Abstract
Nodal follicular lymphoma (FL) is typically composed of follicular or nodular proliferation of small cleaved lymphoid cells, presumably derived from germinal center (GC) B cells. The hallmark of FL is t(14;18)(q32;q21) chromosomal translocation, which juxtaposes anti-apoptotic gene BCL2 to immunoglobulin heavy chain (IGH) promoter. Reflecting this background, FL cells are immunohistochemically positive for BCL2 as well as GC B cell markers CD10 and BCL6. It is known that low grade B-cell lymphomas, including FL, chronic lymphocytic leukemia/small lymphocytic lymphoma, and marginal zone lymphoma, are sometimes associated with marginal zone differentiation or plasmacytic differentiation. The marginal zone differentiation obscures the morphological differences among these, providing diagnostic challenges for histopathologists. In this paper, we present a case of FL, originally mimicking marginal zone lymphoma in the axillary lymph node. Subsequent bone marrow biopsy showed paratrabecular infiltration of small to medium-sized lymphoid cells. Immunohistochemical analysis of the bone marrow biopsy together with histopathology and flow cytometry of the axillary lymph node led to a final diagnosis of FL with marginal zone differentiation in the axillary lymph node and its bone marrow infiltration. Our case illustrates and reconfirms the importance of clinicopathological correlation which leads to a correct diagnosis.
Keywords: Follicular lymphoma, marginal zone differentiation, flow cytometry
Introduction
Nodal follicular lymphoma (FL) is typically composed of follicular or nodular proliferation of small cleaved lymphoid cells, presumably derived from germinal center (GC) B cells [1]. The hallmark of FL is t(14;18)(q32;q21) chromosomal translocation, which juxtaposes anti-apoptotic gene BCL2 to Immunoglobulin heavy chain (IGH) promoter, providing a basis of survival of FL cells. Reflecting these biological backgrounds, not only hematoxylin and eosin (HE) stain, but also immunohistochemical analysis of the lymph node specimen is useful for correct pathological diagnosis of FL. That is, FL cells are immunohistochemically positive for BCL2 as well as GC B cell markers CD10 and BCL6. However, definitive diagnosis of FL requires clinicopathological correlation, for example, between histology and flow cytometry data.
It is known that low grade B-cell lymphomas, including FL, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and marginal zone lymphoma, are sometimes associated with marginal zone differentiation or plasmacytic differentiation [2]. Marginal zone differentiation is represented in terms of microscopic pattern at low power view and/or cellular differentiation. The former includes expansion of the corresponding marginal zone, that is, the zone surrounding GCs, while the latter include monocytoid B-cell-like or plasma cell-like appearance [2]. The marginal zone differentiation of low grade B-cell lymphomas obscures the morphological differences among these, which may provide diagnostic challenges for histopathologists.
In this paper, we present a case of FL, originally mimicking marginal zone lymphoma in the axillary lymph node. Subsequent bone marrow biopsy showed paratrabecular infiltration of small to medium-sized lymphoid cells. Immunohistochemical analysis of the bone marrow biopsy led to a pathological diagnosis of FL, which was consistent with flow cytometry data of the axillary lymph node specimen.
Case report
A 75-year old Japanese man was admitted to Takarazuka Municipal Hospital for examination of the swelling of multiple lymph nodes. Both past and family histories were not remarkable. Physical and computerized tomography examination revealed swelling of lymph nodes of bilateral submandibular, cervical, supraclavicular, axillary, pulmonary hilar and inguinal regions (data not shown). Swelling was also observed in paraaortic, intraabdominal, and intrapelvic lymph nodes (data not shown). The size of the swollen lymph nodes was around 2 to 3 cm in diameter. Spleen was also suspected for the tumor involvement. Blood test on admission showed no remarkable abnormalities in white cell count (5.22 × 109/L; normal range: 5.0-8.0), hemoglobin (14.0 g/dL; normal range: 13.5-17.5), platelet (196 × 109/L; normal range: 120-280), and lactate dehydrogenase (150 U/L; normal range: 100-210). Although total protein was 7.4 g/dL (normal range: 6.5-8.3) and albumin 3.7 g/dL (normal range: 3.5-4.8), the ratio of γ-globulin was 24.8% (normal range: 10.6-20.5) with high titer of antinuclear antibody (1280 fold; normal range: less than 40) showing speckled pattern. Slightly high CRP (0.4 mg/dl; normal range: less than 0.3) and higher level of soluble interleukin-2 receptor (4200 U/ml; normal range: 145-519) were also noted.
