Abstract
Objective: To investigate the expression and clinical significance of annexin A7 in the differentiation and lymphatic metastasis of gastric cancer (GC). Methods: The clinical and pathological data were recorded for analysis. Immunohistochemical staining and Western blot were performed to analyze the expression of ANXA 7 in primary GC tissues. Logistic regression analyses were conducted to evaluate the associations between annexin A7 expression levels and differentiations of GC. Analyses of the ROC were conducted to determine the cut-off value of the ratio of pixel density of annexin A7 for predicting lymphatic metastasis of GC. Results: A total of 162 GC patients were enrolled in this study, and expression rate of annexin A7 was 65.4% in GC. The survival rate of patients with positive expression of annexin A7 was lower than that in patients with negative expression (P=0.000). The results of COX regression showed that the positive expression of annexin A7, submucosal confinement and pathological stage of GC were associated with poor clinical outcomes. The ratio of pixel density value of primary GC tissues with PN 1-3 lymphatic spread was significantly higher than those in tissues with PN 0 lymphatic spread (0.56±0.09 vs. 0.42±0.07, P < 0.05). ROC analysis showed a high area under the curve for the ratio of pixel density value of annexin A7 in primary GC tissues. At a cut-off level of > 0.505, the ratio of pixel density value of annexin A7 exhibited 76.7% sensitivity and 88.3% specificity for detecting lymphatic metastasis of GC. Conclusion: High annexin A7 expression is associated with poor differentiation in GC patients, and it may be a predictor for lymphatic metastasis of GC.
Keywords: Annexin A7, gastric cancer, cancer differentiation, lymphatic metastasis, predictor
Introduction
Despite the developments of surgical techniques and improvements of anticancer drugs in recent years, gastric cancer (GC) is still one of the most common malignancies in China and the second leading cause of cancer death worldwide [1]. The two most important prognostic indicators for patients with GC are the disease stage at diagnosis and nodal status [2]. Therefore, identification of new diagnostic and prognostic biomarkers for GC diagnosis is one of the major goals in this field.
Annexins are Ca2+- and phospholipid-binding proteins, which are associated with cell transformation, cancer progression, and metastasis [3]. Annexin A7, also named as synexin, belongs to the group A annexin family. Previous studies have found that the annexin A7 specifically functions as a cancer promoter candidate in majority of gastrointestinal cancers [4,5]. However, effects of annexin A7 on the proliferation, migration and invasion of GC were still unknown. In this study, therefore, we investigated the expression and clinical significance of annexin A7 in the differentiation and lymphatic spread of GC.
Materials and methods
Patients and specimens
This study enrolled 162 patients with newly diagnosed GC undergoing successful gastrectomy in the Forth Hospital of Hebei Medical University from September 2010 and March 2011, and there was no evidence of any other malignancies. Tumor tissues and adjacent normal mucosa (at least 3 cm away from the edge of tumor mass) were collected at a size of 1.0 cm×0.5 cm×0.5 cm. There was no evidence of any other malignancies. Patients receiving preoperative chemotherapy or radiotherapy were excluded. The clinical data on gender, age, depth of tumor invasion, location, and tumor size were obtained from medical records, and the data on histological differentiation as well as the extent of lymph node involvement were obtained from pathological reports. The histological diagnosis of gastric adenocarcinoma was made according to the recommendations of the World Health Organization. Tumor-node-metastasis status was based on the 7th edition of the American Joint Commission on Cancer Staging System [6]. The study protocol was approved by the Ethical Committee of the Fourth Hospital of Hebei Medical University, and the written informed consent about surgical specimens was given by all patients before gastrectomy. Immunohistochemical staining and Western blot were performed to analyze the expression of ANXA 7 in primary GC tissues. All patients were followed up for at least 3 years, and the methods of follow-up included telephone call, E-mail and outpatient visiting.
