Abstract
Recently, the architectural remodeling of venous vessel wall ranks as the basis of varicose veins development based on the phenotypic state of vascular smooth muscle cells (VSMCs). In this study, we firstly demonstrated an obvious up-regulation of IQ-domain GTPase-activating protein 1 (IQGAP1) in patients with varicose veins. Importantly, following stimulation with PDGF-BB for 4 h, a common inducer of phenotypic switch in VSMCs, a dramatically time-dependent increase in IQGAP1 expression was observed in human venous smooth muscle cells (HUVSMCs), concomitant with the down-regulation of SMC markers [including α-smooth muscle actin (SMA), smooth muscle calponin (CNN), SM22α (SM22)], suggesting a critical function of IQGAP1 during the switch of synthetic VSMC phenotype. Further analysis ascertained that IQGAP1 overexpression significantly inhibited the expression of SMA, SM and CNN, while its silencing dramatically promoted their expression levels. Moreover, the elevated IQGAP1 enhanced cell proliferation, migration and rearrangement. Mechanism assay confirmed that IQGAP1 overexpression notably blocked myocardin levels. Importantly, after transfection with myocardin siRNA, IQGAP1 down-regulation-induced decrease in cell proliferation, migration and cell rearrangement was remarkably attenuated. Together, these results demonstrated that IQGAP1 may regulate the phenotypic switch of VSMCs by myocardin pathway, which is critical for the pathological progression of varicose vein. Therefore, this study supports a prominent insight into how IQGAP1 possesses its benefit function in varicose veins development by regulating vascular remodeling.
Keywords: Varicose vein, IQGAP1, vascular remodeling, VSMC
Introduction
Varicose veins rank as a common venous disease with an estimated prevalence of 5% to 30% of adult population. In USA, over 25 million of adult population with varicose veins has been reported. Normally, it is reasonably benign; however, some severe varicosities can also occur and lead to serious complications including deep vein thrombosis, longstanding venous ulcers and chronic venous insufficiency (CVI), which has high morbidity in middle and elderly people [1,2]. It has been demonstrated that more than 2.5 million people suffer from progressive venous insufficiency each year [3]. Despite the recent advances have been made in identifying the risk factors for varicose veins, the underlying mechanism involved in the pathogenesis and progression of varicose veins is still unclearly.
Recently, vascular remodeling has drawn the increasing attention as its critical role in the development of venous diseases [4,5]. The accumulating evidences confer that high prevalence of venous diseases is associated with the structural reorganization of the venous vessel wall. Morphological research suggested that these usually appear as bulged and twisted corkscrew-like structures with restricted functionality in the lower extremities. Though many factors have been confirmed to be associated with vascular remodeling, recent researches have shown that vascular smooth muscle cells (VSMCs) exert pivotal role during the process of vascular remodeling in varicose veins by regulating their phenotype state [6,7]. Unlike many terminally differentiated cells, SMCs can transform from the differentiated contractile phenotype to a synthetic state, which is characterized by high proliferation, migration, and extracellular matrix production [8]. During this process, the contractile ability of SMCs will reduce and result in the lack of the resistance to environmental stimulation, and subsequently trigger varicose vein. However, the correlated mechanism involved in the switch of SMC phenotype remains undefinedly.
IQ-domain GTPase-activating proteins (IQGAPs) are the recently identified protein family with the evolutionary conserved multistructural domains. Lots of researchers have manifested a critical function of this family in adjusting cell adhesion, migration, proliferation and other biological processes [9-11]. Among of them, IQGAP1, as a scaffolding protein, is the most ubiquitously expressed and can bind several molecules to regulate cell function, such as cell proliferaion, invasion and migration [12,13]. Recent study has demonstrated that IQGAP1 can bind to vascular endothlial growth factor receptor 2 (VEGF2) to regulate endothlial migration and postischemic angiogenesis [12,14]. Furthermore, it is also reported to be associated with platelet-derived growth factor (PDGF)-induced focal adhesion formation and VSMC migration, and its silencing exhibit impaired neointimal formation in response to vascular injury, indicating a potential therapeutic target against vascular migration-related diseases [15]. However, its function in venous disease is still unclearly.
In this study, we firstly explored the correlation between IQGAP1 and varicose veins. Further-more, the function of IQGAP1 on VSMC phenotypic regulation was further investigated. The corresponding underlying mechanism was also analyzed.
Materials and methods
Reagents
Rabbit anti-human SMA polyclonal antibody was obtained from MyBiosource (San Diego, CA). Against human CNN and SM22 antibody was from Abcam (Cambridge, MA). Myocardin Rabbit anti-human polyclonal antibody was purchased from Biocompare (Foster City, CA). PDGF-BB was from R & D company. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG were from Bio-Rad (Hercules, CA). Rabbit polyclonal antibody to cyclinD1 and ki67 was seperately bought from Abgent (San Diego, CA) and Raybiotech, Inc. (Norcross, GA).