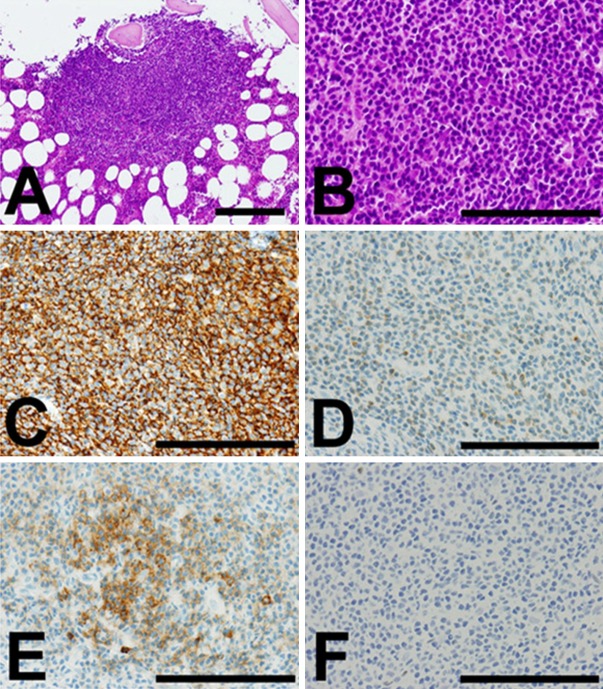
Biopsy of the left axillary lymph node was performed for histopathological diagnosis. HE stain of the resected lymph node showed that small to medium-sized lymphoid cells proliferated circumscribing reactive GCs in a so-called marginal zone pattern (Figure 1A and 1B). Marginal sinus beneath the lymph node capsule was packed with monocytoid tumor cells (Figure 1A). The tumor cells were confined inside the lymph node and did not infiltrate beyond the capsule (Figure 1A). Immunohistochemical analysis showed that the proliferating lymphoid tumor cells were positive for CD20 (Figure 1C), and BCL2 (Figure 1D). They were stained negative for CD3, CD23, and cyclin D1 (data not shown). They were almost negative for CD10, in contrast to reactive GC B-cells stained strongly positive for CD10 (Figure 1E). However, the tumor cells were positively stained for BCL6 as well as the GC B-cells, although the intensity of the staining was weaker in the former than the latter (Figure 1F). Plasma cells sparsely observed in the tumor were considered to be reactive, because they did not show restricted expression of immunoglobulin κ or λ light chains by in situ hybridization (data not shown). From these histological data, marginal zone lymphoma of the lymph node was most suspected but not concluded. On the other hand, flow cytometry of the same axillary lymph node specimen revealed a population of small to medium-sized B-cells with restricted expression of λ chain (κ 13.9%, λ 87.0%). Their CD10-positivity was 55.6% (data not shown), which was not typical for marginal zone lymphoma but rather suggestive of FL. Because of this discrepancy between the histology and flow cytometry of the lymph node, the possibility of low grade B-cell lymphoma was suggested but the histological subtype was not conclusively defined at this stage.
Figure 1.
