Abstract
Background: Immunohistochemical (IHC) expression of Ki67 has been identified as a prognostic and predictive marker in hormone receptor (HR)-positive breast cancer, however, there is little evidence of the association of Ki67 with prognosis in HR-negative patients. We aimed to assess the benefit of Ki67 assessment in HR-negative breast cancers after neoadjuvant chemotherapy (NAC). Methods: In the present study, a total of 183 HR-negative breast cancer patients with Stage II to III that treated with anthracycline and/or taxane-based neoadjuvant chemotherapy between 2004 and 2011 were retrospectively analyzed. Endocrine therapy and trastuzumab was not administered to any patients in this study. Clinical and pathological features of the patients with breast cancer were retrieved from the hospital records. Predictive factors for NAC response and survival were analyzed. Results: Of the 183 patients, 122 (66.6%) were HR- HER2+, and 61 (33.3%) were triple-negative. The clinical response rates were similar across breast cancer subtype. Patients whose tumors contained high Ki67 expression effectively responded to NAC. Ki67 labeling index was a predictive marker for pathologic complete response (pCR). Ki67 expression showed a positive correlation with HER2 status, tumor size, lymph node status, lymphovascular invasion and tumor grade. Furthermore, high Ki67 expression in post-treatment tumors was strongly correlated with poor disease-free survival (DFS), but no correlation of Ki-67 expression with overall survival (OS) was observed. Conclusions: Our results suggest that Ki67 expression in HR-negative breast cancer may improve the assessment of pathological response after NAC, and Ki67 score in residual tumor was an independent prognosticator for DFS in the HR-negative breast cancer patients.
Keywords: Breast cancer, Ki67, neoadjuvant chemotherapy, prognosis
Introduction
Neoadjuvant chemotherapy has been established as a standard treatment strategy for patients with not only local advanced but also operable breast cancer. This strategy allows patients to benefit a reduction in the extent of surgery and provides information on the efficacy of chemotherapy [1]. Recently, it has been demonstrated that patients who achieved a pathologic complete response (pCR) to NAC were likely to also have a favorable long-term outcome in certain subtypes of breast cancer patients [2]. As such, clinical and molecular biomarkers capable of predicting pCR have been assessed following neoadjuvant treatment in breast cancer patients [3,4]. Conventional variables such as tumor size, nodal status and histological grade do not correlate well with sensitivity to specific types of chemotherapy drugs. Several retrospective breast cancer studies have suggested that tumor expression of ER, PgR, epidermal growth factor receptor (EGFR), HER2, Ki-67 and p53 may be associated with chemotherapy sensitivity [5-8].
Compared with other biomarkers, Ki67 expression has been reported to correlate with tumor cell proliferation rate, which is a nuclear protein that is expressed during all phases of the cell cycle, except the G0 phase, and many studies have investigated the IHC expression of Ki67 as a prognostic and predictive marker for breast cancer [9,10]. The St Gallen Consensus Meeting determined that the Ki67 labeling index is chiefly important for distinguishing between “luminal A” and “luminal B” breast cancer subtypes, with a cutoff value of 14% [11]. Furthermore, this indicator is commonly used to predict the magnitude of chemotherapy benefit in luminal-type breast cancers who received a range of NAC regimens, including anthracycline-based and/or taxane-based protocols, and recent studies have clearly identified Ki67 as a prognostic marker for ER-positive breast cancer. Although Ki67 is a well established prognostic marker in ER-positive breast cancer, assessment of cellular proliferation by Ki67 expression is not yet recommended in routine pathological evaluation by the existing guidelines of the American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO), one likely reason is that how Ki67 measurements and thresholds should influence clinical decisions has not been established. Furthermore, there is little evidence to support a role for Ki67 in HR-negative breast cancer prognosis. Sueta et al. [12] reported that Ki67 had no predictive value for pCR in HER2 and triple-negative subtypes with NAC, and Naoki Niikura et al. [13] showed that Ki67 was not associated with survival in the HR-negative group with 716 patients. HR-negative breast cancers, especially triple-negative subtype is generally associated with an aggressive phenotype and a poor prognosis; however, it is true that there are some subpopulations of patients with HR-negative whose prognosis remains good. Therefore, a better understanding of the molecular and histopathological features of HR-negative breast cancers and its heterogeneity is important for the development of a new therapeutic strategy and to improve the prognosis of HR-negative breast cancers. As such, further studies are required to assess the benefit of Ki67 assessment in HR-negative breast cancers.