Immunohistochemistry
The surgically resected samples were formaldehyde-fixed (10%) and paraffin-embedded. The paraffin blocks were serially sectioned into ten 4-μm sections. After xylene dewaxing and rehydration using an ethanol gradient, the samples were incubated at room temperature in H2O2 (3%) for 15 min to remove any endogenous peroxidase activity, followed by washing with PBS. Citrate buffer (0.01 M, pH 6.0) was used for antigen retrieval, and normal goat serum (5%) was used to block the samples at room temperature for 40 min to block the heterogenetic antigens. Rabbit anti-annexin 7 polyclonal antibody (Proteintech Group, Inc., 1:100 dilution) was added, incubated at 4°C overnight, and washed with PBS. A biotin-conjugated, goat anti-rabbit IgG polyclonal antibody (Zhongshan Golden Bridge Inc.; 1:100 dilution) was added, incubated at 37°C for 30 min, and washed with PBS. This was followed by the addition of a horseradish peroxidase (HRP)-conjugated streptavidin working solution. Next, the samples were washed with PBS, and DAB chromogen was applied to the sample for color development. The samples were then restained with hematoxylin, dehydrated, cleared, and mounted with neutral gum. PBS was substituted for the primary antibody as the negative control.
The expression of annexin A7 was scored by multiplying the intensity scores and the percentage area positively stained [7]. Briefly, the intensity score was categorized into four groups: no staining marked 0; weak staining marked 1; moderate staining marked 2 and strong staining marked 3. For the percentage of positively stained cells, the scores were sorted from 0 to 4 (< 5% marked 0; 5%-25% marked 1; 25%-50% marked 2; 51%-75% marked 3; 75% marked 4). After such calculation, the finally composite scores were divided into four grades: 0 and 1 were negative, 2-4 were weakly positive, 5-8 were moderately positive and 9-12 were strongly positive. 2 and more than 2 scores were evaluated as positive results.
Western blot analysis
There were 30 GC tissues with poor differentiation and 30 GC tissues with well/moderate differentiation from the 162 specimens using for Western blot analysis. A RIPA lysis buffer (150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.9, 10 mM NaF, PMSF, and 1× protease inhibitors (complete cocktail tablets, Roche)) was used to isolate total protein from the primary GC tissues. The Bradford assay was used to determine protein concentration. Forty micrograms of total protein for each sample was electrophoretically separated using SDS-PAGE and transferred onto a PVDF membrane (Amresco, USA). The membranes were blocked at room temperature for 1 h in a TBST solution (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween-20) with 5% fat-free milk. The rabbit anti-annexin A7 polyclonal antibody (Proteintech Group Inc., 1:200 dilution) and the rabbit anti-GNPDH polyclonal antibody (1:1,000 dilution) were added and incubated at 4°C overnight. A goat anti-rabbit IgG polyclonal antibody (1:2,000 dilution) with HRP labeling was added and incubated at 37°C for 1 h and washed with TBST. ECL chemiluminescence reagents (Pierce, USA) were used for autoradiography. The ratio of pixel density value of annexin A7 was represented by the annexin A7/GNPDH gray level ratio. The gray level was analyzed using the Quantity One software (Bio-Rad, USA).
Staging of gastric cancer
In this study, we referred to the classification established by the Japanese Research Society for GC [8], which defines early GC as a lesion confined to the mucosal or submucosal layer, and advanced GC as a lesion invading the proper muscle layer or deeper. The nodal classification was determined according to the International Union Against Cancer (UICC) TNM staging system for gastric cancer [6], in which lymph node metastasis is classified into four groups: pN 0, no metastasis; pN 1, 1-2 positive lymph nodes; pN 2, 3-6 positive lymph nodes; pN 3a, 7-15 positive lymph nodes; pN 3b, 15 or more positive lymph nodes.
