Abstract
Malignant mesotheliomas of the testis arise from the tunica vaginalis, formed from the evagination of the abdominal peritoneum into the scrotum. It is an extremely rare tumor representing 0.3% to 5% of all malignant mesotheliomas. We presented an interesting case of 68-year-old male with swelling and slightly painful in the right scrotum. Histologically, the lesion were composed of small tubular, microcystic, gland lined by flattened epithelioid cells and vague signet ring cells set in a myxofibrous stroma, which is resemblance to adenomatoid tumor. But the tumor cells showed significant atypical cytologic morphology and invaded into spermatic cord tissue, which indicated the diagnosis of malignant tumor. Immunohistochemistry study showed positive expression of CK, CK5/6, CK7, Calretinin, D2-40 and Vimentin which indicated the diagnosis of malignant mesothelioma. This case of mesothelioma should be classified as epithelial in type. To our knowledge, the mesothelioma of the tunica vaginalis testis with adenomatoid tumor-like microscopic features is very rare.
Keywords: Mesothelioma, tunica vaginalis testis, histology, adenomatoid
Introduction
Mesotheliomas are relatively rare tumors that arise from the serosal surface of the pleura, peritoneum and pericardium. On rare occasions, they originate from the tunica vaginalis of the testis [1]. Malignant mesotheliomas of the testicular tunica vaginalis occurred in patients aged 12-76 years [1]. Hydrocele, with or without an associated mass, or appreciation of a paratesticular mass accounted for the clinical presentation [2]. Some patients had a history of asbestos exposure [3]. It is histologically classified as epithelioid pattern, sarcomatoid pattern, desmoplastic pattern and biphasic pattern. But some times, it has been considered being difficult to diagnose in a substantial subset of cases [4,5]. Herein, we present a case of malignant mesothelioma of the testicular tunica vaginalis in a 68-year-old Chinese male. Microscopically, aggregates of small tubular, cystic, gland lined by flattened epithelioid cells and vague signet ring cells set in a myxofibrous stroma suggest the probably diagnosis of mesothelioma, adenomatoid tumor, yolk sac tumor or metastatic adenocarcinoma. Finally, histological and immunochemistry study support the diagnosis of malignant mesothelioma well. To our knowledge, the malignant mesothelioma with such adenomatoid features should be classified as epithelioid mesothelioma, and this kind of microscopic presentation is very rare in mesothelioma of tunica vaginalis testis [6-8].
Case presentation
A 68-year-old male referred to hospital for complaining of a swelling and slightly painful in the right scrotum 5-month ago. The patient worked as a farmer and denied the exposure history of asbestos. Physical examination demonstrated that the right scrotum apparently enlarged, various sizes of nodules were palpable with slight tenderness, nodules did not disappeared in a supine position. Laboratory examination revealed values of serum alphafetoprotein (α-AFP), alkaline phosphatase (AP), CA19-9, CA125 and prostate specific antigen (PSA) were in normal level. Scrotal ultrasound revealed that liquid dark area in the right scrotum, multiple hyperechoic masses with blood flow inside could be detected on the tunica vaginalis testis. No lesions in other organs including lung, prostate and gastrointestinal were detected. With the consent of the patient himself, radical orchiectomy was performed. The clinical follow-up was done at 3 months and 6 months after the surgery, respectively. No recurrence or metastasis of the tumor was observed.