The aim of the present study was to evaluation whether Ki67 levels can determine the efficacy of chemotherapy for HR-negative subtypes and validate the prognostic role of Ki67 expression in this cohort. Breast cancer cases were divided into 3 categories according to the Ki67 score: low, < 14% Ki67-positive cells; intermediate, ≥ 14% and ≤ 30% Ki67-positive cells; and high, > 30% Ki67-positive cells, and then pCR, DFS, and OS were compared in breast cancer subgroups at a single large institution according to Ki67 scores.
Materials and methods
Patients and treatment
A total of 183 HR-negative (non-luminal) (both ER and PgR IHC-negative) invasive breast cancer patients treated with anthracycline and/or taxane-based NAC from January 2004 to December 2011 at Guangxi University Affiliated Tumor Hospital (China) were retrospectively recruited to this study. Patients were considered evaluable if they had completed NAC, and patients who had not completed all regimens were excluded. The NAC regimens included FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2, every 3 weeks), AC (doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2, every 3 weeks) followed by T (docetaxel 75 mg/m2 every 3 weeks) each for 4 cycles, and TEC (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks). Chemotherapy was administered for a median of 4 cycles (range 2-6 cycles) before surgery. All the patients underwent mastectomy plus axillary lymph node dissection within 4 weeks after NAC. None of them had received molecular targeted therapy. Of note, the vast majority of the patients in the People’s Republic of China do not receive targeted therapy, for economic reasons. The patients with stage II to stage III breast cancer received radiotherapy after chemotherapy. Major pathological parameters were available, including tumor size, location, histological grade, lymph node status, and ER, PgR, and HER2 status, as determined by conventional IHC. All patients were on a regular follow-up schedule. The primary endpoint was pCR rate and disease-free survival (DFS), defined as the time interval from breast cancer surgery to the first evidence of recurrence (local, regional, or distant). If there was no recurrence, patients were censored on the last follow-up.
Immunohistochemical analysis
ER, PgR, HER-2 status and Ki-67 index were evaluated before and after NAC by immunohistochemistry (IHC). All immunohistochemical analyses were carried out in a single reference laboratory and evaluated by light microscopy blindly and independently by two pathologists. The cutoff value for ER positivity and PgR positivity was 10% positive tumor cells with nuclear staining. HR negative was defined as negative for both ER and PgR. HER2 protein overexpression was defined as 3+, complete membrane staining or fluorescence in situ hybridization [FISH] amplification. Ki67 was scored as the percentage of nuclei-stained cells out of all cancer cells in the invasive front of the tumor regardless of the intensity in × 400 high-power field, 500 to 1000 tumor cells were counted in each case. We classify IHC Ki67 expression into 3 categories according to the score of Ki67: low, < 14% Ki67-positive cells; intermediate, ≥ 14% and ≤ 30% Ki67-positive cells; and high, > 30% Ki67-positive cells. Antibodies, dilutions and suppliers were as follows: ER (M7047, Dako), PgR (M3569, Dako), HER-2 (polyclonal, Dako), Ki-67 (MIB1, Dako).
Evaluation of NAC response
The clinical response to NAC was evaluated by physical and imaging examinations according to Response Evaluation Criteria in Solid Tumors (RECIST). No clinical evidence of tumor in the breast and axillary lymph nodes was defined as a complete response (CR). Reduction in the greatest tumor diameter exceeded 30% was graded as a partial response (PR). Tumor reduction less than 30% or an increase up to 20% in the greatest diameter was considered as a stable disease (SD). Tumors that increase of more than 20% in the greatest diameter or appearance of new disease were considered as a progressive disease (PD). The achievement of pCR (pathologic complete response) on postoperative specimens was defined as the absence of invasive residuals in breast or nodes.