Specimen collections
The obtained human materials were all from volunteers and processed according to the recommendations of The Affiliated Suzhou Hospital of Nanjing Medical University. The study was conducted in compliance with the Helsinki Declaration and all patients gave written informed consent. The greater saphenous vein (GSV) tissues were obtained from 27 patients (aged from 30-65 years), consisting of those undergoing surgery for primary varicose veins. About 25 patients (aged from 29-66 years) were encompassed and recognized as control group, who were performed elective coronary bypass surgery and without the clinical, ultrasonographic and intraoperative signs of varicosis. All specimens were preserved in liquid nitrogen for subsequent experiments.
Cell culture and treatments
Human venous smooth muscle cells (HUVSMCs) were purchased from Matsa Biological Technology co., LTD and mainted in DMEM supplemented with 10% fetal calf serum and100 μg/mL streptomycin/ penicillin. Passages from 6 to 10 were chosen for further study. Cells were incubated with 10 ng/mL PDGF-BB for various times (0, 4 and 8 h). All cells were maintained at 37°C with 5% CO2 humidified atmosphere.
Expression of IQGAP1 in vitro
The recombinant human IQGAP1 was performed to obtain the protein of IQGAP1 according to a method reported previously [16]. Briefly, the human the full-length IQGAP1 cDNA was amplified with its specific primers using PCR. After purification, the PCR products were digested with Xho I and Apa l restriction enzymes, then the fragments were subcloned into the Xho I and Apa l cloning site of the pcDNA3.1 (+) vector (Invitrogen, Carlsbad, CA) to induce IQGAP1expression. Sequences were ascertained by DNA sequencing. The recombinant IQGAP1 protein was purified by Ni-NTA magnetic beads (Qiagen) and followed by assessed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The vector-transfected groups were used as control.
Lentivirus construction and transfection
To silence the expression of IQGAP1 in HUVSMCs, the IQGAP1 shRNA lentivirus plasmids were constructed according to a method reported previously with little changes [17]. Briefly, DNA fragments containing 5’-CAACGA-CATTGCCAGGGATAT-3’ were synthesized from Bioscience Corporation and then were subcloned into human U6 promoter–containing pBluescript SK (+) plasmid (pU6). Then, IQGAP1 shRNA was cloned into pRRL plasmid with PSMA promoter. The non-specific shRNA sequences were subcloned into the same vector and were tanken as control. The 293 cells were then co-transfected with the lentivirus plasmids carrying shRNA, packaging vector MVR (Beijing Zhongyuan Ltd., Beijing, China) and 2 μg of pCMV-VSVG (Clontech, Saint-Germain-en-Laye, France) using Lipofec-tamine® 2000 Reagent (Life Technologies, Carlsbad, CA) for 48 h at 37°C. The cultured medium was collected and filtered using a 0.45 μm filter (Amicon Ultra-15 100 K, Millipore), followed by infecting the cultured HUVSMCs with the collected LV-IQGAP1 shRNA virus. The p24 ELISA kit (Cell Biolabs, Inc., San Diego) was used to determine the virus titers and then was stored at -80°C until used.
Myocardin siRNA transfection
To targeted silence the expression of myocardin, its specific siRNA and scrambled siRNA were obtained from Santa Cruz Biotechnology. When cells were grown into 40-50% confluence, about 2 μg/mL myocardin siRNA or scrambled siRNA (NC) were added to silence the expression of myocardin together with the GeneSilencer® siRNA transfection reagent. About 24 h later, cells were collected and the transfection efficiency was subsequently assessed by RT-PCR and Western blotting.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total cellular RNA was extracted from the above cells using RNeasy Mini Kit with an on-column deoxyribonuclease digestion (QIAGEN, Valencia, CA). Then, the obtained RNA was reverse transcribed to synthesize the first strand cDNA by the Promega Reverse Transcription System (Promega, Southampton, UK). Quantitative real-time RT-PCR was performed on an ABI PRISM 7000 sequence detection system using SYBR Premix Ex TaqTM II Kit (Takara Bio Inc., Otsu, Japan) with the specific primers as follows: SMA (sense: 5’-GCGTGGCTATTCCTTC-GTTA-3’; antisense: 5’-ATGAAGGATGGCTGG-AACAG-3’); SM22 (sense: 5’-AACAGCCTGTA-CCCTGATGG-3’; antisense: 5’-CGGTAGTGCCCA-TCATTCTT-3’); CNN, sense: 5’-AGCTAAGAGAA-GGGCGGAAC-3’; antisense: 5’-CATCTGCAGGC-TGACATTGA-3’); and myocardin (sense: 5’-TGCATGCTGCTGTAAAGTCC-3’; antisense: 5’-TAGCTGAATCGGTGTTGCTG-3’). GAPDH was used for normalization.