Materials and methods
The resected specimens were fixed with 10% neutral-buffered formalin and embedded in paraffin blocks. Tissue blocks were cut into 4-μm slides, deparaffinized in xylene, rehydrated with graded alcohols, and immunostained with the following antibodies: cytokeratin (CK, AE1/AE3, 1:50, DAKO), cytokeratin 5/6 (CK 5/6, 1:200, DAKO), cytokeratin 7 (CK 7, 1:200, DAKO), cytokeratin 20 (CK 20, 1:200, DAKO), vimentin (1:200, DAKO), calretinin (1:100, DAKO), CD30 (1:100, DAKO), carcino-embryonic antigen (CEA, 1:100, DAKO), α-fetoprofein (α-AFP, 1:200, DAKO), thyroid transcription factor 1 (TTF-1, 1:100, DAKO), Prostate Specific Antigen (PSA, 1:100, Santa cruz), S-100 (1:100, Santa Cruz), CD34 (1:100, DAKO), α-inhibin (1:100, DAKO), PLAP (1:100, DAKO), and Ki67 (1:200, DAKO). Sections were stained with a streptavidin-peroxidase system (KIT-9720 Ultrasensitive TM S-P, MaiXin, China). The chromogen used was diaminobenzidine tetrahydrochloride substrate (DAB kit, MaiXin, China), slightly counterstained with hematoxylin, dehydrated and mounted. For the negative controls, the primary antibody was replaced with PBS. This study was prospectively performed and approved by the institutional Ethics Committees of China Medical University and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Results
Gross features
Grossly, testis, tunica vaginalis and spermatic cord was approximately 6.8 × 5.4 × 4.6 cm, the tumors presented as multiple nodules (diameter 0.3-1.0 cm) on the surface of testis and tunica vaginalis with unclear boundary. The cut face of the tumor was grey-white or jelly-like in appearance with moderate hardness texture.
Microscopic features
Histologically, the tumor had an infiltrative growth pattern with invasion of the tunica vaginalis and spermatic cord (Figure 1A). Generally, the tumor cells were set in a fibrous or myxoid stroma containing some scattered chronic inflammatory cells, the tumor are composed of small tubular, microcystic, and single cells lined by flattened epithelioid cells set in a myxofibrous stroma (Figure 1B). Our case also shows microcystic structures, adenoid cystic or signet ring appearances. Focally, plump or signet ring epithelioid cell could also form small nest of cells (Figure 1C). Cytologically, variation in cell size and shape could be identified, cyoplasmic vacuolation was markable, nuclear pleomorphism was also seen as a feature (Figure 1D). Focally, the tumor cells showed slightly eosinophilic cytoplasm. Mitotic activity was not easily identified in our case 1 per 10 high power fields.
Figure 1.
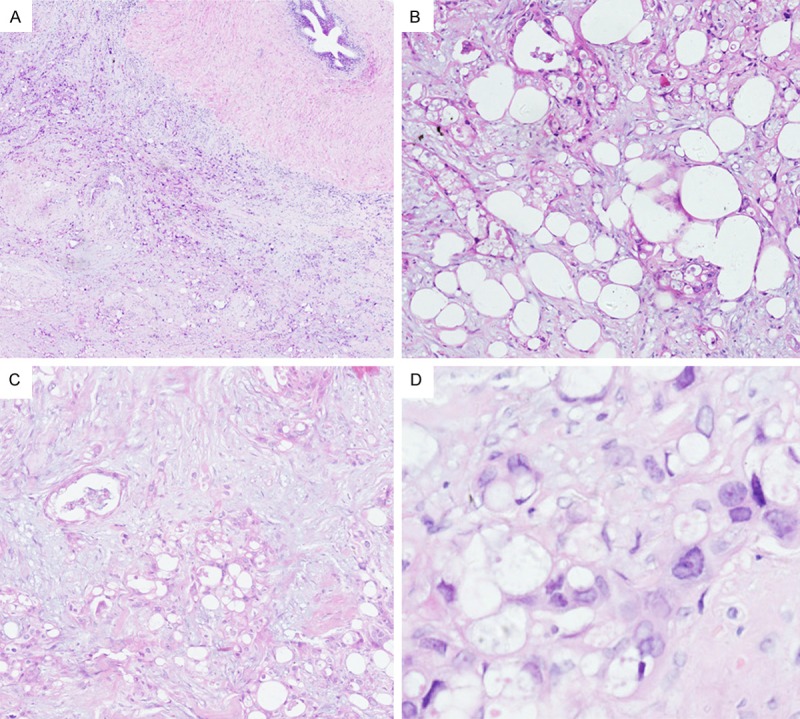
Morphological change of the tumor. (A) Tumor cells had an infiltrative growth pattern with invasion of spermatic cord. (B) The tumor was composed with small tubular, adenoid cystic or signet ring structures set in myxofibrous stroma. (C) Epithelioid cells with slightly eosinophilic cytoplasm formed small tubular, gland and solid nest structures. (D) Variation in cell size and shape could be identified, ctyoplasmic vacuolation was markable, nuclear pleomorphism was also seen as a feature (A, HE × 40. B, HE × 100. C, HE × 100. D, HE × 400).
