Abstract
Brain-invasive meningiomas have an adverse prognosis, so it is important to detect and correctly evaluate brain invasion by light microscopy. Furthermore, the underlying biological mechanisms responsible for brain-invasive growth are incompletely understood. The primary aim of this study was to identify immunohistochemical markers that could improve identification and evaluation of brain invasion in meningiomas. A second aim was to investigate the process of brain invasion using immunohistochemical markers of proliferation, extracellular matrix modulation, and cell adhesion. From a series of 196 human meningiomas, 67 cases were selected for analysis because of the presence of brain tissue in tumor specimens. Fourteen of these 67 meningiomas were brain-invasive. Invasiveness was determined primarily by evaluation of hematoxylin-erytrosin-saffron- (HES-) stained specimens, although glial fibrillary acidic protein (GFAP), anti-collagen IV, and cluster of differentiation 44 (CD44) markers provided additional information. It was important to examine microscopic sections from various levels of the paraffin-embedded tissue block to adequately assess invasiveness. Sections stained using antibodies against Ki-67/MIB-1, phospohistone-H3 (PHH3), matrix metalloproteinase-9 (MMP-9), cathepsin D, plasminogen activator inhibitor-1 (PAI-1), and E-cadherin antigens were used to characterize brain-invasive meningiomas and to investigate the process of brain invasion. Only increased expression of the extracellular matrix modulator MMP-9 correlated with brain-invasive growth (p=0.025). Examination of HES-stained sections identified brain invasion. Use of relevant immunohistochemical markers did not contribute substantially to this evaluation. Evaluation of stepwise sections should be considered when brain-invasive growth is suspected. MMP-9 may be an important mediator of brain-invasive growth.
Keywords: GFAP, EMA, collagen IV, Ki-67/MIB-1, PHH3, MMP-9, cathepsin D, PAI-1, E-cadherin, CD4
Introduction
Meningiomas are derived from arachnoidal cells and are the most common intracranial tumor. They compress the surrounding brain tissue when they expand, and they are most often bound by the pial-glial basement membrane as a border. However, more aggressive meningiomas can demonstrate a brain-invasive growth pattern [1,2]. Invasiveness can be characterized histologically by “irregular, tongue-like protrusions of tumor cells infiltrating underlying parenchyma, without an intervening layer of leptomeninges” together with reactive astrocytosis in adjoining brain tissue [3,4]. The World Health Organization (WHO) 2007 classification considers all brain-invasive meningiomas prognostically equal to WHO grade II [3]. Patients with these tumors are followed more closely postoperatively and are considered for adjuvant radiotherapy to prolong their survival. Therefore, it is important to correctly decide whether a meningioma is brain-invasive or not based on microscopic evaluation. This can be difficult on hematoxylin-erytrosin-saffron- (HES-) sections despite the clear-cut definition. Consequently, appropriate immunohistochemical markers could assist in this evaluation.
Markers that could be used to facilitate histological detection and assist in determining brain-invasive growth in meningiomas include epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), collagen IV, and cluster of differentiation 44 (CD44). EMA and GFAP antibodies stain meningioma and brain tissue, respectively, making it easier to visualize any brain-meningioma interface and to detect brain tissue that is encompassed by an invasive meningioma [5-7]. The leptomeningeal pial-glial basement membrane, which disappears in areas of brain invasion, can be stained with an anti-collagen type IV antibody [4-7]. Anti-collagen type IV antibodies might also help to distinguish meningiomas with a thin surrounding leptomeningeal layer that interdigitate brain parenchyma from true brain-invasive tumors [5,7]. CD44 immunoreactivity is reported to be present in subpial astrocytes when the basement membrane is intact and to disappear in areas where invasive meningiomas disrupt the basement membrane and glia limitans [6,8]. Accordingly, some of these markers might assist in the evaluation of brain-invasive growth in meningiomas.
The biological mechanisms underlying brain-invasive growth in meningiomas are incompletely understood [9,10]. This is especially true for brain-invasive growth by otherwise benign tumors [4,11-13]. Thus, there is a need for independent biological markers that can identify more aggressive and recurrent tumors. Tumor invasion can be described as a stepwise process involving the degradation of the extracellular matrix (ECM), tumor cell adhesion to resident cells or components, increased proliferation, and cell migration into new intracellular/ECM space [13-15]. A better understanding of these invasive mechanisms could lay the groundwork for the development of new therapeutic strategies [13,14].
The present study was designed to investigate the roles of cell proliferation, ECM modulation, and cell adhesion in brain-invasive meningiomas by assessing the presence, absence, and localization of proteins. We used the proliferative markers Ki-67/MIB-1 and phospohistone-H3 (PHH3) to investigate whether brain-invasive meningiomas have greater proliferative potential than non-invasive ones [9,16-19].
To investigate how brain-invasive meningiomas alter the ECM, we used three different markers on ECM modulation, matrix metalloproteinase-9 (MMP-9), cathepsin D, and plasminogen activators inhibator-1 (PAI-1). The zinc-dependent endopeptidase MMP-9 degrades ECM, including collagen IV [13]. The correlation between MMP-9 and brain invasion in meningiomas is of particular interest because strong expression of MMP-9 is correlated with higher grade, increased invasiveness, and poorer survival [20-23], although the results are somewhat ambiguous [5,24].
The proteolytic enzyme cathepsin D is another interesting ECM modulator that is found mainly in lysosomes and endosomes. High expression levels of cathepsin D stimulate proliferative activity and ECM degradation and increase the metastatic potential [25], although the underlying mechanisms seem complex and are incompletely understood [26]. In meningiomas, most studies have found an inversely proportional correlation between cathepsin D expression and tumor grade [13,27-29]. However, the relationship between cathepsin D expression and brain invasion in meningiomas is not well described [29].
Plasminogen activators and their inhibitor PAI-1 are involved in fibrinolysis, ECM degradation, cell migration, and tumor invasion [30]. Elevated levels of PAs in parallel with PAI-1 have been found in several malignant tumors [30], which is somewhat paradoxical [31]. It is hypothesized that PAI-1 can act to protect tumor cells at the tumor-host interface [31]. The interactions between PAs and PAI-1 in cancer development are both intriguing and complex [30,31]. In meningiomas, high levels of PAs and PAI-1 have been correlated with malignancy and invasiveness [31-33], but the association between the expression of PAI-1 and brain-invasive growth is poorly described using immunohistochemical methods [31,32].
Brain invasion requires meningioma cells to adhere to resident cells or to ECM in order to migrate into the intracellular space. Accordingly, we used immunohistochemistry to investigate the immunohistochemical expression of two cell adhesion molecules, E-cadherin CD44. E-cadherin is a Ca2+-dependent transmembrane protein that is involved in cell-cell adhesion in epithelial tissue and that is down-regulated in several malignant and invasive cancers [9,34-38]. Decreased expression is inversely correlated to WHO grade, poor patient outcome, and soft tissue invasion in meningiomas [35,36,38-40], although reports are conflicting [34,37,41,42]. Few studies have looked at E-cadherin expression in brain-invasive meningiomas, and there is no clear association between expression of E-cadherin and invasiveness [9,37,38].