Statistical analysis
Absolute numbers and percentages were computed to describe the data. Continuous variables were expressed as mean±SD and were compared using the unpaired t test for normally distributed and Mann-Whitney U test for non-normally distributed variables. Categorical variables were expressed as absolute or relative frequencies and were compared using chi-square analyses or the Fisher exact test, as appropriate to the cell frequencies. Logistic regression analyses adjusted for clinical data were conducted to evaluate the associations between annexin A7 expression levels and differentiations of GC. For the stepwise regression analysis, nominal variables as well as numerical variables were included. Analyses of the receiver operating characteristic (ROC) were conducted to determine the cut-off value of the ratio of pixel density of annexin A7 for predicting the lymphatic metastasis of GC. The cumulative survival was analyzed according to the Kaplan-Meier method. The proportional hazard assumption was checked for each categorical variable by use of Cox regression. SPSS 19.0 for Windows (SPSS Inc., Chicago, Illinois) was used for statistical analysis. Values of P < 0.05 were considered statistically significant.
Results
In this study, we evaluated the association of expression of annexin A7 with differentiation and lymphatic metastasis of GC in more details. A total of 162 GC patients were enrolled. After 3-year follow-up, 5 cases were lost.
Correlation between expression of ANXA 7 and clinicopathological factors of GC
The expression rate of annexin A7 was 65.4% (106/162) in the primary GC tissues and 31.5% (51/162) in adjacent normal mucosa by immunohistochemistry (P < 0.001). As shown in Table 1 and Figure 1, positive annexin A7 correlated with differentiation, and pathological stage, ly-mph node involvement as well as submucosal confinement (all P < 0.05). However, no differences were found in age, gender distribution, and tumor size between different expression of annexin A7. The cumulative survival rate was 45.9% in all patients who were finished 3-year follow-up. Kaplan-Meier analysis was made to analyze the relationship between annexin A7 expression and the survival curve that was drawn as Figure 2. The survival rate of patients with positive expression of annexin A7 was lower than that in patients with negative expression (29.4% vs. 76.4%, P < 0.001).
Table 1.
Correlation between expression of annexin A7 and clinicopathological factors of GC tissues
| Characteristic | Negative annexin A7 (n=56) | Positive annexin A7 (n=106) | P value |
|---|---|---|---|
| Age (yr.) | 0.767 | ||
| < 60 | 23 (41.1%) | 41 (38.7) | |
| ≥ 60 | 33 (58.9) | 65 (61.3) | |
| Gender distribution | 0.536 | ||
| male | 42 (75.0) | 84 (79.2) | |
| female | 14 (25.0) | 22 (20.8) | |
| Differentiation | 0.018 | ||
| well | 4 (7.1) | 3 (2.8) | |
| moderate | 22 (39.3) | 23 (21.7) | |
| poor | 30 (53.6) | 80 (75.5) | |
| Pathological stage | 0.042 | ||
| I | 15 (26.8) | 15 (14.2) | |
| II | 14 (25.0) | 17 (16.0) | |
| III | 24 (42.9) | 60 (56.6) | |
| IV | 3 (5.4) | 14 (13.2) | |
| Submucosal confinement | 0.047 | ||
| early | 10 (17.9) | 8 (7.5) | |
| advanced | 46 (82.1) | 98 (92.5) | |
| Lymph node involvement | 0.047 | ||
| PN 0 | 6 (10.7) | 18 (16.1) | |
| PN 1-2 | 24 (42.9) | 27 (24.1) | |
| PN 3a-3b | 26 (46.4) | 61 (59.8) | |
| Tumor size | 0.188 | ||
| < 5 | 15 (26.8) | 19 (17.9) | |
| ≥ 5 | 41 (73.2) | 87 (82.1) |
Figure 1.