Representative histological images of the tumor cells in the axillary lymph node. (A, B) Hematoxylin and eosin (HE) stain. (A) Original maginification: × 100. Bar: 200 μm. (B) Original magnification: × 400. Bar: 100 μm. (C-F) Immunohisotchemistry. Original magnification: × 400. Bar: 100 μm. (C) CD20. (D) BCL2. (E) CD10. (F) BCL6. Positive cells were stained brown in immunohistochemistry. In each figures, a reactive germinal center (GC) is indicated by arrowheads at the right lower quadrant of the figure. At low power view in HE stain, the tumor cells were proliferating surrounding GC (indicated by arrowheads) in a marginal zone pattern (A). Perinodal adipose tissue was seen outside the capsule at the left lower quadrant of the figure. High power view showed monocytoid B-cell-like appearance of the tumor cells (B). The proliferating tumor cells surrounding the GC were positive for CD20 (C), and BCL2 (D). They were almost negative for CD10, in contrast to reactive GC B-cells stained strongly positive for CD10 (E). However, the tumor cells were weakly positive for BCL6 while the GC B-cells were strongly positive (F).
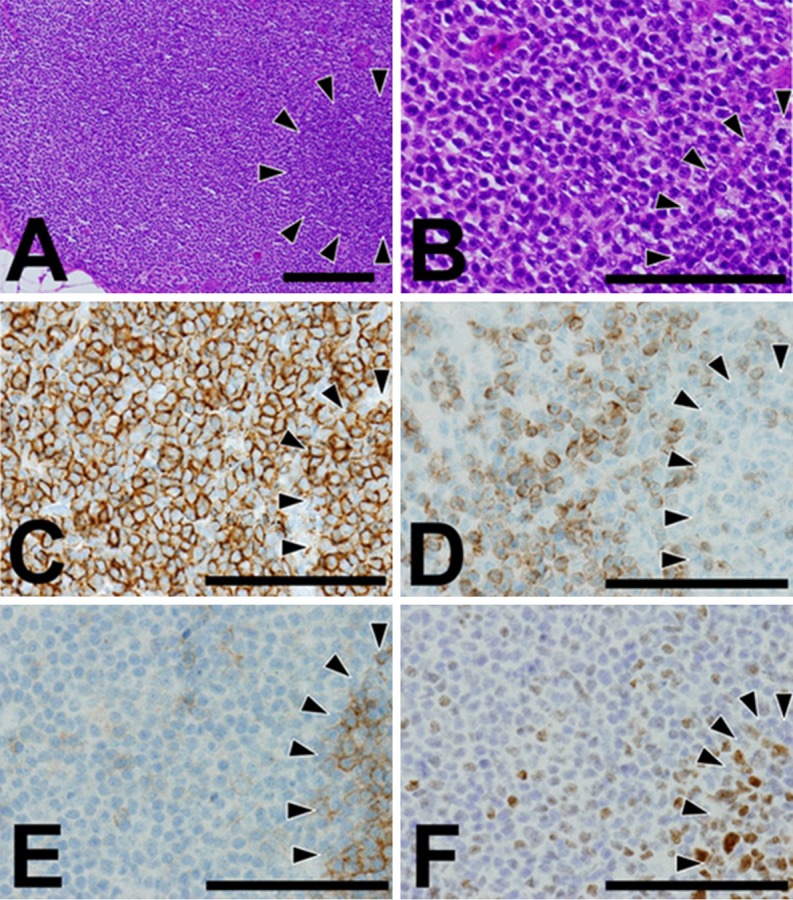
Subsequently, bone marrow biopsy was performed to determine the clinical stage of the lymphoma. The HE image of the bone marrow biopsy showed paratrabecular nodules formed by small to medium-sized centrocyte-like cells without clear GCs (Figure 2A and 2B). Immunohistochemical analysis of the nodules showed that the lymphoid cells were positive for CD20 (Figure 2C), BCL2 (Figure 2D) and CD10 (Figure 2E), but they were negative for CD3 (data not shown) and BCL6 (Figure 2F). They were also immunohistochemically negative for CD34 and terminal deoxynucleotidyl transferase, excluding the possibility of acute lymphoblastic leukemia or hematogone (data not shown). These histological data led to a pathological diagnosis of infiltration of FL cells in the bone marrow, which was consistent with flow cytometry data of the axillary lymph node specimen.