The cell-surface glycoprotein CD44 is a multifunctional receptor that is expressed on most human cells. Normally, CD44 is crucial for regulating of cell adhesion, proliferation, migration, angiogenesis, inflammation, and cell signaling [43]. Its principal ligand, hyaluronan, is an important ECM component that is also abundant in brain tissue [44,45]. Several studies show enhanced CD44 expression in aggressive and invasive cancers [43], suggesting that CD44 may act as a cell surface receptor for proteolytically active MMP-9 [46]. In meningiomas, anti-CD44 antibodies generally stain fibroblastic and lower grade tumors weaker than they stain meningothelial and transitional subtypes and higher grade tumors [8,29,42,47-50]. Few studies have addressed the relationship between brain-invasive growth and CD44 expression in meningiomas without finding a clear association [8,29].
This study was designed to identify immunohistochemical markers that could be used to assess brain-invasive growth in meningiomas and to investigate the process of brain invasion using immunohistochemical markers of proliferation, ECM modulation, and cell adhesion. Furthermore, we analyzed clinical, histopathological, and survival data to determine whether there were differences between brain-invasive and non-invasive meningiomas.
Methods
Patients and samples
The material was collected at St. Olavs Hospital, Trondheim University Hospital. All neurosurgical care in Mid-Norway (680 110 habitants in 2011) is centralized at this hospital [51]. All patients treated for primary, intracranial meningioma from January 1, 1991 to December 31, 2000, were identified by searching the electronic patient file database at the Department of Pathology and Medical Genetics using SNOMED number M953xx. This retrieved the records of 202 patients treated for meningioma, and we carefully reviewed all of the available microscopic slides of each patient. Six patients were excluded due to inadequate microscopic specimens. The present study included all patients (n=67) whose microscopic specimen showed brain tissue.
Clinical data
Clinical data were collected from medical records at St. Olavs Hospital and at other local hospitals. The recorded clinical data include sex, preoperative WHO performance score, tumor localization, resection grade, postoperative radiation, and recurrence and/or death. Tumor location was determined by CT or MRI analysis. Evaluation of gross total removal (GTR) or subtotal resection (STR) was registered from the surgeon’s report [52]. Information about patient death was collected from the Norwegian Cause of Death Registry. The date of follow-up completion was January 1, 2009; there was a minimum follow-up of 8 years and a maximum follow-up period of 18 years.
The Regional Committee for Medical and Health Research Ethics approved the study (project number 4.2006.947).
Histopathology
A Nikon 80i light microscope was used for the microscopical analyses. Formalin-fixed and paraffin-embedded HES-stained sections from the 67 cases were reviewed without knowledge of prior grading or patient outcome. All available specimens for each case were paraffin embedded.
Brain-invasive growth was defined as “irregular, tongue-like protrusions of tumor cells infiltrating underlying parenchyma, without an intervening layer of leptomeninges” plus reactive astrocytosis in adjoining brain tissue (Figure 1A) [3,4]. Meningioma infiltration alongside Robin-Virchow spaces was not considered brain invasion. Brain invasion was definitively determined using findings from the HES-stained section if there were conflicting findings from the GFAP-, CD44-, and anti-collagen IV-stained sections. Histological grading was performed using the WHO 2007 classification grading criteria [3].
Figure 1.
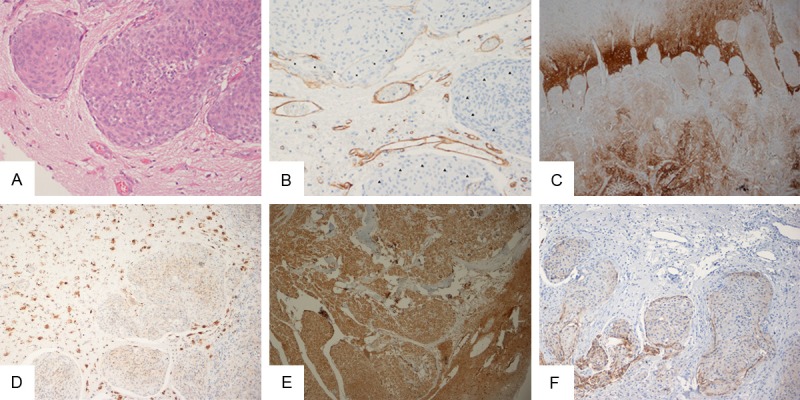
(A) Brain invasion as seen in a HES-stained specimen; (B) Collagen IV immunoreactivity (marked by asterisks) as seen in the pia around the main front of the meningioma towards the brain parenchyma. Protruding islands of meningioma tissue without immunoreactive pia are marked with arrowheads. Immunoabeled endothelium serves as an internal positive control; (C) Strong CD44 immunoreactivity in gliotic brain tissue that shows areas of meningioma invasion; (D) Cathepsin D labeled brain-invasive meningioma (right) and neurons (left); (E) Brain-invasive meningioma and brain tissue strongly stained with MMP-9; (F). E-cadherin labeled meningioma cells that collectively invade brain tissue. Magnification, 20× (A, B), 4× (C), 10× (D-F).
The following histological parameters were assessed as present or absent in all cases: mitoses per 10 high-power fields (40x objective), sheeting, necrosis, small cell formation, hypercellularity, macronucleoli, nuclear pleomorphism, apoptosis, vesicular nuclei, fibrosis, soft tissue infiltration, hypervascularization, lymphocytes, lipidization, hemosiderin, and ps ammoma bodies. The parameters were evaluated in accordance with accepted definitions [53].
Immunohistochemistry
The paraffin-embedded tissue block for each case with the most representative microscopic slide was chosen for immunohistochemical staining analysis. A list of commercial antibodies and protocol information is shown in Table 1. Representative 4 ìm thick sections of tumor tissue were incubated with primary antibodies after heat-induced epitope-retrieval (HIER) using DAKO PT Link (Dako, Glostrup, Denmark) and quenching of endogenous peroxidase activity with 3% hydrogen peroxide. All antibodies were incubated at room temperature for 40 minutes, except for PHH3, which was incubated for 60 minutes. Immunohistological staining was performed using the DAKO Autostainer and the DAKO EnVision™ detection system with diaminobenzidine (DAB+) as the chromogene. Sections were counterstained with hematoxylin.
Table 1.