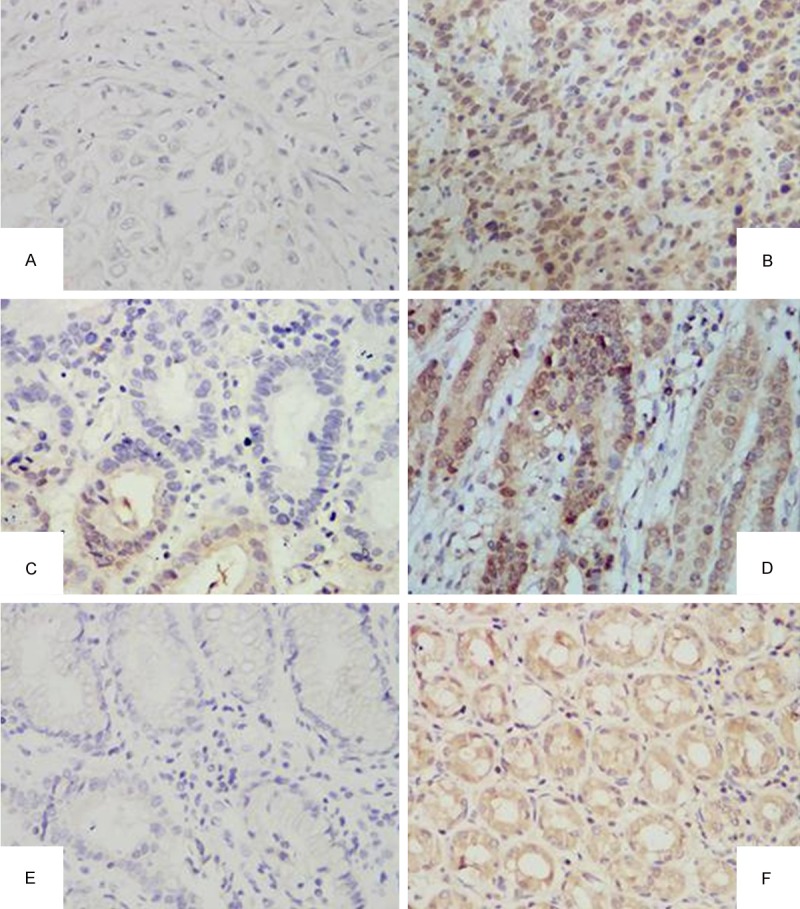
Expression of annexin A7 in gastric carcinoma and its adjacent tissue. A. Negative expression of annexin A7 in poor differentiation GC tissue (×400); B. Positive expression of annexin A7 in poor differentiation GC tissue (×400); C. Negative expression of annexin A7 in high differentiation GC tissue (×400); D. Positive expression of annexin A7 in high differentiation GC tissue (×400); E. Negative expression of annexin A7 in adjacent tissue (×400); F. Positive expression of annexin A7 in adjacent tissue (×400).
Figure 2.
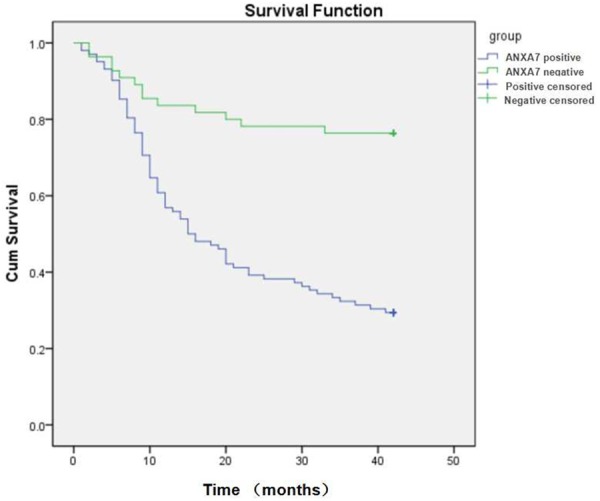
Kaplan-Meier analysis showed that the survival rate of patients with positive expression of annexin A7 was lower than that in patients with negative expression (29.4% vs. 76.4%, P=0.000).
The results of COX regression showed that the positive expression of annexin A7, submucosal confinement and pathological stage of GC were associated with poor clinical outcomes (Table 2).
Table 2.