Immunohistochemistry
Immunohistochemical staining showed that the tumor cells were diffusely positive for CK, CK5/6, CK7, Calretinin, D2-40, focally positive for Vimentin, negative for WT-1, CK20, CEA, CD30, α-AFP, S-100, TTF-1, PSA, PLAP, α-inhibin, CD34 and CDX-2 (Figure 2). Ki67 index was approximate 50% for tumor cells. According to the morphological, immunohistochemical findings and the previously reports, the tumor was diagnosed as a malignant mesothelioma of the tunica vaginalis testis.
Figure 2.
Immunohistochemical staining of the tumor. (A) The cells were entirely positive for CK. (B) The tumor cells were strong positive for Calretinin. (C) Negative expression of CEA was present in the tumor cells. (D) Ki67 index was approximately 50% in mesothelima (A, CK × 100. B, Calretinin × 100. C, CEA × 100. and D, Ki67 × 100).
Discussion
Most mesotheliomas arise from the pleura representing 68% to 85% of all malignant mesothelioma cases, whereas 9.1% to 24.1% of malignant mesotheliomas arise from the peritoneum. Mesothelioma of the tunica vaginalis testis is a distinctly rare tumor, representing only 0.3% to 5% of all mesotheliomas [1,2]. Mesothelioma of the tunica vaginalis testis arises from the serosal membrane of the tunica vaginalis and has a mesenchymal origin. It mostly occurred between ages 55 and 75 [1]. Paratesticular mesotheliomas often demonstrated a palpable tumor associated with a hydrocele or presented with localized soreness or swelling, acute hydrocele, recurrent hydrocele, haematocele and diffuse thickening of the spermatic cord [1-5]. The patient in our case is a 68-year-old male and has relative classic clinical features of mesothelioma according to the previous reports.
According to the microscopical presentation of the HE staining, a variety of neoplasms that involve the paratesticular region must be distinguished from mesothelioma, including, adenomatoid tumor, yolk sac tumor, embryonal carcinoma, and metastatic adenocarcinoma. Benign adenomatoid tumor might be the most important differential diagnosis. The tumor is typically located in the epididymis, often at the lower pole. It is most common in the ages 30 to 50 [9]. It is typically small and well demarcated and devoid of an infiltrative appearance on gross sectioning. Histologically, these tumors are composed of aggregates of small tubular, cystic or single cells lined by flattened epithelioid cells set in a myxofibrous stroma. Most if not all of these histopathologic features were also appreciated in our cases; however, more importantly, no invasive growth and significant atypical cytologic features should be identified in true adenomatoid tumors [10-12]. In our case, low power microscopy illustrated the obviously invasive growth pattern of the tumor cells, and the high power microscopy illustrated significant atypical cytologic features, as well as the Ki67 index (50%), which could be distinguished from adenomatoid tumors.