Clinical outcome assessment
All patients were followed-up until the date of death or when censored at the latest date (December 30th 2013). The median duration of follow-up for all of the patients in this study was 47 months. Overall survival was defined as the time from the date of operation to death or when censored at the latest date if patients were still alive. DFS was defined as the length of time from the date of operation to events such as local relapse or distant metastases, the occurrence of a new primary tumor, or death without evidence of cancer.
Statistical analysis
Analyses were conducted using SPSS v16.0 (SPSS Inc., Chicago, IL). Correlations of Ki67 expression with other clinicopathological parameters were evaluated using the chi-square test. Univariate and multivariate analyses to determine independent prognostic factors were performed by the Cox proportional model. Variables with a P < 0.05 were accepted for the multivariate model. Kaplan-Meier and the log-rank test were employed to evaluate the distribution of DFS and OS. All P values reported in this analysis were two sided, and a P value of less than 0.05 was considered significant.
Results
Patients’ characteristics
Table 1 summarizes the characteristics of patients in this study. The median age of the enrolled patients was 49 years (range 25-70 years), and 57.9% of these patients were premenopausal. All the patients were defined as ER and PgR negative both before and after NAC. Of the 183 patients, 122 (66.7%) were HR- HER2+, and 61 (33.3%) were triple-negative, 121 (66.1%) of the cases were defined as axillary lymph node positive.
Table 1.
Patient and baseline tumor characteristics
| Factor | Number (%) |
|---|---|
| All | 183 (100) |
| Median age, years (range) 49 (25-70) | |
| Age (y) | |
| ≤ 50 | 116 (63.4) |
| > 50 | 68 (36.6) |
| Menstrual status | |
| Premenopausal | 106 (57.9) |
| Postmenopausal | 77 (42.1) |
| Clinical stage | |
| II | 70 (38.3) |
| III | 113 (61.7) |
| Tumor size (cm) | |
| ≤ 2.0 | 49 (26.8) |
| > 2.0 | 134 (73.2) |
| Clinical lymph node status | |
| Negative | 62 (33.9) |
| Positive | 121 (66.1) |
| Histological grade | |
| G1 | 32 (17.5) |
| G2 | 70 (38.3) |
| G3 | 81 (44.2) |
| HR, HER2 subtype | |
| HR- HER2+ | 122 (66.7) |
| HR- HER2- | 61 (33.3) |
| Ki67 status | |
| Low (< 14%) | 45 (24.6) |
| Intermediate (14-30%) | 60 (32.8) |
| High (> 30%) | 78 (42.6) |
| Neoadjuvant treatment | |
| FEC | 90 (49.2) |
| TEC | 55 (30.1) |
| AC followed by T | 38 (20.7) |
| Clinical response | |
| CR/PR | 156 (85.2) |
| SD/PD | 27 (14.8) |
| Pathological response | |
| pCR | 35 (19.1) |
| non-pCR | 148 (80.9) |
HR, hormone receptor; HER-2, human epidermal receptor 2; FEC, 5-fluorouracil. + epirubicin + cyclophosphamide; TEC, docetaxel + epirubicin + cyclophosphamide; AC followed by T, doxorubicin + cyclophosphamide followed by docetaxel; CR/CR, complete response or partial response; SD/PD, stable disease or progression of disease; pCR, pathological complete response. HER-2, human epidermal receptor 2.
Correlation of Ki67 expression with clinicopathological parameters before and after NAC
Correlations of Ki67 expression with other clinicopathological parameters were evaluated using the chi-square test. As show in Table 2, Ki67-high tumors were significantly associated with advanced tumor stage (P < 0.001), lymph node positivity (P < 0.001), high tumor grade (P = 0.026), lymphovascular invasion (P = 0.019) and HER2 positivity (P < 0.001) of the tumor. There was no relationship between the age, menopausal status and Ki67 positivity.
Table 2.