Antibodies and immunohistochemical procedures used in this study
| Antibody | Manufacturer | Host | Clonality | Clone/Epitope | Positive control | Dilution | HIER* |
|---|---|---|---|---|---|---|---|
| EMA | Dako Denmark AS, Glostrup, DK | Mouse | Monoclonal | E-29 | Mamma | 1:750 | pH 9 |
| GFAP | Dako Denmark AS, Glostrup, DK | Rabbit | Polyclonal | Human medulla-blastoma | 1:2500 | pH 9 | |
| Collagen IV | Dako Denmark AS, Glostrup, DK | Mouse | Monoclonal | CIV 22 | Appendix | 1:25 | pH 6, 1 |
| Ki-67/MIB-1 | Dako Denmark AS, Glostrup, DK | Mouse | Monoclonal | MIB-1 | Human medulla-blastoma | 1:100 | pH 6 |
| PHH3 | Upstate, EMD Millipore Corp., Billerica, MA, USA | Rabbit | Polyclonal | Ser10 | Human medulla-blastoma | 1:2000 | pH 9 |
| MMP-9 | Dako Denmark AS, Glostrup, DK | Rabbit | Polyclonal | Tonsil | 1:10 | pH 9 | |
| Cathepsin D | Leica Biosystems Newcastle Ltd, Newcastle, UK | Mouse | Monoclonal | C5 | Liver | 1:200 | pH 9 |
| PAI-1 | Abcam Inc., Cambridge MA, USA | Mouse | Monoclonal | 1D5 | Appendix | 1:500 | pH 9 |
| E-Cadherin | Dako Denmark AS, Glostrup, DK | Mouse | Monoclonal | NCH-38 | Tonsil | 1:100 | pH 9 |
| CD44 | Dako Denmark AS, Glostrup, DK | Mouse | Monoclonal | DF1485 | Tonsil | 1:25 | pH 6, 1 |
Heat-induced epitope retrieval.
The expression of MMP-9, cathepsin D, PAI-1, E-cadherin, and CD44 were evaluated semi-quantitatively using an immunohistochemical scoring model that assessed the sum of the extent of staining (1: 0-30%; 2: 30-60%; 3: 60-100%) and intensity (1: weak; 2: moderate; 3: strong). The immunohistochemical score was correlated with brain-invasive status, WHO grade, and recurrence (Table 2). The expression of all immunohistochemical markers was evaluated both in areas with strong immunoreactivity centrally in the tumor tissue and adjacent to the brain parenchyma. These two evaluations were compared. PHH3 was only evaluated centrally in specimens, because there were so few labeled cells in the tumor tissue adjoining the brain parenchyma.
Table 2.
Associations between expression of immunohistochemical markers and brain-invasive status, WHO grade, and recurrence
| Marker | Brain invasion | WHO 2007 grade | Recurrence | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Yes | No | P-value | Grade II | Grade I | P-value | Yes | No | P-value | |
|
|
|
|
|||||||
| n=14 | n=53 | n=33 | n=34 | n=23 | n=44 | ||||
| Proliferation | |||||||||
| Ki-67/MIB-1 | |||||||||
| Mean rank: | 41.18 | 32.10 | 0.121 | 44.85 | 23.47 | < 0.001 | 35.20 | 33.38 | 0.716 |
| U=271 | z=-1.55 | U=919 | z=4.49 | U=479 | z=-0.36 | ||||
| Median: | 3.1 | 1.7 | 3.2 | 1.4 | 1.7 | 1.7 | |||
| PHH3 | |||||||||
| Mean rank: | 33.82 | 34.05 | 0.968 | 44.37 | 23.59 | < 0.001 | 37.0 | 32.43 | 0.349 |
| U=374 | z=0.04 | U=915 | z=4.57 | U=437 | z=-0.94 | ||||
| Median: | 2 | 2 | 4 | 1 | 2 | 2 | |||
| ECM modulation | |||||||||
| MMP-9 | |||||||||
| Mean rank: | 41.50 | 32.02 | 0.025 | 36.50 | 31.57 | 0.153 | 36.87 | 32.50 | 0.228 |
| U=266 | z=-2.24 | U=644 | z=1.43 | U=440 | z=-1.21 | ||||
| Cathepsin D | |||||||||
| Mean rank: | 37.07 | 33.19 | 0.486 | 38.18 | 29.94 | 0.069 | 33.85 | 34.08 | 0.961 |
| U=328 | z=-0.70 | U=699 | z=1.82 | U=510 | z=0.05 | ||||
| PAI-1 | |||||||||
| Mean rank: | 35.32 | 33.65 | 0.759 | 35.70 | 32.35 | 0.450 | 31.37 | 35.38 | 0.391 |
| U=353 | z=-0.31 | U=617 | z=0.76 | U=567 | z=0.86 | ||||
| Cell adhesion | |||||||||
| E-cadherin | |||||||||
| Mean rank: | 33.43 | 34.15 | 0.899 | 36.08 | 31.99 | 629.5 | 33.39 | 34.32 | 0.859 |
| U=379 | z=0.13 | U=630 | z=0.88 | U=520 | z=0.19 | ||||
| CD44 | |||||||||
| Mean rank: | 37.42 | 31.89 | 0.333 | 33.73 | 32.29 | 0.751 | 31.17 | 34.00 | 0.554 |
| U=281 | z=-0.97 | U=552 | z=0.32 | U=525 | z=0.59 | ||||
Statistically significant p-values are in bold. P-values were determined using the Mann-Whitney U test. ECM, extracellular matrix.
PHH3-positive nuclei were recorded in 10 consecutive high-power fields; only positively-stained nuclei with chromatin changes equivalent with mitotic figures were counted. The Ki-67/MIB-1 labeling index was defined as the percentage of distinctly immunoreactive tumor cells out of the total number of cells inside an ocular grid reticle (0.058 mm2). The labeling index for Ki-67/MIB-1 was both calculated as the mean of three individual counts in central areas with high proliferative activity and counted once in areas that were in the vicinity of brain tissue, because of sparse tumor tissue.
Statistical analysis
SPSS version 18.0 (SPSS in., Chicago, IL) was used for statistical analyses. P-values less than 0.05 were regarded as being statistically significant.
The chi-square exact test was performed to analyze associations between clinical or tumor variables and brain-invasive growth (Table 3), and, similarly, to calculate p-values for the associations between histopathological parameters and brain-invasive growth (statistics not shown). Fischer’s exact test was used when the expected cell frequency was less than five. The chi-square test was also performed when one variable consisted of more than two groups even though the expected cell frequency was less than five (see ‘localization’ and ‘cause of death’ in Table 3).
Table 3.
Clinical and tumor characteristics
| Clinical and tumor characteristics | Description | n | Brain-invasive (n=14) | Non-brain-invasive (n=53) | P-value |
|---|---|---|---|---|---|
| Sex | Female | 51 | 8 | 43 | 0.082 |
| Male | 16 | 6 | 10 | ||
| WHO performance score | 0-1 | 56 | 13 | 43 | 0.435 |
| 2-4 | 11 | 1 | 10 | ||
| Localization | Falcine | 11 | 3 | 8 | 0.915 |
| Convexity | 33 | 7 | 26 | ||
| Basal | 13 | 2 | 11 | ||
| Tentorial and posterior fossa | 10 | 2 | 8 | ||
| Surgeon’s evaluation of resection grade | Gross total removal | 50 | 8 | 42 | 0.092 |
| Subtotal resection | 17 | 6 | 11 | ||
| Post-operative radiation | Yes | 10 | 5 | 5 | 0.027 |
| No | 57 | 9 | 48 | ||
| Recurrence | Yes | 23 | 5 | 18 | 1.0 |
| No | 44 | 9 | 35 | ||
| Death by January 1, 2009 (n=28) | Tumor related | 7 | 2 | 5 | 0.929 |
| Other cancer entity | 7 | 2 | 5 | ||
| Other | 13 | 3 | 10 | ||
| Unknown | 1 | 0 | 1 |
Statistically significant p-values are in bold numbers.