Western blotting
Cells were homogenized and lysed with RIPA lysis buffer (Beyotime, Nantong, China) to extract the total protein, followed by protein concentration measurements by the micro-BCA protein assay (Pierce). A total of 150 μg protein per lane was separately electrophoresed by 12% SDS-PAGE, and then were transferred to a PVDF membrane (Schleicher & Schuell, Germany). To blocking the nonspecific binding, the membranes were incubated with 5% nonfat milk in TBST buffer at 4°C overnight. Then, the indicated primary antibodies against IQGAP1, SMA, SM-22, CNN, cyclinD1, Ki67 and myocardin were added for 1 h. The membrane was then incubated with secondary antibodies conjugated with HRP (Jackson Immuno Research), followed by visualizing with the LumiGLo reagent (Pierce).
MTT assay
After precondition with the indicated treatments, the culture medium were replaced with fresh medium containing 500 μg/ml MTT reagents and incubated for further 5 h at 37°C. Then, 200 μl isopropanol was added to dissolve formazan production. Cell viability was assessed by determining the absorbance of MTT at 590 nm using a micro-ELISA reader (Bio-Rad). All samples were performed in triplicate.
Spheroid generation assay
To evaluate the effect of the indicated treatment on cell rearrangement, the formation of 3-dimensional spheroid HUVSMCs was performed according to the previous description [18]. Briefly, HUVSMCs were suspended in culture medium containing 0.25% (w/v) carboxymethylcellulose, and then were seeded in nonadherent round-bottom 96-well plates with 3000 cells/spheroid. About 24 h later, the formation of spheroid was analyzed and results were exhibited with mean spheroid circumference of 14-18 spheroids per experimental group.
Cell migration assay
Cell migration was evaluated by the scratch wound assay in vitro. After seeded in 24-well plates (12, 000 cells/well), cells were treatment with the indicated conditions. Then, a single scratch wound was generated with 200 μl disposable pipette tip and the scratch wounds were photographed with an inverted microscope and digital camera. The ImageJ software was used to quantitate the scratch wound widths. The corresponding results were shown as the percentage of wound closure setting the initial scratch width as 100%.
Statistical analysis
The results presented are the average of at least three experiments and reported as means ± SD. All experiments were calculated using SPSS 11.0. Statistical analyses were performed by an independent Student t-test. A P < 0.05 was considered as statistically significant and is indicated by asterisks.
Results
Elevated IQGAP1 was determined in patients with varicose veins
Given that IQGAP1, as a scaffold protein, exerts a pivotal role in neointimal formation after vascular injury by regulating cell migration and adhesion [12]. However, little is known about its roles in varicose veins. To explore the possible role of IQGAP1 in the development of varicose vein, we collected vein tissues from primary varicose vein patients and control patients. As shown in Figure 1A, an obviously higher mRNA level of IQGAP1 was observed in varicosity patients, compared with control groups. Simultaneously, the protein levels of IQGAP1 also dramatically increased in tissues from varicose vein patients (Figure 1B). Together, these results indicated that IQGAP1 might be possessing a critical function in the pathological process of varicose vein.
Figure 1.
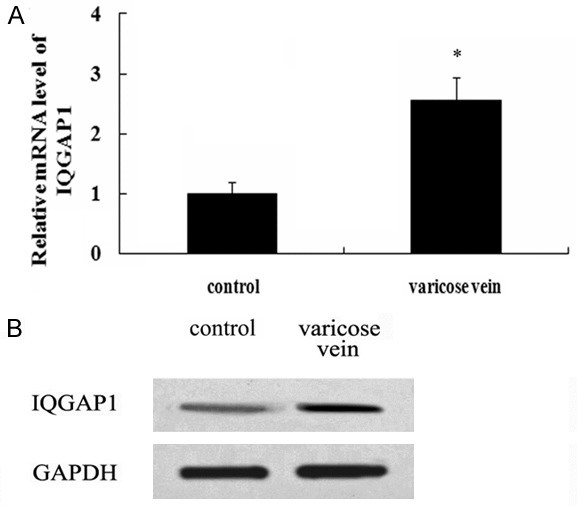
Abundant CXCR7 was corroborated in varicose vein tissues. The mRNA levels of IQGAP1 in 27 patients with varicose vein and 25 control patients were assessed by RT-PCR (A). The corresponding protein levels were detected by western blotting (B). *P < 0.05 versus control groups.