The Relationship between expression level of Ki67 and the patients’ characteristics
| Factor | Low Ki67 (n) | Intermediate Ki67 (n) | High Ki67 (n) | P value |
|---|---|---|---|---|
| Age (y) | 0.358 | |||
| ≤ 50 | 34 | 39 | 43 | |
| > 50 | 11 | 21 | 35 | |
| Menstrual status | 0.214 | |||
| Premenopausal | 29 | 42 | 51 | |
| Postmenopausal | 16 | 18 | 27 | |
| Tumor size (cm) | < 0.001 | |||
| ≤ 2.0 | 16 | 30 | 3 | |
| > 2.0 | 29 | 30 | 75 | |
| Lymph node status | < 0.001 | |||
| Negative | 29 | 28 | 5 | |
| Positive | 16 | 32 | 73 | |
| Histological grade | 0.026 | |||
| G1-2 | 40 | 43 | 19 | |
| G3 | 5 | 17 | 59 | |
| HER2 status | < 0.001 | |||
| Positive | 14 | 36 | 72 | |
| Negative | 31 | 24 | 6 | |
| lymphovascular invasion | 0.019 | |||
| Absent | 33 | 34 | 17 | |
| Present | 12 | 26 | 61 | |
Correlation of Ki67 expression with response to neoadjuvant chemotherapy
Thirty-five out of the 183 patients (19.1%) achieved a pathologic complete remission. We have examined whether Ki67 level affected the pathological response to neoadjuvant chemotherapy, the results show that tumors with high Ki67 showed significantly improved pCR rates in the HR-negative breast cancer (Table 3). The clinical response rates (PR + CR) were comparable between high Ki67 group (88.5%) and low (82.2%), intermediate group (85.0%). In univariate logistic regression, positive HER2 status (OR = 1.72, 95% CI 0.69-3.26, P = 0.035), high Ki67 (OR = 3.61, 95% CI 1.33-7.82, P = 0.001) and tumor grade 3 (OR = 2.06, 95% CI 0.79-6.64, P = 0.031) were significant predictors for a pCR (Table 4). No significant correlation with pCR rate was detected for age, menopause status, tumor size, nodal status, and the treatment regimen (Table 4). In the multivariate analysis, tumors with high Ki67 expression showed only a great trend toward a higher pCR rate (OR = 2.58, 95% CI 1.24-9.18; P = 0.006), whereas HER2 status showed borderline significant (P = 0.054).
Table 3.
Clinical and pathological response after neoadjuvant chemotherapy by Ki67 labeling index
| Ki67 labeling index | P value | |||
|---|---|---|---|---|
|
|
||||
| Low (n = 45) % | Intermediate (n = 60) % | High (n = 78) % | ||
| Clinical response | ||||
| PR + CR | 37 (82.2) | 51 (85.0) | 69 (88.5) | 0.158 |
| SD + PD | 8 (17.8) | 9 (15.0) | 9 (11.5) | |
| Pathological response | ||||
| pCR | 5 (11.1) | 8 (13.3) | 22 (26.9) | 0.001 |
| non pCR | 40 (88.9) | 52 (86.7) | 56 (73.1) | |
CR + CR, complete response and partial response; SD + PD, stable disease and progression of disease; pCR, pathological complete response.
Table 4.
Univariate and multivariate logistic regression models of baseline characteristics predictive of pCR
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Oddis ratio | 95% CI | P value | Oddis ratio | 95% CI | P value | |
| Age | 1.53 | 0.69-3.26 | 0.244 | |||
| Menstrual status | 1.06 | 0.42-2.08 | 0.362 | |||
| Tumor size | 1.56 | 0.62-4.33 | 0.075 | |||
| Tumor grade | 2.06 | 0.79-6.64 | 0.031 | 1.82 | 0.71-7.32 | 0.086 |
| Lymph node status | 1.38 | 0.66-3.21 | 0.048 | |||
| HER2 status | 1.72 | 0.49-3.66 | 0.035 | 1.49 | 0.61-4.96 | 0.054 |
| Ki67 labeling index | 3.61 | 1.33-7.82 | 0.001 | 2.58 | 1.24-9.18 | 0.006 |
| NAC regimen | 1.27 | 0.48-1.66 | 0.105 | |||
HER-2, human epidermal receptor; NAC, neoadjuvant chemotherapy.