Prognostic significance of selected variables concerning survival at follow-up according to univariable Cox regression analyses
| Variables | Differentiation of GC | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P value | |
| Positive annexin A7 | 2.940 | 1.309-6.604 | 0.009 |
| Submucosal confinement | 9.565 | 1.670-54.771 | 0.011 |
| Pathological stage | 2.226 | 1.200-4.127 | 0.011 |
| Lymph node involvement | 1.252 | 0.465-3.370 | 0.656 |
| Tumor size | 0.481 | 0.173-1.342 | 0.162 |
| Gender | 0.578 | 0.223-1.493 | 0.257 |
| Age | 1.110 | 0.499-2.467 | 0.799 |
Annexin A7 expression confirmed by Western blot
Western blot was performed to further determine whether the over-expression of annexin A7 in the immunohistochemical staining of the primary GC tissue was a reliable result. Faintly stained band was present in all samples, whereas relatively strong annexin A7 band was observed in the poorly-differentiated tissues with lymph node involvement (Figure 3). Annexin A7 antibody showed an immunoreactive band with an apparent molecular weight was at 47-kDa. The ratio of pixel density value of GC tissues with PN 1-3 lymphatic spread was significantly higher than those in tissues with PN 0 lymphatic spread (0.56±0.09 vs. 0.42±0.07, P < 0.05). Figure 4 shows a histogram of the ratio of pixel density value comparison between both groups.
Figure 3.
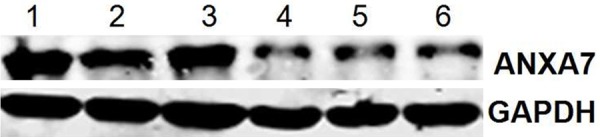
Western blot shows the expression of annexin A7 is increased in primary GC tissue with lymph node involvement. Cases 1, 2, and 3 indicated the band of primary GC tissue with PN 1-3, and Cases 4, 5, and 6 indicated the band of primary GC tissue with PN 0. The protein band in GC tissue with PN 1-3 was stronger.
Figure 4.
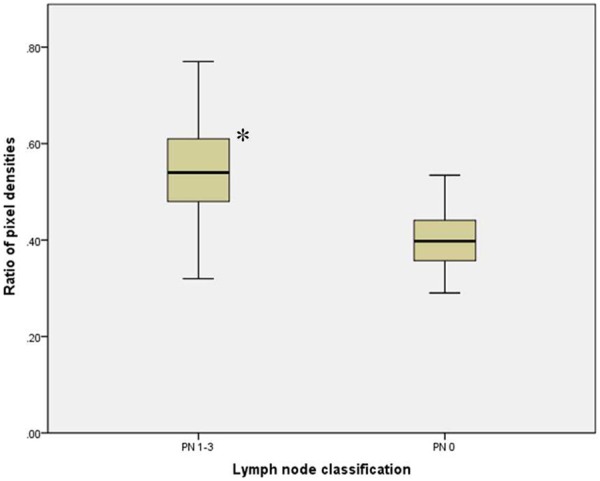
Ratio of pixel densities in primary GC tissues with different lymph node classification. Note: compared with LN 0, P < 0.05.
Predicting effect of ratio of the pixel density value of annexin A7 on lymphatic metastasis of GC
Receiver-operating characteristic analysis shows a higher area under the curve for the ratio of pixel density value of annexin A7 in primary GC tissues with lymph node involvement. At a cut-off level of > 0.505, the ratio of pixel density value of annexin A7 exhibited 76.7% sensitivity and 88.3% specificity for detecting lymphatic metastasis of GC. Area under the ROC curve was 0.882 (95% CI: 0.796-0.967, P < 0.001) (Figure 5).
Figure 5.
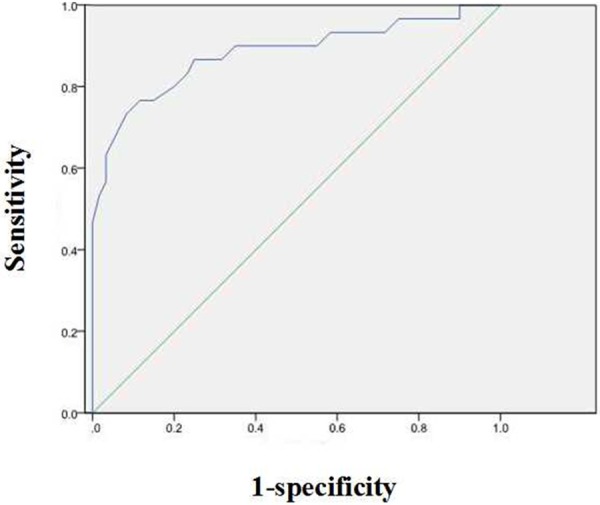
Receiver-operating characteristic analysis shows a higher area under the curve for ratio of the pixel density value of annexin A7 in primary GC. At a cut-off level of > 0.505, the pixel density value of annexin A7 exhibited 76.7% sensitivity and 88.3% specificity for detecting lymphatic metastasis of primary GC tissues. Area under the ROC curve: 0.882, 95% CI: 0.796-0.967, P < 0.001.