IQGAP1 expression correlates with VSMC synthetic phenotype
Accumulating evidence has proven that PDGF-BB is a potential mediator to switch the VSMC phenotype to a synthetic state by regulating SMC specific gene expression, which is the characterization of varicose vein [19,20]. In this study, following stimulation with PDGF-BB for 4 h, the mRNA levels of IQGAP1 were significantly up-regulated (Figure 2A). Furthermore, a higher expression level of IQGAP1 mRNA was detected at 8 h post stimulation with PDGF-BB. Importantly, with the increasing of IQGAP1, the mRNA levels of SMC markers SMA, SM22 and CNN were notably down-regulated, suggesting an obvious correlation between IQGAP1 and SMC marker molecules (including SMA, SM22 and CNN) associated with the contratile phenotype (Figure 2B). The similar results were also confirmed in protein levels (Figure 2C), implying that IQGAP1 might be positively associated with synthetic VSMC phenotype.
Figure 2.
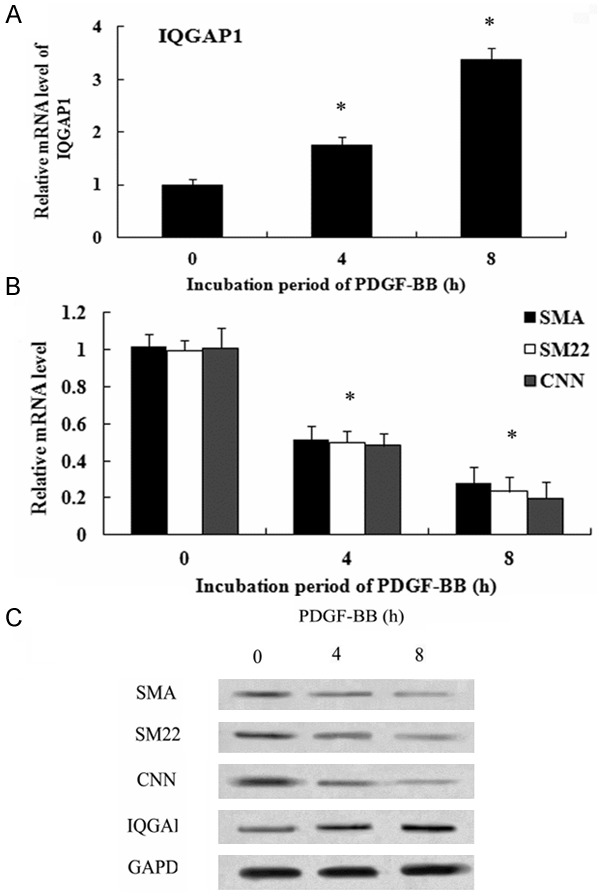
IQGAP1 Expression Correlates with VSMC Synthetic Phenotype. The cultured HUVSMCs were incubated with 10 ng/mL PDGF-BB for the indicated times (0, 4 and 8 h). Then, the mRNA levels of IQGAP1 were examined (A). Furthermore, the mRNA levels of SMC-specific markers (SMA, SM and CNN) were analyzed by RT-PCR (B). Antibodies against SMA, SM and CNN were used to detect their protein levels (C).
IQGAP1 negatively regulated the expression of VSMC-specific contractile protein
To evaluate the function of IQGAP1 on VSMC contractile phenotype-specific gene expression, the recombinant pcDNA3.1 (+)-IQGAP1 was transfected into HUVSMCs. Western blotting analysis demonstrated that the pcDNA3.1 (+)-IQGAP1 transfection strikingly up-regulated IQGAP1 expression, compared with control and vector groups (Figure 3A). Simultaneously, IQGAP1 overexpression obviously blocked the expression levels of SMA, SM22 and CNN mRNA (Figure 3B), concomitant with the decrease in their protein levels (Figure 3C). When transfection with IQGAP1 shRNA, the protein levels of IQGAP1 were dramatically decreased (Figure 3D). Furthermore, IQGAP1 silencing induced a 3.45-fold increase in SMA mRNA levels, 2.58-fold increase in SM22 mRNA levels and 2.21-fold increase in CNN mRNA levels, respectively (Figure 3E). Consistent with the above results, the similar up-regulation in the protein levels of SMA, SM22 and CNN were also observed after transfection with IQGAP1 shRNA (Figure 3F). Therefore, these results told that IQGAP1 could negatively regulate the expression of VSMC-specific contractile protein, indicating a potential role in the regulation of SMC phenotype switch.
Figure 3.