Prognostic value of Ki67 expression on long-term outcome
We evaluated the clinical variables at baseline predicting for DFS using logistic regression analyses, clinicopathological factors in tumors both in pre- and post-treatment specimens were estimated. Age (P = 0.024), tumor size (P = 0.006), tumor histopathologic grade (P = 0.037), pre-NAC node metastasis (P = 0.035), post-NAC node metastasis (P = 0.018), post-NAC HER2 status (P = 0.023), post-NAC Ki67 labeling index (P = 0.003), clinical complete response (P = 0.001) and pathological complete response (P = 0.003) were identified as independent predictive factors for DFS in univariate analysis (Table 5). In multivariate analysis, age (P = 0.038), tumor size (P = 0.026), post-NAC node metastasis (P = 0.037), post-NAC HER2 status (P = 0.021), post-NAC Ki67 labeling index (P = 0.008), clinical complete response (P = 0.001) and pathological complete response (P = 0.024) remained significant. A Kaplan-Meier analysis showed that high Ki67 labeling index (> 30%) after NAC was strongly associated with decreased disease-free (P = 0.004; Figure 1), and there was no significant correlation with overall survival (P = 0.18; Figure 2). Our results indicate that Ki67 expression after NAC is an independent prognostic factor for disease-free survival in HR-negative breast cancer patients.
Table 5.
Univariate and multivariate logistic regression models of clinical variables predictive of DFS
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Age | 2.31 | 1.03-4.43 | 0.024 | 3.05 | 1.15-6.42 | 0.038 |
| Menstrual status | 1.34 | 0.65-3.12 | 0.252 | |||
| Tumor size | 2.34 | 1.18-5.32 | 0.006 | 2.64 | 1.02-5.66 | 0.026 |
| Tumor grade | 1.87 | 0.76-3.65 | 0.037 | 1.66 | 0.81-4.22 | 0.204 |
| Pre axillary lymph node | 1.86 | 0.83-3.29 | 0.035 | 1.62 | 0.65-4.18 | 0.203 |
| Post axillary lymph node | 2.85 | 1.12-5.26 | 0.018 | 3.52 | 1.07-6.25 | 0.037 |
| Pre HER2 status | 1.02 | 0.54-2.27 | 0.813 | |||
| Post HER2 status | 2.21 | 0.93-4.87 | 0.023 | 3.02 | 1.10-6.03 | 0.021 |
| Pre Ki67 labeling index | 1.35 | 0.61-2.83 | 0.439 | |||
| Post Ki67 labeling index | 3.64 | 1.20-8.59 | 0.003 | 3.86 | 1.19-9.21 | 0.008 |
| cCR | 4.35 | 1.18-10.86 | 0.001 | 5.02 | 1.24-12.16 | 0.001 |
| pCR | 3.48 | 1.22-6.97 | 0.003 | 2.39 | 1.16-5.85 | 0.024 |
Pre before, neoadjuvant chemotherapy; Post, after neoadjuvant chemotherapy; cCR, clinical complete response; pCR, pathological complete response.
Figure 1.
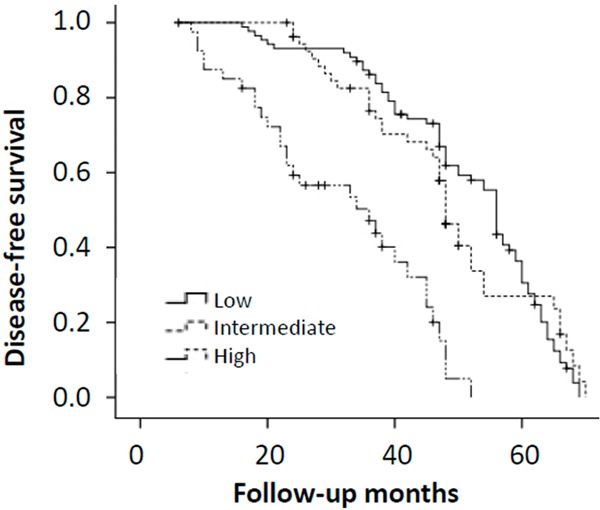
Kaplan-Meier curves of disease-free survival (DFS) in each group according to Ki67 score. Log-rank test was significant for PFS (P = 0.004).
