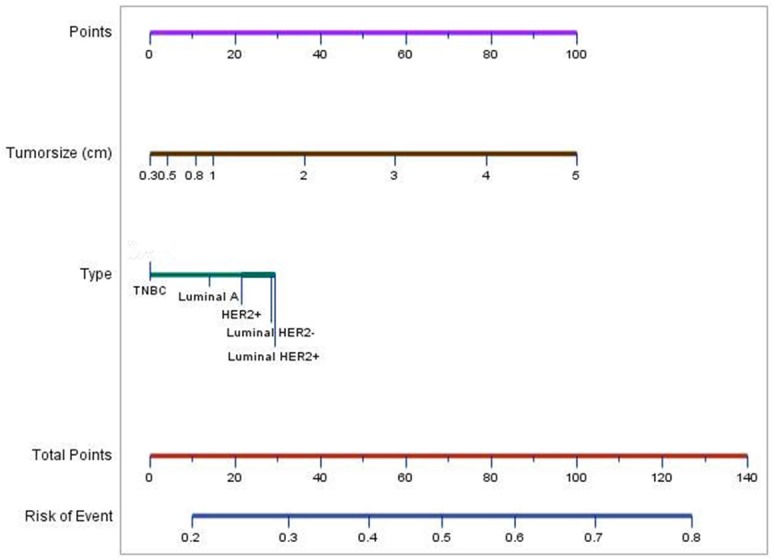
Nomogram to calculate the possibility of ALNM in breast carcinoma. To calculate the risk of ALNM, first, identify the points of tumor size and type in the first line respectively; second, combine the two points in the line of total points; last, identify the risk of ALNM in the lowest line.
Discussion
Our study shows the highest occurrence of LN metastases in triple positive breast cancer, and the lowest occurrence in TNBC. But for each biomarker used for subtying, no statistically significance was found. It shows a greater prognositic value of the combined phenotype. In addition, tumor size shows a positive correlation with the LN involvement.
The accurate prediction of LN status is a prerequisite for treatment decision. Principally, palpable LNs in patients with advanced breast cancer should be preceded with ALD without cytological or histological confirmation. For patients without any evidence of LN metastases, SNB should be performed to confirm the status of ALNs [5]. Accurate and convenient prediction of the LN status is critical for regional management of breast cancer.
The high throughput technology has brought a new breakthrough in the last decade, and valuable information has been provided [12]. Peru et al classified the breast cancer into subtypes by the genetic information for the first time [13]. However, due to the high economic outlay, the genetic classification has not been widely used clinically. Instead, IHC based molecular type shows high consistency with the genetically intrinsic type and highly cost-efficiency [14]. In our study, significant difference in the distribution of ALN positivity was found between different subtypes of breast cancer. It may suggest the different aggressiveness for different types. So LN involvement is an intrinsic characteristic of each molecular type. Definitely, the involvement of the LNs indicates a higher aggressiveness, more than a just later period of the tumor progression [15,16]. The distribution of LN involvement in each molecular subtype is similar with the study of Van Calster et al [17] and Noguchi M et al [16]. For the differences of the relevant results, different inclusion criteria and different standard for categorization contributes a lot. The standard of positivity for ER is ranging from 1% to 10%. The patients in our study were all in T1 or T2 study, which may reduce the influence of the tumor size. However, tumor size which is still a powerful influencing factor.
Predictors used to identify the ALN status has been studied before [9,15,18-24]. Tumor size is the most valuable predictor for ALN status in patients with breast cancer, which confers different strategies for people in the different T stage [23]. Besides, potential predictors include lymphavascular invasion (LVI) [10,15,25], age at diagnosis [10], and so on.
There are several limits in our study. First, there is paucity of the potential predictors: histological grade, lymph vascular invasion (LVI), other biomarkers (Ki67, P53). Different standard were used to determine the tumor grade, so histological grade was not assessed in our study. Ki67 were not assessed in all of our patients, so the categorization of molecular types was made by the PR in those patients who did not receive the Ki67 assessment. So a comprehensive regression model was not reached. 2 the study was not conducted in a prospective way, which may introduce unexpected bias.
In our study, LN involvement is an intrinsic characteristic for molecular subtype of breast cancer. Triple positive and triple negative breast cancer accounts the most and least possibility of LN involvement. But new combined biomarkers or new convenient technology is needed to predict the LN status correctly in the future.
Acknowledgements
The authors thank Li Sun from Zhongshan Hospital, Fudan University and Peng Zhang from School of Public Health, Fudan University for the assistance of statistical analysis.
Disclosure of conflict of interest
None.