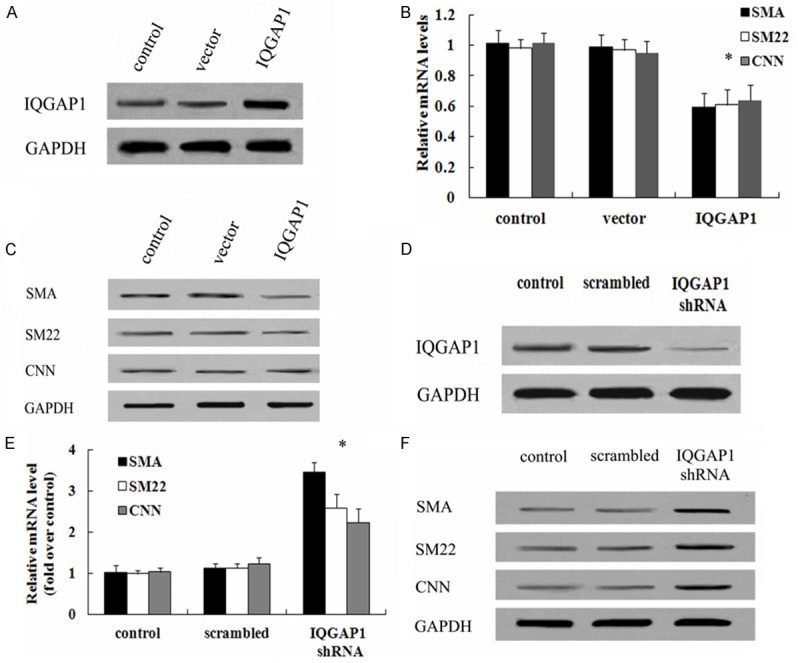
Effect of IQGAP1 on the expression of VSMC-specific contractile protein. To investigate the function of IQGAP1 on the levels of VSMC-specific genes, cells were transfected with recombinant pcDNA3.1 (+)-IQGAP1 vectors. The corresponding transfection effect was evaluated by western blotting (A). The effect of IQGAP1 overexpression on SMC-specific marker (SMA, SM and CNN) expression levels of mRNA (B) and protein (C) was validated by RT-PCR and western blotting. Additionally, the IQGAP1 shRNA was also introduced to analyze the above results. The silencing effect on IQGAP1 protein levels were demonstrated (D). The function of IQGAP1down-regulation in the mRNA levels (E) and protein levels (F) of SMA, SM and CNN was also assessed. *P < 0.05.
IQGAP1 overexpression regulated the switch of SMC phenotype from contractile to synthetic
It is well accepted that the synthetic SMC is characterized with high proliferation, migration and remodeling. To assess the putative function of IQGAP1 on venous remodeling processes, the critical functional properties of VSMC were explored. Following transfection with the recombinant IQGAP1, the relative MTT absorbance was gradually increased in a time-dependent manner by MTT assay, compared with control group (Figure 4A). Furthermore, IQGAP1 overexpression also enhanced the protein expression of cyclin D1 and Ki-67, both are the marker for cell proliferation. These results confirmed that IQGAP1 up-regulation promoted cell proliferation. Further analysis demonstrated that the elevated IQGAP1 expression enhanced the rearrangement of HUVSMCs in 3-dimensional spheroids (Figure 4C). Moreover, increased IQGAP1 levels increased the rate of cell migration (Figure 4D). Therefore, the above results indicated that IQGAP1 up-regulation promoted the phenotype switch of SMC from contractile to synthetic, suggesting an important function in varicose vein.
Figure 4.
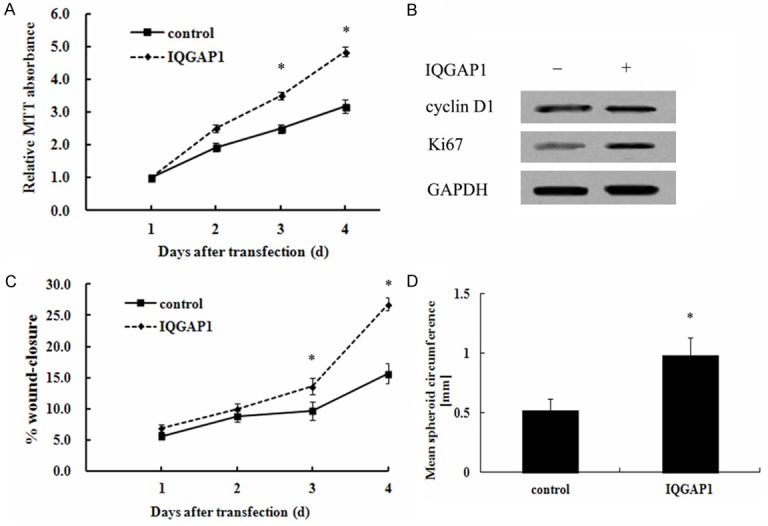
Function of IQGAP1 on the phenotypic switch of SMCs. Following transfection with the recombinant IQGAP1 vectors, the effect of IQGAP1 overexpression on cell proliferation was determined by MTT assay (A). The corresponding effect on the protein levels of proliferate-related markers (cyclin D1 and Ki67) were analyzed by western blotting (B). The scratch wound assay was performed in the presence of 10 ng/mL PDGF-BB to evaluate the effect of IQGAP1 on cell migration (C). Furthermore, the corresponding effect on cell rearrangement was also evaulated by the 3-dimensional spheroid formation assay (D). *P < 0.05.