Discussion
Gastric cancer remains one of the most deadly human malignancies [9], and GC invasion with subsequent peritoneal metastasis is a major cause of death in patients with advanced GC [10]. However, the molecular mechanisms of tumor formation and progression to develop rational approaches to the diagnosis and treatment of GC are still unknown. Annexin A7 is a member of the multigene annexin superfamily of Ca2+- regulated and phospholipids-binding proteins. Previous studies have shown that the deregulation, loss of heterozygosity and subcellular localization of annexin A7 are associated with the occurrence, invasion, metastasis and progression of majority of gastrointestinal cancers. It appears to promote the development and malignancies of liver cancer, gastric cancer, nasopharyngeal carcinoma, and colorectal cancer [3].
The present study was designed to investigate whether annexin A7 might be used as markers for differentiations and lymphatic metastasis of GC at the protein levels. The results found that the expression rate of annexin A7 was 65.4% in GC patients, which were agreed with previous studies. The expression rate of annexin A7 in different differentiated tissues of GC were evaluated by immunohistochemical and Western blot methods. In this study, increased annexin A7 expression was observed in different primary GC tissues, and high expression level of ANXA 7 was identified as an independent factor associated with poor survival rate. Furthermore, an elevated annexin A7 expression level correlated with the lymphatic metastasis of primary GC, which indicated that annexin A7 may be a predictor for lymph node involvement of GC in human beings.
The annexin A7 gene is located on human chromosome 10q21, where multiple potential tumor suppressor genes (TSG) exist. The annexin A7 gene codes for a membrane-associated Ca2+- activated GTPase and a protein kinase C (PKC) substrate. Previous studies have found that encoding annexin A7 protein has two hypotypes with molecular masses of 47 and 51 kDa [11,12], and this was confirmed in our study which showed the band with an apparent molecular weight was at 47-kDa. The annexin A7 protein is predominantly distributed in membranes and to a lesser extent in the nucleus, and contains a long N-terminal domain of ~200 amino acids rich in glycine, tyrosine and proline residues. In this study, we verified this conclusion, and we found that and the pixed density values were 0.56±0.09 and 0.42±0.07 in different levels of lymph node involvement in primary GC tissues, respectively.
Annexin A7 reversibly binds to acidic phospholipids in a Ca2+- dependent manner. It is involved in exocytotic secretion and in aggregation of chromaffin granules. It causes liposome aggregation and forms classical voltage-gated Ca2+ channels in cellular and artificial membranes, which can be stabilized in long open states by GTP [13-17]. Hsu et al. found that annexin A7 expression seemed to be significantly correlated with cell differentiation, metastasis and TNM grade of GC, and over expression of annexin A7 might play important roles in the differentiation and metastasis of GC. However, this discovery is currently still controversial, and the results are different in recent studies. The reasons of different results in these studies may be as follows: first, both of them were small scale studies. Second, there may be different histopathological types in both studies; further classification was not performed in the two studies because of small samples. Third, the methods used in our study were also different from the previous ones.
The limitations of this study included small sample size, no long-term survival observations and without doing qRT-PCR for RNA expression studies. Therefore, further studies were needed to explore the correlation.
In summary, as we expected, we found characteristics such as the positive expression of annexin A7, submucosal confinement and pathological stage of GC were associated with poor clinical outcomes. Receiver-operating characteristic analysis showed a higher area under the curve for the ratio of the pixel density value of annexin A7 in primary GC tissue. At a cut-off level of > 0.505, the ratio of pixel density value of annexin A7 could be used for detecting lymphatic metastasis of GC with high sensitivity and specificity. To our knowledge, this is the first study to evaluate the expression of annexin A7 in GC tissue quantitively. The results could contribute to a new diagnosis and therapeutic strategy to control development and metastasis of GC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81072033).