Mann-Whitney U tests were performed to determine if there were differences in the expression of various immunohistochemical markers between brain-invasive and non-invasive, WHO grade I and II, and recurrent and not recurrent meningiomas (Table 2). The markers’ distributions of immunoreactivity were assessed by visual inspection as similar between brain-invasive and non-invasive, WHO grade I and II, and recurrent and non-recurrent meningiomas.
Time to recurrence (TTR) was used as the survival endpoint because meningiomas are generally slow growing tumors and thus death due to causes other than meningioma can bias the survival analyses. TTR is a measure of the time from diagnosis to recurrence of the disease or to disease-related death, while death from other causes is censored. The date of recurrence was defined using radiology data from CT and/or MRI reports. The Kaplan-Meier method was used to create survival plots related to TTR, with p-values determined by log rank calculations and a maximum follow-up period of 15 years.
Results
Patients
The clinical and tumor characteristics of the 67 patients are shown in Table 3. The tumor samples were from 34 WHO grade I and 33 WHO II grade meningiomas. Postoperative radiation therapy was given to all patients with curative intent. Brain-invasive meningiomas were often treated with adjuvant radiation therapy (p=0.027).
Immunohistochemical markers for evaluating brain-invasion in meningiomas
The brain-invasion status changed in 6 cases from the original evaluation of HES slides to the final review based on careful inspection of the HES-, EMA-, GFAP-, CD44-, and anti-collagen IV-stained slides (Table 4). The level at which the paraffin-embedded tissue block was cut was important in 2 of the 6 cases, since cutting slices for new slides revealed areas with focal brain invasion that were otherwise not apparent. In a third case, brain invasion was observed only in the original HES section and not in the immunohistochemical slides with tissue sections from deeper in the tumor. Thus, the cutting level of the paraffin-embedded tissue block was critical for correct evaluation of brain-invasive growth in 3 of the 14 brain-invasive meningiomas.
Table 4.
Comparison of the number of meningiomas that were considered brain-invasive at the first and final evaluations
| First evaluation | ||||
|---|---|---|---|---|
|
|
||||
| Brain-invasive | Non-brain-invasive | Total | ||
| Final review | Brain-invasive | 12 | 2 | 14 |
| Non-brain-invasive | 4 | 49 | 53 | |
| Total | 16 | 51 | 67 | |
After closer microscopic evaluation and strict adherence to the definition of brain-invasive meningioma, 4 of the 6 cases were changed from ‘brain-invasive’ to ‘non-brain-invasive’ in the final review (Table 4). The HES-stained sections were conclusive for performing this evaluation, although the other immunohistochemical markers provided some additional information (Figure 1A). This is explained in more detail below.
Slides stained for GFAP made it easier to identify even small areas of brain tissue and thus allowed us to examine of all meningioma-brain interfaces. We did not find that GFAP provided additional valuable information about brain-invasiveness beyond that obtained from HES-stained slides. Specifically, GFAP expression was not altered in brain-invasive areas.
EMA immunoreactivity was generally strong and widespread and was thus well suited for verification of the meningioma diagnosis. No specific staining pattern was observed, either in areas adjacent to the brain tissue or in components that invaded the brain. EMA staining did not have any decisive impact on the question of brain invasion.
The anti-collagen IV antibody stained the leptomeninges and vessels (Figure 1B). In cases with an indistinct meningioma-brain interface in HES-stained specimens, collagen IV immunoreactivity in the pia provided additional support for the conclusion that a meningioma was non-brain-invasive. However, some structures that were identified morphologically as pia were unlabeled or were only partially labeled by the anti-collagen IV antibody in several of the non-brain-invasive meningiomas. On the contrary, a thin immunolabeled pia-like structure sometimes covered brain-invasive meningioma protrusions.
Brain tissue, and especially gliotic astrocytes, was labeled strongly by the CD44 antibody (Figure 1C). Similar to GFAP immunostaining, CD44 immunostaining made it easier to detect of brain tissue. We did not observe a decreased CD44 immunoreactivity in areas where there was brain tissue invasion. In fact, immunoreactivity in brain tissue seemed generally stronger towards the glia limitans (Figure 1C). No correlation was found between CD44 expression and specific meningioma subtypes.
Immunohistochemical characterization of brain-invasive meningiomas
Table 2 shows the expression of markers of proliferation, ECM modulation, and cell adhesion and their association with brain-invasive status, WHO grade, and recurrence. None of the markers was significantly correlated with the risk of recurrence.
Regarding the ECM modulator MMP-9, its immunoreactivity was cytoplasmic and heterogeneously distributed in the meningioma tissue. Glial cells were immunoreactive, whereas leptomeninges and vessels were MMP-9 negative. The only marker that displayed significantly higher expression in brain-invasive meningiomas was MMP-9 (p=0.025). Cathepsin D expression showed a trend for higher expression in grade II meningiomas (p=0.069). Cathepsin D immunoreactivity was otherwise cytoplasmic and abundant (Figure 1D). Lymphocytes, neurons, and macrophages were labeled by antibodies to cathepsin D while leptomeninges were not.
Concerning cell adhesion markers, the cytoplasmic immunoreactivity of PAI-1 was generally widespread and moderate to strong (Figure 1E). Brain tissue and leptomeninges were not labeled by antibodies to PAI-1, while the endothelium was stained. E-cadherin expression was membranous and similar to that observed in carcinomas (Figure 1F). The meninges were predominantly negative for E-cadherin expression.
There were no differences in the expression patterns of MMP-9, cathepsin D, PAI-1, E-cadherin, and CD44 in central tumor tissue compared with areas adjoining brain tissue. This was true for both brain-invasive and non-brain-invasive cases.
Regarding markers of proliferation in brain-invasive cases, the Ki-67/MIB-1 labeling indices in central tissue and in the meningioma adjoining brain parenchyma were 3.1 and 3.0, respectively. The expression of Ki-67/MIB-1 and PHH3 was significantly higher in WHO grade II tumors (p < 0.001). Immunostaining of PHH3 made it easier to detect suspicious mitotic figures at lower microscopic magnification. However, we observed that a few distinct mitotic figures were not labeled, while some cells without appearances typical of either tumor cells or mitotic figures were immunoreactive (leukocytes, for instance). Some background staining was also observed in slides of PHH3, especially in areas with abundant vessels. This could make it difficult to count positive cells.
Histological features and meningioma subtypes
Regarding correlations between histological features and brain-invasiveness, only the presence of macronucleoli showed a statistically significant association with brain-invasion (p=0.03) (data not shown). Of the 14 brain-invasive meningiomas, 4 had otherwise benign histology according to WHO classification. We observed two meningiomas of the clear cell subtype and one of the lymphoplasmacyte-rich type. Neither was brain-invasive.