Figure 2.
Representative histological images of the tumor cells in the bone marrow. (A, B) HE stain. (A) Original magnification: ×100. Bar: 200 μm. (B) Original magnification: × 400. Bar: 100 μm. (C-F) Immunohisotchemistry. Original magnification: × 400. Bar: 100 μm. (C) CD20. (D) BCL2. (E) CD10. (F) BCL6. Positive cells were stained brown in immunohistochemistry. At low power view in HE stain, the tumor cell nodules were formed in the vicinity of the bone trabeculae (A). High power view showed centrocyte-like appearance of the tumor cells (B). The proliferating tumor cells were positive for CD20 (C), BCL2 (D), and CD10 (E), but negative for BCL6 (F).
In terms of immunohistochemical results of CD10 and BCL6 showing GC phenotype, there was a discrepancy between the tumor cells of the axillary lymph node and those in the bone marrow. However, in light of the correlation of the flow cytometry and the histopathology of the lymph node and the bone marrow, our final diagnosis was established as FL with marginal zone differentiation in the axillary lymph node and its infiltration into the bone marrow.
Discussion
In this paper, we report a case of FL with marginal zone differentiation with hyperplastic GCs in the lymph node. Parafollicular involvement of CLL/SLL was ruled out based on immunohistochemical data. With the aid of bone marrow examination and flow cytometry, correct histopathological diagnosis was obtained. Immunohistochemical data on GC markers CD10 and BCL6 was discordant between the tumor cells in the axillary lymph node and those in the bone marrow. However, this is not unexpected; morphological discrepancy in the lymphoma is often recognized between lesions of the lymph node and those in the bone marrow [3]. Serological test of the patient showed high titer of antinuclear antibody, consistent with marginal zone differentiation of the lymphoma.
Yamada et al. reported four cases of FL with marginal zone differentiation, in which the tumor nodules were tiny or indistinct [4]. Our case is similar to the patient 4 of Yamada et al., which was associated with multiple reactive GCs [4]. The case 4 had the precedence of FL, which made Yamada et al. suspicious of the recurrence of the previous FL [4]. However, our case has no previous history of FL, which made our initial diagnosis of the lymph node challenging and inconclusive.
In the case of FL with marginal zone differentiation, detection of BCL2 translocation in the marginal zone components as well as the FL nodules provide evidence that the both of the two components of the tumor are clonally related [5,6]. This is also consistent with the notion that the tumor is FL with marginal zone differentiation and not a composite tumor of FL and marginal zone lymphoma. Chromosomal abnormality in our case was 52, XY, +Y, +add(1)(p11), +3, +7, +18, add(21)(p11.2), +22[13]/46, XY[7], which did not include t(14; 18) translocation of BCL2 locus. However, it contained aberrations of chromosome 3, particularly trisomy 3, that were associated with marginal zone lymphoma [7,8]. Chromosomal abnormalities reported for FL with marginal zone differentiation include those in chromosomes 21, 22, and X, some of which seemed to be shared with our case [8]. It was reported that some cases of FLs with marginal zone differentiation did not harbor t(14;18) translocation of BCL2 locus [9]. Therefore, the absence of the BCL2 translocation does not preclude the diagnosis of follicular lymphoma in our case. Goodlad et al. reported that FLs with marginal zone differentiation is a higher risk variant among FLs [7]. Therefore, careful follow-up will be desirable for FLs with marginal zone differentiation, such as our case.