Expression levels of myocardin was attenuated after IQGAP1 overexpression
Myocardin, as a critical regulator of contractile and quiescent smooth muscle cell phenotype, is known to be down-regulated in SMC of varicose veins and important for maintaining the contractile phenotypic state. To further clarify the underlying mechanism involved in IQGAP1-mediated SMC phenotype switch, the expression levels of IQGAP1 were discussed. As shown in Figure 5A, IQGAP1 overexpression inhibited the mRNA levels of myocardin, compared with control group. Consistently, blocking IQGAP1 expression significantly up-regulated myocardin mRNA levels (Figure 5B). Further protein analysis confirmed that IQGAP1 up-regulation obviously abrogated myocardin protein levels (Figure 5C). The corresponding increase in myocardin protein level was also observed in IQGAP1 shRNA-treated groups (Figure 5D).
Figure 5.
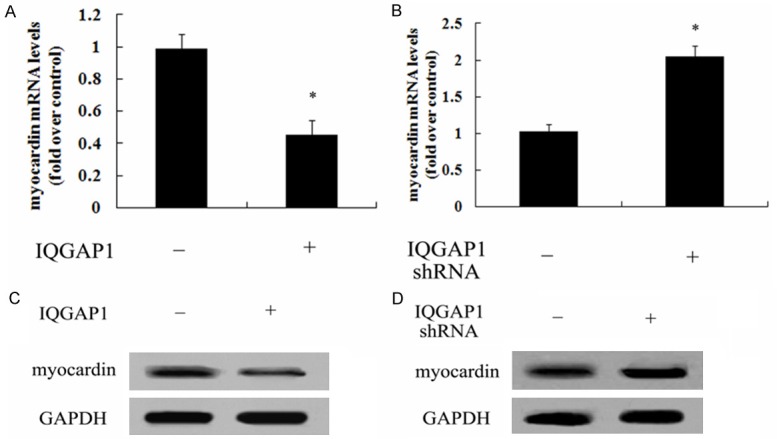
IQGAP1 regulated the expression of myocardin. After overexpression of IQGAP1 in HUVSMCs, the mRNA levels of myocardin were analyzed by RT-PCR (A). The corresponding protein levels of myocardin were also detected by western blotting (B). Following transection with IQGAP1 shRNA, the corresponding effect on myocardin mRNA levels (C) and protein levels (D) was also monitored. *P < 0.05.
Myocardin was accounted for IQGAP1-mediated SMC phenotype state
To further elucidate the underlying mechanism involved in IQGAP1-regulated SMC phenotype state, myocardin siRNA was introduced. As shown in Figure 6A, IQGAP1 shRNA transfection obviously promoted the expression of myocardin mRNA; after transfection with IQGAP1 siRNA, this increase triggered by IQGAP1 silencing was dramatically decreased. The similar down-regulation in myocardin protein levels were also determined (Figure 6B). Further mechanism analysis confirmed that IQGAP1 down-regulation notably attenuated cell proliferation, which was evidently ameliorated when silencing myocardin expression with myocardin siRNA (Figure 6C). Moreover, myocardin was related to IQGAP1-regulated cell rearrangement as myocardin silencing remarkably antagonized IQGAP1 shRNA-induced inhibitor effect on cell rearrangements (Figure 6D). Additionally, IQGAP1 shRNA transfection significantly inhibited cell migration (Figure 6E). As expected, myocardin siRNA treatment strikingly restored the above inhibitory effect on cell migration. Together, these results suggested that IQGAP1 majorly regulated SMC phenotype state by myocardin pathway.
Figure 6.