Survival data
Figure 2 shows a Kaplan-Meier plot of the material divided by brain-invasive status in relation to TTR (p=0.615). In the present study there was not a significant difference in survival between patients with WHO grade I and II meningiomas (p=0.691). In the original cases (n=196), from which these cases (n=67) that had microscopically detected brain tissue were selected, there was a statistically significant difference between grade I and II tumors (p=0.047) [54].
Figure 2.
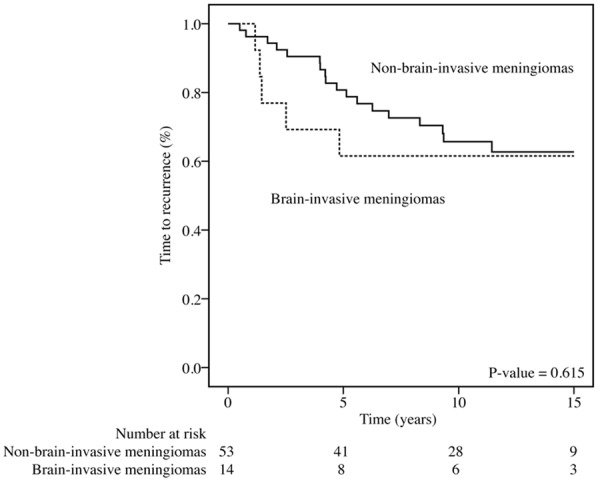
Fifteen-year survival rates for patients with brain-invasive or non-invasive meningiomas (Kaplan-Meier plot). TTR, time to recurrence. The p-value was determined using the log-rank test.
Discussion
In this study, we investigated whether immunohistochemical markers could aid in the histological assessment of brain-invasive growth in meningiomas and then explored the process of brain invasion using immunohistochemical markers of proliferation, ECM modulation, and cell adhesion. First, we observed that HES-stained sections cut from different levels of the paraffin-embedded tissue block were sufficient to identify brain-invasive growth. Second, we found that the ECM modulator MMP-9 might be involved in the biological process of brain invasion in meningiomas.
Our first finding was that sections that were cut stepwise and HES-stained are adequate for evaluating the presence or absence of brain invasion. This point of view is based on our experience with the immunohistochemical markers EMA, GFAP, CD44, and collagen IV. The value of observing deeper tissue sections was also clear: 3 of the 14 brain-invasive meningiomas would not have been identified as such if stepwise sections had not been analyzed. Thus, examination of stepwise sections is recommended for determining whether a meningioma is brain-invasive.
None of the immunohistochemical markers used to evaluate brain invasion provided additional information that changed the evaluation made on the HES-stained sections. Nevertheless, it was our experience that the markers complemented each other by highlighting different tissue structures, thus increasing our understanding and assessment skills of brain-invasive growth in meningiomas. As an example, HES-stained sections were satisfactory for making the meningioma diagnosis, for detecting brain tissue, and for evaluating brain-invasion. GFAP- and CD44-stained slides made it easier to detect brain tissue and were useful for confirming our identification of all relevant brain-meningioma interfaces. Similarly, observing EMA-stained sections confirmed the meningioma diagnosis and showed the relationship of meningioma tissue to brain tissue. Regardless of the markers used, we have to emphasize the importance of always carefully searching for brain tissue and reporting its presence or absence in relation to meningioma tissue, as this has important prognostic implications [1,3]. Our results do not support the findings of Zeltner and co-workers that decreased GFAP and CD44 expression at the tumor-brain interface of invasive meningiomas correlates with the disappearance of astrocytes in these areas (Figure 1C) [6]. The reason for this is not clear, but we speculate whether increased expression of CD44 and GFAP in gliotic astrocytes may have masked the decreased number of astrocytes. We did not find a correlation between CD44 expression and brain-invasive growth, nor was there a correlation with WHO grade, in contrast with the findings of others [8,29]. This may be because there is no such relationship, because the studies used antibodies of different manufactures or to different CD44 isoforms, or because of study limitations.
Use of an anti-collagen IV antibody was helpful in assessing brain-invasive growth in meningiomas because this antibody labeled leptomeninges and thus made it easier to identify the morphology of pia in HES-stained slides. We confirmed the finding by Nakasu et al. that using an anti-collagen IV antibody is useful for assessing pia as an intervening basement membrane in tight meningioma-brain interfaces when there is suspected brain invasion [5]. However, we also observed that variable immunostaining of pia could lead to misinterpretation of non-invasive meningiomas as brain-invasive and that weak collagen IV immunoreactivity around projections of invasive meningiomas could be misinterpreted as non-brain-invasive growth. The difficulties in interpreting collagen IV-stained sections may be due to the methodological aspects of immunohistochemistry or may be due to the fact that pia is a fragile structure that can easily be damaged when a sample is processed for immunohistochemical analysis. Alternatively, brain invasion is a dynamic, gradual, and complex process, and the collagen IV staining pattern may reflect this. In support of this view, Schittenhelm et al. reported that brain invasion can precede pial-glial basement membrane disruption in otherwise benign meningiomas [7], while brain invasion in higher grade meningiomas seems to start with disruption of the basement membrane [6]. Furthermore, based on their interpretation of collagen IV-stained specimens, Fritz et al. described the formation of a new basement membrane that was closer to the meningioma than the pial-like basement membrane. Hence, they hypothesized that basement membrane degradation and synthesis take place in parallel [4]. Accordingly, collagen IV staining for routine assessment of brain-invasiveness in meningiomas is of limited value.
Our second finding was that MMP-9 is highly expressed in brain-invasive meningiomas. This supports the notion that MMP-9 is important for ECM modulation and brain-invasiveness by meningiomas [13,22]. This makes sense from a physiological standpoint because MMP-9 has collagenolytic activity and collagen IV is a main component of the pia. MMP-9 is an enzyme and the technique used in this study stained the whole protein without providing information whether the enzyme is active or not. Similar to von Randow et al., we did not find a specific expression pattern for MMP-9 nor did we find increased expression of MMP-9 in the invasive front [13]. Furthermore, the ubiquitous expression of MMP-9 supports a role for this protein in meningioma tumorigenesis as in other tumors [55,56]. Accordingly, MMP family members may exert additional effects, including modulation of extracellular tissue, angiogenesis, and invasion [57], which are also features typical of meningiomas [3]. We observed only a weak trend of higher MMP-9 expression in grade II and recurrent meningiomas. This might explain the conflicting results concerning MMP-9 and brain tumor prognosis in the literature [5,20-24,58]. The expression of MMP-9 in meningiomas in general and especially in brain-invasive meningiomas is interesting because MMP-9 might represent a therapeutic target for meningioma treatment [13,21,58-60].