Figure 2.
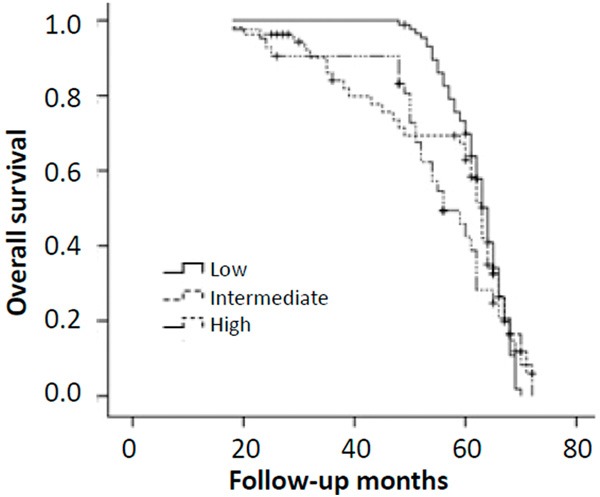
Kaplan-Meier curves of overall survival (OS) in each group according to Ki67 score. Log-rank test was not significant for OS (P = 0.18).
Discussion
Recent studies have reported a predictive value of Ki67 for response to NAC in breast cancer, but that the role of Ki67 differs depending on HR status or tumor subtypes. Many studies suggested that in patients with HR-positive tumors, Ki67 is a predictive marker for chemotherapeutic efficacy, and it is helpful for defining good prognosis and poor prognosis [12,14,15]. In contrast, there is little evidence to support the predictive and prognostic value of Ki67 expression in HR-negative breast cancer after NAC. However, one clinical trial with 552 breast cancer patients following NAC showed that Ki67 independently improved the prediction of treatment response in a group of luminal tumors as well as triple-negative tumors [16]. Munzone et al. [17] also found that Ki67 was associated with prognosis in node-negative, triple-negative groups. Since the role of Ki67 in HR-negative breast cancer was controversial, and neoadjuvant treatment allows directly testing response prediction [18], we have analyzed the predictive value of Ki67 expression in the NAC-administered HR negative breast cancer patients.
In the present study, we classify IHC Ki67 expression into 3 categories according to the levels of Ki67: low (< 14%), intermediate (14-30%), and high (> 30%). Some studies have reported that Ki67 had no predictive value for pCR in HR-HER2+ and triple-negative subtypes, because greater chemotherapy sensitivity was generally observed in this tumors [19]. However, we detected a significant higher pCR rate for tumors with high Ki67 expression within the HR-negative tumors, and the pCR rate in low and intermediate groups were comparable. However, the clinical response rate (CR + PR) was comparable between tumors with high Ki67 expression and low or intermediate Ki67 expression. That may be explained that the clinical response rate was relative high in HR-negative breast cancer. Our findings are consistent with several studies [14,16,17], indicated that pretherapeutic Ki67 could be used as a predictive parameter for pathological response to NAC in breast cancer across tumor subtypes. We also evaluated the utility of commonly used tumor characteristics to predict pCR after NAC. Pretreatment Ki67, HER2 status, lymph node status as well as tumor histopathologic grade was significant predictor of pCR by multivariate analysis. The exact reason underlying this finding is unclear, but it may be explained that tumors with positive HER2, positive lymph node and higher histopathologic grade were consistently characterized by higher rates of proliferation, which were likely to respond well to chemotherapy.