References
- 1.Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, Dekhne NS. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007;110:1687–96. doi: 10.1002/cncr.22981. [DOI] [PubMed] [Google Scholar]
- 2.Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 2012;21:289–95. doi: 10.1016/j.breast.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirapati P, Sotiriou C, Kunkel S, Farmer P, Pradervand S, Haibe-Kains B, Desmedt C, Ignatiadis M, Sengstag T, Schutz F, Goldstein DR, Piccart M, Delorenzi M. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AR, Bosserman LD, Burstein HJ, Cody HR, Hayman J, Perkins CL, Podoloff DA, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2014;32:1365–83. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 6.Rao R, Euhus D, Mayo HG, Balch C. Axillary Node Interventions in Breast Cancer: A Systematic Review. JAMA. 2013;310:1385–94. doi: 10.1001/jama.2013.277804. [DOI] [PubMed] [Google Scholar]
- 7.Howland NK, Driver TD, Sedrak MP, Wen X, Dong W, Hatch S, Eltorky MA, Chao C. Lymph node involvement in immunohistochemistry-based molecular classifications of breast cancer. J Surg Res. 2013;185:697–703. doi: 10.1016/j.jss.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones T, Neboori H, Wu H, Yang Q, Haffty BG, Evans S, Higgins S, Moran MS. Are breast cancer subtypes prognostic for nodal involvement and associated with clinicopathologic features at presentation in early-stage breast cancer? Ann Surg Oncol. 2013;20:2866–72. doi: 10.1245/s10434-013-2994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Kim SH, Suh YJ, Shim BY, Kim HK. Predictors of axillary lymph node metastases (ALNM) in a Korean population with T1-2 breast carcinoma: triple negative breast cancer has a high incidence of ALNM irrespective of the tumor size. Cancer Res Treat. 2010;42:30–6. doi: 10.4143/crt.2010.42.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga JY, Alran S, Salmon RJ, Fourchotte V, Vincent-Salomon A, Sastre-Garau X, Antoine M, Uzan S, Sigal-Zafrani B, De Rycke Y. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6:e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumors. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehm T, Maul H, Gebauer S, Scharf A, Baier P, Sohn C, Jager W, Gebauer G. Prediction of axillary lymph node status of breast cancer patients by tumor biological factors of the primary tumor. Strahlenther Onkol. 2005;181:580–6. doi: 10.1007/s00066-005-1374-y. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M, Kurosumi M, Iwata H, Miyauchi M, Ohta M, Imoto S, Motomura K, Sato K, Tsugawa K. Clinical and pathologic factors predicting axillary lymph node involvement in breast cancer. Breast Cancer. 2000;7:114–23. doi: 10.1007/BF02967442. [DOI] [PubMed] [Google Scholar]
- 17.Van Calster B, Vanden BI, Drijkoningen M, Pochet N, Cheng J, Van Huffel S, Hendrickx W, Decock J, Huang HJ, Leunen K, Amant F, Berteloot P, Paridaens R, Wildiers H, Van Limbergen E, Weltens C, Timmerman D, Van Gorp T, Smeets A, Van den Bogaert W, Vergote I, Christiaens MR, Neven P. Axillary lymph node status of operable breast cancers by combined steroid receptor and HER-2 status: triple positive tumours are more likely lymph node positive. Breast Cancer Res Treat. 2009;113:181–7. doi: 10.1007/s10549-008-9914-7. [DOI] [PubMed] [Google Scholar]
- 18.Chua B, Ung O, Taylor R, Boyages J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. Anz J Surg. 2001;71:723–8. doi: 10.1046/j.1445-1433.2001.02266.x. [DOI] [PubMed] [Google Scholar]
- 19.Markopoulos C, Kouskos E, Gogas H, Mandas D, Kakisis J, Gogas J. Factors affecting axillary lymph node metastases in patients with T1 breast carcinoma. Am Surg. 2000;66:1011–3. [PubMed] [Google Scholar]
- 20.Patani NR, Dwek MV, Douek M. Predictors of axillary lymph node metastasis in breast cancer: a systematic review. Eur J Surg Oncol. 2007;33:409–19. doi: 10.1016/j.ejso.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Rivadeneira DE, Simmons RM, Christos PJ, Hanna K, Daly JM, Osborne MP. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6. doi: 10.1016/s1072-7515(00)00310-0. discussion 6-8. [DOI] [PubMed] [Google Scholar]
- 22.Xie F, Yang H, Wang S, Zhou B, Tong F, Yang D, Zhang J. A logistic regression model for predicting axillary lymph node metastases in early breast carcinoma patients. Sensors (Basel) 2012;12:9936–50. doi: 10.3390/s120709936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip CH, Taib NA, Tan GH, Ng KL, Yoong BK, Choo WY. Predictors of axillary lymph node metastases in breast cancer: is there a role for minimal axillary surgery? World J Surg. 2009;33:54–7. doi: 10.1007/s00268-008-9782-7. [DOI] [PubMed] [Google Scholar]
- 24.Zetterlund L, Stemme S, Arnrup H, de Boniface J. Incidence of and risk factors for sentinel lymph node metastasis in patients with a postoperative diagnosis of ductal carcinoma in situ. Br J Surg. 2014;101:488–94. doi: 10.1002/bjs.9404. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Lu X, Wang ZC, Iglehart JD, Zhang X, Richardson AL. Predicting features of breast cancer with gene expression patterns. Breast Cancer Res Treat. 2008;108:191–201. doi: 10.1007/s10549-007-9596-6. [DOI] [PubMed] [Google Scholar]