Regarding proliferation markers, both PHH3 and Ki-67/MIB-1 labeling indices were significantly increased in grade II meningiomas, in agreement with published reports [19,61,62]. However, the indices were not significantly increased in brain-invasive meningiomas. In fact, the proliferative activity was almost equal in brain-invasive areas compared with the rest of the tumor. This may be because there are more hot spots for counting Ki-67/MIB-1 reactive cells in the central part of the specimen. Nevertheless, tumor cells with high proliferative activity do not seem to be a prerequisite for brain-invasive growth, but the literature is conflicting and this issue is not clear [9,17,18]. These differences in proliferative potential in tumor samples may also, for instance, be due to the clonal diversity of tumor cells [63,64].
The immunohistological markers cathepsin D, PAI-1, and E-cadherin were not expressed at significantly higher levels in brain-invasive meningiomas. Several forms of the protease cathepsin D are expressed in normal cells and serve housekeeping functions. This protein may also play a role in cancer development, progression, and invasion [25,26]. Because it is expressed at physiological levels in normal cells and because there are many variants of cathepsin D, it has been difficult to estimate its prognostic impact. Special visualization techniques may be required for it to be useful in determining prognosis [26]. This may explain why, in contrast to others, we found a trend of higher cathepsin D expression in WHO grade II tumors [13,27-29]. Our observations did not tie PAI-1 to brain invasion, in contrast with other studies [31-33]. It is possible that there is no relationship between PAI-1 expression and brain invasion. Alternatively, the discrepancy can be due to differences in techniques [32]. Finally, a local imbalance between PAI-1 and hyaluronan may be more important than the PAI-1 expression per se [31]. Concerning E-cadherin, our results lend support to other studies that indicate E-cadherin expression is independent of malignancy grade [34,37,41,42], ant that there is no clear correlation between E-cadherin expression and brain-invasive growth [9,37,38]. Down-regulation of E-cadherin, a shift from E-cadherin to N-cadherin expression, and an epithelial-to-mesenchymal transition are associated with single cell invasion, whereas cancers with collective cell invasion, which can be induced by podoplanin, retain E-cadherin expression [14,65,66]. We found that the typical adherent brain-invasive protrusions in meningiomas expressed E-cadherin. Taken together with the fact that meningiomas have been shown to express podoplanin [67], this may point towards podoplanin involvement in the biological mechanism of brain invasion in meningiomas.
Regarding the histological features of meningiomas, only the presence of macronucleoli showed a statistically significant correlation with brain-invasive growth. This finding must be confirmed in additional larger studies. None of the other WHO-defined features associated with malignancy in meningiomas correlated brain invasion, underlining the importance of thorough inspection for brain-invasive growth on microscopic examination.
The survival analyses did not reveal an increased risk of recurrence for patients with brain-invasive meningiomas. There was a clear trend, however, for brain-invasive meningiomas to relapse more frequently within the first 5 years post-surgery, and the graph was in accordance with expected survival prognosis for WHO grade II tumors [3]. The difference in survival between patients with grade I and grade II meningiomas was significant in the material comprising all the retrieved specimens available for histological review (n=196) but non-significant in this study; hence, the composition of the material in this study must have changed when only specimens with observed brain tissue were included and this seems to have altered the survival data [54]. Resection grade, which is an independent prognostic factor for meningiomas, may be such a factor that biases the prognostic value of WHO grade more in a smaller material.
The selection method could have anticipated the results, because established morphological criteria were used to select cases with brain parenchyma and not immunohistochemical stained specimens. Further, the selection of the material might have been biased as only HES-stained sections from one level of each paraffin-embedded tissue block was assessed at the time of selection, while later in the study we found that assessing of sections from various levels of the paraffin-embedded tissue block was necessary to correctly evaluate brain-invasive status in meningiomas. It is not necessary that the tumor is invasive over the whole surface and if the surgeon does not send the representative parts to the pathological department, there is a risk of misclassification. The limited sample size, which is the most common reason to commit a type II error i.e. to retain a false null hypothesis, could explain the poor correlation between some of the immunohistochemical markers and brain-invasive status, WHO grade, or recurrence. We did not perform any adjustment methods when performing multiple comparisons. Especially when performing multiple explorative tests is it likely that some tests will be significantly associated. In addition, methods other than immunohistochemistry or multiparameter approaches might be better suited to evaluate the biological mechanisms underlying invasiveness of meningiomas [68]. Use of immunohistochemical markers to investigate dural invasion of meningiomas is also of interest, because widespread dural invasion can complicate surgical removal, although it does not change the malignancy grade. Finally, it is difficult to say whether we used the best immunohistochemical markers to carry out the aims of the study. Cathepsin B might represent well-suited immunohistochemical marker for investigating brain-invasive meningiomas [69]. Further studies are needed to fully explore the phenomenon of brain invasion in meningiomas.
In conclusion, HES-stained sections are adequate for assessing brain invasion in human meningiomas. The use of relevant immunohistochemical markers did not improve this assessment. When brain invasion is considered, stepwise sections should be examined. The extracellular matrix modulator MMP-9 may be an important mediator of brain-invasive growth.
Acknowledgements
We would like to thank the following: David Scheie (MD, PhD) for constructive discussions; Jon D. Andresen for help with designing the figures; and Ivar S. Nordrum (MD, PhD, Prof.), Haakon Skogseth (PhD), Jørgen Sugar (MD), Martin Wohlwend and Line N. Hansen for constructive comments during the preparation of the manuscript.
Disclosure of conflict of interest
We have no financial conflicts of interest associated with this work. The manuscript has not been published previously and is not being submitted to any other journal. None of the authors have any financial support to disclose or any industry affiliation. None of the authors have any personal or institutional financial interests in materials or devices described in the submission.