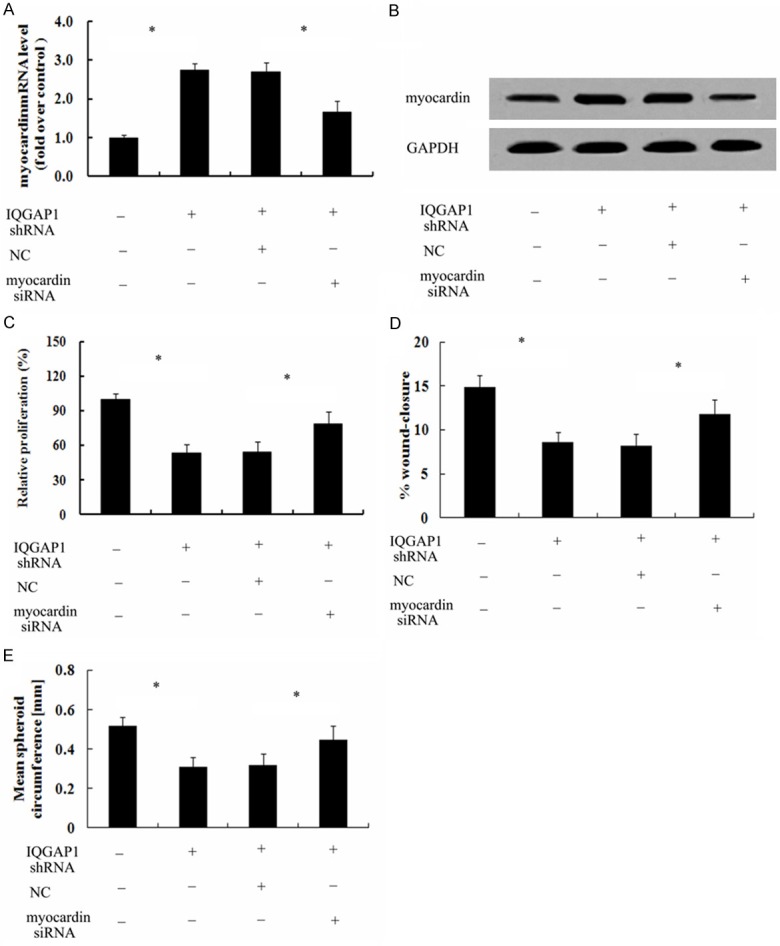
IQGAP1 majorly mediated SMC phenotype state by the myocardin pathway. To analyze the function of myocardin on IQGAP1 silencing-induced cell phenotype state, the transfection of myocardin siRNA was performed. The corresponding effect on myocardin mRNA (A) and protein levels (B) was evaluated by RT-PCR and western blotting. The inhibitor effect of IQGAP1 down-regulation on cell proliferation (C), migration (D) and rearrangement (E) was further explored. *P < 0.05.
Discussion
IQGAP1 was identified in 1994 as a widely expressed IQ domain-containing protein with a similar region sequence to the Ras GTPase-activating proteins. As a scaffold protein, IQGAP1 plays a pivotal role in regulating the actin cytoskeleton and cell migration by interacting directly with actin [13]. It is known that neovascularization is pivotal for the recurrence of varicose veins after operation. Previous studies have demonstrated that IQGAP1 is demonstrated to be as a regulator during the process of post-ischemic neovascularization by regulating endothelial cell migration [21]. Furthermore, IQGAP1 functions as a VEGFR2-associated scaffold protein to promote endothelial cell migration and proliferation, and its silencing imparied VEGF-induced angiogenesis, indicating a critial role of IQGAP1 during vasculogenesis [12]. However, the specific function of IQGAP1 in vascular biology and disease, such as varicose vein, remains unexplored. In this study, we firstly confirmed an obvious up-regulation of IQGAP1 expression in patients with varicose vein, implying a crucial function of IQGAP1 in the development of varicose veins.
The phenotypic state of vascular smooth muscle cells (VSMCs) is known as the pathological basis of vascular-related diseases, such as varicose veins, atherosclerosis and restenosis RS [7,22]. Normally, VSMC exhibited the contractile phenotype by regulating its sepcific genes, including α-smooth muscle actin (SMA), smooth muscle calponin (CNN) and SM22α (SM22). When response to some stimulation such as injury, the quiescent contractile vSMCs will reduce the expression of SMC-specific genes and promote their proliferation, migration, collagen synthesis to remodel the phenotype state into synthetic state, which ultimately trigger varicose vein [6,23]. PDGF-BB is common inducer to regulate phenotypic switch in VSMCs [20]. In this study, the expression levels of IQGAP1 were dose-dependently up-regulated following PDGF-BB stimulation. Importantly, this increase was accompanied with the down-regulation of SMC-specific markers including SMA, SM22 and CNN, implying an obvious association between IQGAP1 and synthetic VSMC phenotype.