Recently, it has been reported that higher levels of Ki-67 were significantly associated with premenopausal status, larger tumor size, higher tumor grade, lymphatic and vascular invasion, lymph node positivity, HR negativity as well as HER2 positivity [20-22]. In the present study, we have analyzed the correlation between Ki67 and other clinicopathological parameters before and after NAC. As expected, we found a positive correlation between Ki67 expression and HER2 status. Besides, Ki67 expression showed a positive correlation with lymph node status, lymphovascular invasion, tumor grade and tumor size. However, there was not any significant association between age, menopausal status and Ki67 levels in the HR-negative patients. The possible explanation is that breast tumors which are HER2 positive, lymph node positivity and higher tumor grade tend to have higher proliferation rates. However, the detailed relationship between Ki67 and other clinicopathological parameters has not been adequately investigated and requires further investigation.
Even though many studies have demonstrated the prognostic value of Ki67, the debate on the prognostic role of Ki67 in breast cancer is still open. In the majority of those studies, it was reported that higher Ki67 expression was associated with poor prognosis [23,24]. However, there is little evidence to support a prognostic role for Ki67 in HR-negative breast cancer patients [5,13,25]. Among these studies, the breast cancer populations and treatment differ widely, the assays for Ki67 were performed with different methods, the cutoffs to designate “positive” and “negative” or “high” and “low” Ki67 populations differ widely, which may influence the survival analysis. In our study, to exclude the heterogeneity of breast cancer subtype and treatment, we chose only the HR negative (non-luminal) breast cancer subgroup. All patients had received modified radical mastectomy of breast cancer following anthracycline and/or taxane-based chemotherapy, and after the surgery, they only received the same chemotherapy regimens and local radiotherapy; none of them received molecular targeted therapy or endocrinotherapy. When we chose 30% of Ki67 labeling index as a cutoff value, the univariate analysis showed that age, tumor size, tumor histopathologic grade, pre-NAC node metastasis, post-NAC node metastasis, post-NAC HER2 status, post-NAC Ki67 labeling index, cCR and pCR were significantly associated with disease-free survival. Post-NAC Ki67 labeling index was the only factor that was significantly associated with disease-free survival by multivariate analysis, suggesting that Ki67 labeling index after NAC is a prognostic factor for disease-free survival. A Kaplan-Meier analysis showed that patients whose breast tumors showed low Ki67 expression after NAC displayed a longer disease-free survival. These results suggested that the level of Ki67 expression is a prognostic factor predicting disease-free in HR negative breast cancer patients, and 30% is a suitable cutoff. Furthermore, for patients with high Ki67 levels in residual disease, new treatment strategies have to be found. Interestingly, this association could not be shown between the Ki67 levels and overall survival, Ki67 was not an independent prognostic factor for overall survival in this study. One possibility is that breast cancers patients often displayed a long overall survival, and the follow-up time was relatively short. Other possible explanations include statistical chance or potential imbalances in baseline prognostic factors. Thus, to better define the impact of Ki67 on overall survival in HR-negative breast cancers, further studies are required.
There are some limitations of our study. First, it was a retrospective study using a nonrandomized database; therefore, this study suffers from the bias associated with any retrospective study, such as inherent selection bias. Second, our study included a small sample size and follow-up time was relatively short, and more accuracy could have been obtained with a larger sample size and longer follow-up.
In conclusion, we examined the reliability of Ki67 as a predictor of pCR afteranthracycline and/or taxane-containing neoadjuvant chemotherapy and found that Ki67 labeling index could be used as a means of better reflecting tumor response to chemotherapy in HR-negative breast cancer. Furthermore, a high Ki67 expression in residual tumors was strongly correlated with poor disease-free, but not overall survival. It is thus necessary to establish additional strategies to improve disease-free survival for patients whose residual tumors show high Ki67 expression after NAC.
Acknowledgements
This study was supported by the Key Projects of Health Department in Guangxi Zhuang Autonomous Region (No. 2010079), and Science and Technology Research Fund of Guangxi Zhuang Autonomous Region Science and Technology Department. (No. 13550053-12).
Disclosure of conflict of interest
None.