Abbreviations
- CD44
Cluster of differentiation 44
- ECM
Extracellular matrix
- EMA
Epithelial membrane antigen
- GFAP
Glial fibrillary acidic protein
- GTR
Gross total removal
- HES
Hematoxylin-erytrosin-saffron
- MMP-9
Matrix metalloproteinase-9
- Pas
Plasminogen activators
- PAI-1
Plasminogen activators inhibitor-1
- PHH3
Phospohistone-H3
- STR
Subtotal resection
- WHO
World Health Organization
References
- 1.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Vranic A, Popovic M, Cor A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery. 2010;67:1124–32. doi: 10.1227/NEU.0b013e3181eb95b7. [DOI] [PubMed] [Google Scholar]
- 3.Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Tumours of the meninges. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System 4. Lyon: IARC press; 2007. pp. 163–172. [Google Scholar]
- 4.Fritz J, Roser F, Tatagiba M, Bornemann A. The basement membrane at the tumour-brain interface of brain-invasive grade I meningiomas. Neuropathol Appl Neurobiol. 2005;31:339–342. doi: 10.1111/j.1365-2990.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakasu S, Fukami T, Jito J, Matsuda M. Microscopic anatomy of the brain-meningioma interface. Brain Tumor Pathol. 2005;22:53–57. doi: 10.1007/s10014-005-0187-0. [DOI] [PubMed] [Google Scholar]
- 6.Zeltner L, Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Kim YJ, Bornemann A. The astrocytic response towards invasive meningiomas. Neuropathol Appl Neurobiol. 2007;33:163–168. doi: 10.1111/j.1365-2990.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 7.Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Bornemann A. Patterns of SPARC expression and basement membrane intactness at the tumour-brain border of invasive meningiomas. Neuropathol Appl Neurobiol. 2006;32:525–531. doi: 10.1111/j.1365-2990.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 8.Figarella-Branger D, Roche PH, Daniel L, Dufour H, Bianco N, Pellissier JF. Cell-adhesion molecules in human meningiomas: correlation with clinical and morphological data. Neuropathol Appl Neurobiol. 1997;23:113–122. [PubMed] [Google Scholar]
- 9.Utsuki S, Oka H, Sato Y, Kawano N, Tsuchiya B, Kobayashi I, Fujii K. Invasive meningioma is associated with a low expression of E-cadherin and beta-catenin. Clin Neuropathol. 2005;24:8–12. [PubMed] [Google Scholar]
- 10.Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. 2013;13:337. doi: 10.1007/s11910-013-0337-4. [DOI] [PubMed] [Google Scholar]
- 11.Rempel SA, Ge S, Gutierrez JA. SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res. 1999;5:237–241. [PubMed] [Google Scholar]
- 12.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 13.von Randow AJ, Schindler S, Tews DS. Expression of extracellular matrix-degrading proteins in classic, atypical, and anaplastic meningiomas. Pathol Res Pract. 2006;202:365–372. doi: 10.1016/j.prp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Price JT, Thompson EW. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin Ther Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217. [DOI] [PubMed] [Google Scholar]
- 15.Tews DS. Adhesive and invasive features in gliomas. Pathol Res Pract. 2000;196:701–711. doi: 10.1016/S0344-0338(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 16.Torp SH, Lindboe CF, Granli US, Moen TM, Nordtomme T. Comparative investigation of proliferation markers and their prognostic relevance in human meningiomas. Clin Neuropathol. 2001;20:190–195. [PubMed] [Google Scholar]
- 17.Madsen C, Schroder HD. Ki-67 immunoreactivity in meningiomas--determination of the proliferative potential of meningiomas using the monoclonal antibody Ki-67. Clin Neuropathol. 1997;16:137–142. [PubMed] [Google Scholar]
- 18.Suwa T, Kawano N, Oka H, Ito H, Kameya T. Invasive meningioma: a tumour with high proliferating and “recurrence” potential. Acta Neurochir (Wien) 1995;136:127–131. doi: 10.1007/BF01410613. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima S, Terasaki M, Sakata K, Miyagi N, Kato S, Sugita Y, Shigemori M. Sensitivity and usefulness of anti-phosphohistone-H3 antibody immunostaining for counting mitotic figures in meningioma cases. Brain Tumor Pathol. 2009;26:51–57. doi: 10.1007/s10014-009-0249-9. [DOI] [PubMed] [Google Scholar]
- 20.Okada M, Miyake K, Matsumoto Y, Kawai N, Kunishio K, Nagao S. Matrix metalloproteinase-2 and matrix metalloproteinase-9 expressions correlate with the recurrence of intracranial meningiomas. J Neurooncol. 2004;66:29–37. doi: 10.1023/b:neon.0000013474.01161.58. [DOI] [PubMed] [Google Scholar]
- 21.Barresi V, Vitarelli E, Tuccari G, Barresi G. MMP-9 expression in meningiomas: a prognostic marker for recurrence risk? J Neurooncol. 2011;102:189–196. doi: 10.1007/s11060-010-0312-8. [DOI] [PubMed] [Google Scholar]
- 22.Nordqvist AC, Smurawa H, Mathiesen T. Expression of matrix metalloproteinases 2 and 9 in meningiomas associated with different degrees of brain invasiveness and edema. J Neurosurg. 2001;95:839–844. doi: 10.3171/jns.2001.95.5.0839. [DOI] [PubMed] [Google Scholar]
- 23.Siddique K, Yanamandra N, Gujrati M, Dinh D, Rao JS, Olivero W. Expression of matrix metalloproteinases, their inhibitors, and urokinase plasminogen activator in human meningiomas. Int J Oncol. 2003;22:289–294. [PubMed] [Google Scholar]
- 24.Mizoue T, Kawamoto H, Arita K, Tominaga A, Kurisu K. Secretion of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by meningiomas detected by cell immunoblot analysis. Acta Neurochir. 1999;141:481–486. doi: 10.1007/s007010050328. [DOI] [PubMed] [Google Scholar]
- 25.Masson O, Bach AS, Derocq D, Prebois C, Laurent-Matha V, Pattingre S, Liaudet-Coopman E. Pathophysiological functions of cathepsin D: Targeting its catalytic activity versus its protein binding activity? Biochimie. 2010;92:1635–1643. doi: 10.1016/j.biochi.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Nicotra G, Castino R, Follo C, Peracchio C, Valente G, Isidoro C. The dilemma: does tissue expression of cathepsin D reflect tumor malignancy? The question: does the assay truly mirror cathepsin D mis-function in the tumor? Cancer Biomark. 2010;7:47–64. doi: 10.3233/CBM-2010-0143. [DOI] [PubMed] [Google Scholar]
- 27.Castilla EA, Prayson RA, Abramovich CM, Cohen ML. Immunohistochemical expression of cathepsin D in meningiomas. Am J Clin Pathol. 2003;119:123–128. doi: 10.1309/W0H7-05HA-JL73-T0EQ. [DOI] [PubMed] [Google Scholar]
- 28.Lusis EA, Chicoine MR, Perry A. High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol. 2005;73:219–223. doi: 10.1007/s11060-004-5233-y. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz J, Martinez A, Hernandez S, Zimman H, Ferrer M, Fernandez C, Saez M, Lopez-Asenjo JA, Sanz-Ortega J. Clinicopathological variables, immunophenotype, chromosome 1p36 loss and tumour recurrence of 247 meningiomas grade I and II. Histol Histopathol. 2010;25:341–349. doi: 10.14670/HH-25.341. [DOI] [PubMed] [Google Scholar]
- 30.Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43–66. doi: 10.1007/978-0-387-79962-9_4. [DOI] [PubMed] [Google Scholar]