Disclosure of conflict of interest
None.
References
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 2.Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S, Lenz HJ. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointestinal Oncol. 2011;2:77–84. doi: 10.3978/j.issn.2078-6891.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo C, Liu S, Greenaway F, Sun MZ. Potential role of annexin A7 in cancers. Clinica Chimica Acta. 2013;423:83–89. doi: 10.1016/j.cca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Sun MZ, Liu S, Tang J. Proteomics investigation of mouse hepatocarcinoma cell lines with different lymph node metastasis capacities. Chem J Chin Univ. 2009;30:517–24. [Google Scholar]
- 5.Jimenez CR, Knol JC, Meijer GA, Fijneman RJ. Proteomics of colorectal cancer: overview of discovery studies and identification of commonly identified cancer-associated proteins and candidate CRC serum markers. J Proteomics. 2010;73:1873–95. doi: 10.1016/j.jprot.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual 7th edition. New York: Springer; 2010. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava M, Bubendorf L, Raffeld M, Bucher C, Torhorst J, Sauter G, Olsen C, Kallioniemi OP, Eidelman O, Pollard HB. Prognostic impact of ANX7-GTPase in metastatic and HER2-negative breast cancer patients. Clin Cancer Res. 2004;10:2344–50. doi: 10.1158/1078-0432.ccr-03-0278. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava M, Bubendorf L, Nolan L, Glasman M, Leighton X, Miller G, Fehrle W, Raffeld M, Eidelman O, Kallioniemi OP, Srivastava S, Pollard HB. ANX7 as a bio-marker in prostate and breast cancer progression. Dis Markers. 2001;17:115–20. doi: 10.1155/2001/239602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torosyan Y, Dobi A, Naga S, Mezhevaya K, Glasman M, Norris C, Jiang G, Mueller G, Pollard H, Srivastava M. Distinct effects of annexin A7 and p53 on arachidonate lipoxygenation in prostate cancer cells involve 5-lipoxygenase transcription. Cancer Res. 2006;66:9609–16. doi: 10.1158/0008-5472.CAN-06-1574. [DOI] [PubMed] [Google Scholar]
- 13.Gerelsaikhan T, Vasa PK, Chander A. Annexin A7 and SNAP23 interactions in alveolar type II cells and in vitro: a role for Ca (2+) and PKC. Biochim Biophys Acta. 2012;1823:1796–806. doi: 10.1016/j.bbamcr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chander A, Gerelsaikhan T, Vasa PK. Annexin A7 trafficking to alveolar type II cell surface: possible roles for protein insertion into membranes and lamellar body secretion. Biochim Biophys Acta. 2013;1833:1244–55. doi: 10.1016/j.bbamcr.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mears D, Zimliki CL, Atwater I, Rojas E, Glassman M, Leighton X, Pollard HB, Srivastava M. The Anx7 (+/-) knockout mutation alters electrical and secretory responses to Ca (2+) -mobilizing agents in pancreatic beta-cells. Cell Physiol Biochem. 2012;29:697–704. doi: 10.1159/000186926. [DOI] [PubMed] [Google Scholar]
- 16.Taniuchi K, Yokotani K, Saibara T. BART inhibits pancreatic cancer cell invasion by PKCα inactivation through binding to ANX7. PLoS One. 2012;7:e35674. doi: 10.1371/journal.pone.0035674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu PI, Huang MS, Chen HC, Hsu PN, Lai TC, Wang JL, Lo GH, Lai KH, Tseng CJ, Hsiao M. The significance of Annexin A7 expression and its correlation with poor cellular differentiation and enhanced metastatic potential of gastric cancer. J Surg Oncol. 2008;97:609–14. doi: 10.1002/jso.21046. [DOI] [PubMed] [Google Scholar]