There are some factors that made the diagnosis of the present case challenging. First, HE image of the lymph node tumor closely mimicked marginal zone lymphoma. Not only the pattern of tumor cell proliferation, but also monocytoid B-cell-like appearance of the tumor cells suggested a straightforward diagnosis of marginal zone lymphoma ‘at a glance’. Instead of typical follicular nodules of FL, the tumor was associated with multiple reactive GCs. Second, the patient had no past history of FL, which put FL at lower rank in the list of differential diagnosis. Third, the FL cells in the lymph node and those in the bone marrow exhibited discrepancy in terms of morphology as well as immunophenotype. Morphologically, the former mimicked marginal zone lymphoma, while the latter showed typical follicular pattern. Immunohistochemistry demonstrated that the former showed CD10-negative and BCL6-positive pattern, while the latter CD10-positive and BCL6-negative. It is known that bone marrow lesion does not always recapitulate the morphological aspects of the lymph node lesions of lymphomas and vice versa [3]. Hypothetically, the morphological and phenotypic outlook of the lymphoma depends on where it resides, presumably reflecting the differences of niche for the lymphoma, for example, between the lymph node and the bone marrow.
In conclusion, we present a case of FL with marginal zone differentiation in the axillary lymph node in a patient without previous history of FL. The final diagnosis was obtained not only with a detailed examination of the lymph node pathology, but also with the aid of bone marrow examination and flow cytometry. The diagnosis of FL with marginal zone differentiation may be of clinical significance in light of the prognosis of the patient. Our case illustrates and reconfirms the importance of clinicopathological correlation which leads to a correct diagnosis.
Acknowledgements
We thank all the colleagues in the Department of Surgical Pathology, Hyogo College of Medicine, and Department of Pathology, Takarazuka Municipal Hospital, for preparation of pathological specimen.
Disclosure of conflict of interest
None.
References
- 1.Harris NL, Swerdlow SH, Jaffe ES, Ott G, Nathwani BN, de Jong D, Yoshino T, Spagnolo D. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. In: Follicular lymphoma. 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 220–226. [Google Scholar]
- 2.Harris NL, de Leval L, Ferry JA. Follicular lymphoma. In: Jaffe ES, Harris NL, Vardiman JW, Campo E, Arber DA, editors. Hematopathology. Philadelphia, PA: Saunders/Elsevier; 2011. pp. 267–290. [Google Scholar]
- 3.Brunning RD, Arber DA. Bone marrow. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Maryland Heights, MO: Mosby/Elsevier; 2011. pp. 1927–2012. [Google Scholar]
- 4.Yamada K, Maeshima AM, Taniguchi H, Kawabata Y, Nomoto J, Maruyama D, Kim SW, Watanabe T, Kobayashi Y, Tobinai K, Tsuda H. Follicular lymphoma with marked monocytoid or plasmacytoid differentiation and tiny or indistinct follicles: a case study of four patients. Leuk Lymphoma. 2011;52:804–813. doi: 10.3109/10428194.2011.555570. [DOI] [PubMed] [Google Scholar]
- 5.Schmid U, Cogliatti SB, Diss TC, Isaacson PG. Monocytoid/marginal zone B-cell differentiation in follicle centre cell lymphoma. Histopathology. 1996;29:201–208. doi: 10.1111/j.1365-2559.1996.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 6.Yegappan S, Schnitzer B, Hsi ED. Follicular lymphoma with marginal zone differentiation: microdissection demonstrates the t(14;18) in both the follicular and marginal zone components. Mod Pathol. 2001;14:191–196. doi: 10.1038/modpathol.3880284. [DOI] [PubMed] [Google Scholar]
- 7.Goodlad JR, Batstone PJ, Hamilton D, Hollowood K. Follicular lymphoma with marginal zone differentiation: cytogenetic findings in support of a high-risk variant of follicular lymphoma. Histopathology. 2003;42:292–298. doi: 10.1046/j.1365-2559.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 8.Torlakovic EE, Aamot HV, Heim S. A marginal zone phenotype in follicular lymphoma with t(14;18) is associated with secondary cytogenetic aberrations typical of marginal zone lymphoma. J Pathol. 2006;209:258–264. doi: 10.1002/path.1981. [DOI] [PubMed] [Google Scholar]
- 9.Gradowski JF, Jaffe ES, Warnke RA, Pittaluga S, Surti U, Gole LA, Swerdlow SH. Follicular lymphomas with plasmacytic differentiation include two subtypes. Mod Pathol. 2010;23:71–79. doi: 10.1038/modpathol.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]