- 31.Arai Y, Kubota T, Nakagawa T, Kabuto M, Sato K, Kobayashi H. Production of urokinase-type plasminogen activator (u-PA) and plasminogen activator inhibitor-1 (PAI-1) in human brain tumours. Acta Neurochir. 1998;140:377–385. doi: 10.1007/s007010050112. discussion 385-376. [DOI] [PubMed] [Google Scholar]
- 32.Kandenwein JA, Park-Simon TW, Schramm J, Simon M. uPA/PAI-1 expression and uPA promoter methylation in meningiomas. J Neurooncol. 2011;103:533–539. doi: 10.1007/s11060-010-0411-6. [DOI] [PubMed] [Google Scholar]
- 33.Kondraganti S, Gondi CS, McCutcheon I, Dinh DH, Gujrati M, Rao JS, Olivero WC. RNAi-mediated downregulation of urokinase plasminogen activator and its receptor in human meningioma cells inhibits tumor invasion and growth. Int J Oncol. 2006;28:1353–1360. [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada S, Ishizawa K, Hirose T. Expression of E-cadherin and catenins in meningioma: ubiquitous expression and its irrelevance to malignancy. Pathol Int. 2005;55:1–7. doi: 10.1111/j.1440-1827.2005.01786.x. [DOI] [PubMed] [Google Scholar]
- 35.Pecina-Slaus N, Nikuseva Martic T, Deak AJ, Zeljko M, Hrascan R, Tomas D, Musani V. Genetic and protein changes of E-cadherin in meningiomas. J Cancer Res Clin Oncol. 2010;136:695–702. doi: 10.1007/s00432-009-0708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecina-Slaus N, Cicvara-Pecina T, Kafka A. Epithelial-to-mesenchymal transition: possible role in meningiomas. Front Biosci (Elite Ed) 2012;4:889–96. doi: 10.2741/E427. [DOI] [PubMed] [Google Scholar]
- 37.Brunner EC, Romeike BF, Jung M, Comtesse N, Meese E. Altered expression of beta-catenin/E-cadherin in meningiomas. Histopathology. 2006;49:178–187. doi: 10.1111/j.1365-2559.2006.02440.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwechheimer K, Zhou L, Birchmeier W. E-Cadherin in human brain tumours: loss of immunoreactivity in malignant meningiomas. Virchows Arch. 1998;432:163–167. doi: 10.1007/s004280050151. [DOI] [PubMed] [Google Scholar]
- 39.Zhou K, Wang G, Wang Y, Jin H, Yang S, Liu C. The potential involvement of E-cadherin and beta-catenins in meningioma. PLoS One. 2010;5:e11231. doi: 10.1371/journal.pone.0011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 41.Figarella-Branger D, Pellissier JF, Bouillot P, Bianco N, Mayan M, Grisoli F, Rougon G. Expression of neural cell-adhesion molecule isoforms and epithelial cadherin adhesion molecules in 47 human meningiomas: correlation with clinical and morphological data. Mod Pathol. 1994;7:752–761. [PubMed] [Google Scholar]
- 42.Panagopoulos AT, Lancellotti CL, Veiga JC, de Aguiar PH, Colquhoun A. Expression of cell adhesion proteins and proteins related to angiogenesis and fatty acid metabolism in benign, atypical, and anaplastic meningiomas. J Neurooncol. 2008;89:73–87. doi: 10.1007/s11060-008-9588-3. [DOI] [PubMed] [Google Scholar]
- 43.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 44.Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Exp Cell Res. 2011;317:383–391. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Frischknecht R, Seidenbecher CI. The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 2008;4:249–257. doi: 10.1017/S1740925X09990226. [DOI] [PubMed] [Google Scholar]
- 46.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewy-Trenda I, Omulecka A, Janczukowicz J, Papierz W. CD44 expression in human meningiomas: an immunohistochemical analysis. Pol J Pathol. 2004;55:33–37. [PubMed] [Google Scholar]
- 48.Suzuki SO, Iwaki T, Kitamoto T, Mizoguchi M, Fukui M, Tateishi J. Differential expression of CD44 variants among meningioma subtypes. Clin Mol Pathol. 1996;49:M140–6. doi: 10.1136/mp.49.3.m140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cachia D, Alderson L, Smith T, Yunus S. Widely metastatic meningioma. Arch Neurol. 2012;69:1372–1373. doi: 10.1001/archneurol.2012.1. [DOI] [PubMed] [Google Scholar]
- 50.Rooprai HK, Liyanage K, King A, Davies D, Martin K, Pilkington GJ. CD44 expression in human meningiomas: An immunocytochemical, immunohistochemical and flow cytometric analysis. Int J Oncol. 1999;14:855–860. doi: 10.3892/ijo.14.5.855. [DOI] [PubMed] [Google Scholar]
- 51.Statistics-Norway. SSB: Population, by sex, age and municipality. 1. January 2011. Oslo: Statistics Norway; 2011. [Google Scholar]
- 52.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Backer-Grondahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- 54.Backer-Grøndahl T, Moen BH, Sundstrøm SH, Torp SH. Histopathology and prognosis in human meningiomas. APMIS. 2014;122:856–66. doi: 10.1111/apm.12248. [DOI] [PubMed] [Google Scholar]
- 55.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 56.Groblewska M, Siewko M, Mroczko B, Szmitkowski M. The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol. 2012;50:12–19. doi: 10.2478/18691. [DOI] [PubMed] [Google Scholar]
- 57.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das A, Tan WL, Smith DR. Expression of extracellular matrix markers in benign meningiomas. Neuropathology. 2003;23:275–281. doi: 10.1046/j.1440-1789.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 59.Tummalapalli P, Spomar D, Gondi CS, Olivero WC, Gujrati M, Dinh DH, Rao JS. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int J Oncol. 2007;31:1039–1050. [PMC free article] [PubMed] [Google Scholar]
- 60.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 61.Torp SH, Lindboe CF, Gronberg BH, Lydersen S, Sundstrom S. Prognostic significance of Ki-67/MIB-1 proliferation index in meningiomas. Clin Neuropathol. 2005;24:170–174. [PubMed] [Google Scholar]
- 62.Abry E, Thomassen IO, Salvesen OO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract. 2010;206:810–815. doi: 10.1016/j.prp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210–218. doi: 10.3171/jns.2004.101.2.0210. [DOI] [PubMed] [Google Scholar]
- 64.Urbschat S, Rahnenfuhrer J, Henn W, Feiden W, Wemmert S, Linsler S, Zang KD, Oertel J, Ketter R. Clonal cytogenetic progression within intratumorally heterogeneous meningiomas predicts tumor recurrence. Int J Oncol. 2011;39:1601–1608. doi: 10.3892/ijo.2011.1199. [DOI] [PubMed] [Google Scholar]
- 65.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 67.Shintaku M, Honda T, Sakai T. Expression of podoplanin and calretinin in meningioma: an immunohistochemical study. Brain Tumor Pathol. 2010;27:23–27. doi: 10.1007/s10014-009-0262-z. [DOI] [PubMed] [Google Scholar]
- 68.Gay E, Lages E, Ramus C, Guttin A, El Atifi M, Dupre I, Bouamrani A, Salon C, Ratel D, Wion D, Berger F, Issartel JP. The heterogeneity of meningioma revealed by multiparameter analysis: infiltrative and non-infiltrative clinical phenotypes. Int J Oncol. 2011;38:1287–1297. doi: 10.3892/ijo.2011.944. [DOI] [PubMed] [Google Scholar]
- 69.Strojnik T, Zidanik B, Kos J, Lah TT. Cathepsins B and L are markers for clinically invasive types of meningiomas. Neurosurgery. 2001;48:598–605. doi: 10.1097/00006123-200103000-00029. [DOI] [PubMed] [Google Scholar]