References
- 1.Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 2.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 3.Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010;28:1821–1828. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang GC, Qian XK, Guo ZB, Ren CY, Yao M, Li XR, Wang K, Zu J, Liao N. Pre-treatment hormonal receptor status and Ki67 index predict pathologic complete response to neoadjuvant trastuzumab/taxanes but not disease-free survival in HER2-positive breast cancer patients. Med Oncol. 2012;29:3222–3231. doi: 10.1007/s12032-012-0242-8. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefoi H, Diebold-Berger S, Therasse P, Hamilton A, Van De Vijver M, MacGrogan G, Shepherd L, Amaral N, Duval C, Drijkoningen R. Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann Oncol. 2003;14:406–413. doi: 10.1093/annonc/mdg108. [DOI] [PubMed] [Google Scholar]
- 7.Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C, Mors R, Haegele P, Eber M, Ghnassia JP. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer. 2004;40:205–211. doi: 10.1016/s0959-8049(03)00675-0. [DOI] [PubMed] [Google Scholar]
- 8.Magkou C, Nakopoulou L, Zoubouli C, Karali K, Theohari I, Bakarakos P, Giannopoulou I. Expression of the epidermal growth factor receptor (EGFR) and the phosphorylated EGFR in invasive breast carcinomas. Breast Cancer Res. 2008;10:R49. doi: 10.1186/bcr2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart M, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Wood W, Coates A, Gelber R, Thürlimann B, Senn HJ. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sueta A, Yamamoto Y, Hayashi M, Yamamoto S, Inao T, Ibusuki M, Murakami K, Iwase H. Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer; is it equally useful across tumor subtypes? Surgery. 2014;155:927–935. doi: 10.1016/j.surg.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Niikura N, Masuda S, Kumaki N, Xiaoyan T, Terada M, Terao M, Iwamoto T, Oshitanai R, Morioka T, Tuda B, amura T, Saito Y, Suzuki Y, Tokuda Y. Prognostic significance of the Ki67 scoring categories in breast cancer subgroups. Clin Breast Cancer. 2014;14:323–329. e3. doi: 10.1016/j.clbc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka T, Hosoda M, Yamamoto M, Taguchi K, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y, Yamashita H. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer. 2013 doi: 10.1007/s12282-013-0474-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell’Orto P, Maiorano E, MacGrogan G, Braye SG, Öhlschlegel C. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J. Clin. Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wend-tland R, Bani MR, Schrauder M. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nole F, Mastropasqua M, Rotmensz N, Colleoni M, Esposito A, Adamoli L. Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat. 2012;134:277–282. doi: 10.1007/s10549-012-2040-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann M, Pusztai L. Use of standard Markers and incorporation of molecular markers into breast cancer therapy. Cancer. 2011;117:1575–1582. doi: 10.1002/cncr.25660. [DOI] [PubMed] [Google Scholar]
- 19.Jones RL, Salter J, A’Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M. Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2010;119:315–323. doi: 10.1007/s10549-009-0329-x. [DOI] [PubMed] [Google Scholar]
- 20.Klintman M, Bendahl PO, Grabau D, Lövgren K, Malmström P, Fernö M. The prognostic value of Ki67 is dependent on estrogen receptor status and histological grade in premenopausal patients with node-negative breast cancer. Mod Pathol. 2010;23:251–259. doi: 10.1038/modpathol.2009.167. [DOI] [PubMed] [Google Scholar]
- 21.Spyratos F, Ferrero-Poüs M, Trassard M, Hacene K, Phillips E, Tubiana-Hulin M, Le Doussal V. Correlation between MIB-1 and other proliferation markers. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 22.Faratian D, Munro A, Twelves C, Bartlett J. Membranous and cytoplasmic staining of Ki67 is associated with HER2 and ER status in invasive breast carcinoma. Histopathology. 2009;54:254–257. doi: 10.1111/j.1365-2559.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones RL, Salter J, A’Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116:53–68. doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanei T, Shimomura A, Shimazu K, Nakayama T, Kim S, Iwamoto T, Tamaki Y, Noguchi S. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2011;37:155–161. doi: 10.1016/j.ejso.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Ikeda K, Ogawa Y, Ishikawa T, Hirakawa K. Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at Stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res. 2011;13:R122. doi: 10.1186/bcr3068. [DOI] [PMC free article] [PubMed] [Google Scholar]