Yolk sac tumor could also present the microcystic, reticular, and glandular-alveolar pattern. But clinically, yolk sac tumor always happens in children and adolescents. It always presents asymptomatic right scrotal mass and typically locates in the testis. Most patients are lack of acute hydrocele, recurrent hydrocele, haematocele or diffuse thickening of the spermatic cord. Increased serum α-AFP could be seen in 90% patients [13]. The above clinical features are very helpful to exclude the diagnosis of yolk sac tumor. In addition, the microscope findings and negative expression of α-AFP did not support the diagnosis of yolk sac tumor [13,14]. Embryonal carcinoma some times could invade into epididymal and testicular tunica vaginalis, and gland pattern could be seen in microscope findings. These features might mimic with mesotheliomas, but the tumor cells usually arranged in various structures, such as solid, papillary and slit shaped pattern, the cell morphology of embryonal carcinoma is usually undifferentiated, cell nucleus cytoplasm ratio is very high, and mitotic activity is common [15], these features are not seen in our case. In addition, CD30 negative expression is helpful to exclude the diagnosis of embryonal carcinoma [16].
In the age group in which mesotheliomas most commonly occur, metastatic carcinoma is also a serious consideration. The vast majority of intrascrotal metastases involve only the testicular parenchyma, and a gross distribution similar to that of mesothelioma would be unusual. The adenocarcinoma including prostate, pulmonary adenocarcinoma and gastrointestinal should be distinguishable from mesothelioma on both morphologic and immunohistochemical grounds. Clinical images showed no other tumor like lesion in other organs beside the tunica vaginalis testis lesion, which supports that the tumor of tunica vaginalis testis is primary. In addition, immunohistochemical staining is negative for PSA, TTF-1, CDX2, which is very helpful for leading to the correct diagnosis of mesotheliomas of tunica vaginalis testis.
Immunohistochemistry staining showed diffused positive for CK, CK5/6, CK7, Calretinin; focally positive for vimentin; negative for CEA, CK20, which support the diagnosis of mesothelioma of tunica vaginalis testis. Negative expression of PLAP, α-inhibin is helpful to exclude the diagnosis of seminoma and some sex cord/gonadal stromal tumors. In general, mesotheliomas of tunica vaginalis testis are very rare. Microscopically about 75% of mesotheliomas of tunica vaginalis testis will be purely epithelial in type while the others are biphasic, with varying amounts of the sarcomatoid morphology. Most of the tumors are epithelial with papillary, tubulopapillary, or solid patterns. While our case shows the prominent adenomatoid feature such like: microcystic structures, adenoid cystic or signet ring appearances, aggregates of small tubular, cystic or single cells lined by flattened epithelioid cells set in a myxofibrous stroma. This mesothelioma should be classified as epithelial in type.
In conclusion, our reported case demonstrates a primary malignant tumor of tunica vaginalis testis in a 68-year-old male. The clinical examination, microscopic features and immunochemistry staining support the diagnosis of mesothelioma with prominent adenomatoid features. To our knowledge, mesothelioma with such features is very rare, especially at tunica vaginalis testis.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81272606, No. 81071905) to Dr. EH Wang and National Natural Science Foundation of China to Dr. LH Yang (No. 81301930), Dr. JH Yu (81301837) , Dr. LX Yong (81401885), Dr. HT Xu (81372497).
Disclosure of conflict of interest
None.
References
- 1.Jones MA, Young RH, Scully RE. Malignant mesothelima of the tunica vaginalis. A clinicopathologic analysis of 11 cases with review of the literature. Am J Surg Pathol. 1995;19:815–825. doi: 10.1097/00000478-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Antman K, Hassan R, Eisner M, Ries LA, Edwards BK. Update on malignant mesothelioma. Oncology (Williston Park) 2005;19:1301–1309. [PubMed] [Google Scholar]
- 3.Mensi C, Pellegatta M, Sieno C, Consonni D, Riboldi L, Bertazzi PA. Mesothelioma of tunica vaginalis testis and asbestos exposure. BJU Int. 2012;110:533–537. doi: 10.1111/j.1464-410X.2012.10932.x. [DOI] [PubMed] [Google Scholar]
- 4.Chekol SS, Sun CC. Malignant mesothelioma of the tunica vaginalis testis: diagnostic studies and differential diagnosis. Arch Pathol Lab Med. 2012;136:113–117. doi: 10.5858/arpa.2010-0550-RS. [DOI] [PubMed] [Google Scholar]
- 5.Trpkov K, Barr R, Kulaga A, Yimaz A. Mesothelioma of tunica vaginalis of “uncertain malignant potential”an evolving concept: case report and review of the literature. Diagn Pathol. 2011;6:78. doi: 10.1186/1746-1596-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Kawashima K, Serizawa H, Miura H, Kyeongil K. Adenomatoid mesothelioma with intranuclear inclusion bodies: A case report with cytological and histological findings. Diagn Cytopathol. 2014;42:436–40. doi: 10.1002/dc.22932. [DOI] [PubMed] [Google Scholar]
- 7.Lingamfelter DC, Cavuoti D, Gruszecki AC. Fatal hemopericardial tamponade due to primary pericardial mesothelioma: a case report. Diagn Pathol. 2009;4:44. doi: 10.1186/1746-1596-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissferdt A, Kalhor N, Suster S. Malignant mesothelioma with prominent adenomatoid features: a clinicopathologic and immunohistochemical study of 10 cases. Ann Diagn Pathol. 2011;15:25–29. doi: 10.1016/j.anndiagpath.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.González Resina R, Carranza Carranza A, Congregado Córdoba J, Conde Sánchez JM, Congregado Ruiz CB, Medina López R. Paratesticular adenomatoid tumor: a report of nine cases. Actas Urol Esp. 2010;34:95–100. [PubMed] [Google Scholar]
- 10.Jain S, Dhingra KK, Kohli K, Mahajan N, Khurana N. Adenomatoid tumor of epididymis with signet ring change: a morphological curiosity. Cytopathology. 2012;23:202–203. doi: 10.1111/j.1365-2303.2011.00850.x. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez Maestro M, Tur Gonzalez R, Alonso Dorrego JM, Jesus De la Peña Barthel J, Nistal Martin De Serrano M. Adenomatoid tumors of the epididymis and testicle: report of 9 cases and bibliographic review. Arch Esp Urol. 2009;62:137–141. doi: 10.4321/s0004-06142009000200009. [DOI] [PubMed] [Google Scholar]
- 12.Llarena Ibarguren R, Rodríguez JG, Olano Grasa I, Azurmendi Arín I, Cantón Aller E, Pertusa Peña C. Adenomatoid tumor of the epididymis. Report of five cases. Arch Esp Urol. 2008;61:831–834. doi: 10.4321/s0004-06142008000700013. [DOI] [PubMed] [Google Scholar]
- 13.Kao CS, Idrees MT, Young RH, Ulbright TM. Solid pattern yolk sac tumor: a morphologic and immunohistochemical study of 52 cases. Am J Surg Pathol. 2012;36:360–367. doi: 10.1097/PAS.0b013e31823c510b. [DOI] [PubMed] [Google Scholar]
- 14.Cao D, Humphrey PA. Yolk sac tumor of the testis. J Urol. 2011;186:1475–1476. doi: 10.1016/j.juro.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Williamson SR, Kum JB, Shah SR, Einhorn LH, Eble JN, Cheng L, Ulbright TM, Idrees MT. Signet ring cell carcinoma of the testis: clinicopathologic and molecular evidence for germ cell tumor origin-a case report. Am J Surg Pathol. 2012;36:311–315. doi: 10.1097/PAS.0b013e31823fb7f2. [DOI] [PubMed] [Google Scholar]
- 16.Giannatempo P, Paolini B, Miceli R, Raggi D, Nicolai N, Farè E, Catanzaro M, Biasoni D, Torelli T, Stagni S, Piva L, Mariani L, Salvioni R, Colecchia M, Gianni AM, Necchi A. Persistent CD30 expression by embryonal carcinoma in the treatment time course: prognostic significance of a worthwhile target for personalized treatment. J Urol. 2013;190:1919–1924. doi: 10.1016/j.juro.2013.04.057. [DOI] [PubMed] [Google Scholar]