To further corroborate the function of IQGAP1 on vascular remodeling, the IQGAP1 overexpression and silencing cell models were constructed. Intriguingly, IQGAP1 up-regulation attenuated SMC specific maker expression of SMA, SM22 and CNN. Consistently, knockdown of endogenous IQGAP1 significantly up-regulated the expression of SMC specific genes, indicating that IQGAP1 may benefit for the formation of synthetic SMC state. It is widely accepted that the switch of SMC into the synthetic state is characterized with higher proliferation and migration ability [24,25]. As expected, IQGAP1 overexpression promoted SMC proliferation, concomitant with the increase in cyclin D1 and Ki67 expression, both of these are the marker for cells. Moreover, higher migration ability was also confirmed in this condition. Importantly, the corresponding enhanced rearrangement of HUVSMCs was also observed in IQGAP1-overexpressed groups. The corresponding inhibitor effect on SMC phenotype switch was demonstrated following IQGAP1 silence, indicating that IQGAP1 can positively regulate SMC phenotype switch.
Interestingly, the down-regulation expression of myocardin was determined when IQGAP1 was overexpressed. The similar increased effect on its expression was also conferred after silencing IQGAP1 levels. Myocardin is recognized as a critical determinant for maintaining the contractile SMC phenotype by inhibiting NF-κB-dependent cell cycle progression and stimulating the specific VSMC gene expression [26-28]. Additionally, it is also a strong inhibitor of VSMC migration and proliferation. To further clarify the underlying mechanism of IQGAP1-triggered SMC phenotypic switch, myocardin pathway was introduced. As expected, after blocking myocardin expression with its specific siRNA, IQGAP1 silencing-induced increase in myocardin levels was obviously attenuated, accompany with the corresponding down-regulation in cell proliferation, migration and rearrangement. Accordingly, we can conclude that IQGAP1 induces the SMC phenotype switch in part via the myocardin pathway.
In conclusion, this research explored a potential therapeutic function of IQGAP1 in varicose veins. Here, IQGAP1 was overexpressed in patients with varicose veins. Further analysis suggested that IQGAP1 may positively regulate the phenotypic switch of SMC by regulating myocardin pathway, which is critical for the pathological progression of varicose vein. Therefore, these findings support a prominent insight into how IQGAP1 exerts its beneficial role in the development of varicose vein by regulating vascular remodeling. Recent research has confirmed that neovascularization formation is associated with the recurrence after varicose vein operation [29]. Moreover, IQGAP1 has been proven to be related with post-ischemic neovascularization [21]. Whether IQGAP1 affects the process of varicose vein by regulating angiogenesis still needs to be further explored.
Disclosure of conflict of interest
None.
References
- 1.Regadera J, Prachaney P, Espana G, Condezo-Hoyos L, Rubio M, Gonzalez MC, de Pablo ALL, Arribas SM. Initial lesions of the elastic fibers and extracellular matrix in varicose veins: an inmunohistochemical and confocal microscopy study. FASEB J. 2012;26:833.15. [Google Scholar]
- 2.Nagaraj H, Hebbar AK, Rajaput AS, Kumar B. Prospective clinical study of surgical management of varicose veins of lower limb and its complications. 2014 [Google Scholar]
- 3.Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188:1–8. doi: 10.1016/S0002-9610(03)00284-8. [DOI] [PubMed] [Google Scholar]
- 4.Kockx MM, Knaapen MW, Bortier HE, Cromheeke KM, Boutherin-Falson O, Finet M. Vascular remodeling in varicose veins. Angiology. 1998;49:871–877. doi: 10.1177/000331979804901101. [DOI] [PubMed] [Google Scholar]
- 5.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR Jr, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Wali MA, Eid RA. Smooth muscle changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2001;37:123–135. doi: 10.1540/jsmr.37.123. [DOI] [PubMed] [Google Scholar]
- 7.Badier-Commander C, Couvelard A, Henin D, Verbeuren T, Michel JB, Jacob MP. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193:398–407. doi: 10.1002/path.819. [DOI] [PubMed] [Google Scholar]
- 8.Rensen S, Doevendans P, Van Eys G. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateer SC, Wang N, Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- 11.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species-dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Chen YC. Structure and function of IQ-domain GTPase-activating protein 1 and its association with tumor progression (Review) Biomed Rep. 2014;2:3–6. doi: 10.3892/br.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal. 2012;24:826–834. doi: 10.1016/j.cellsig.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno T, Urao N, Ashino T, Sudhahar V, Inomata H, Yamaoka-Tojo M, McKinney RD, Fukai T, Ushio-Fukai M. IQGAP1 links PDGF receptor-β signal to focal adhesions involved in vascular smooth muscle cell migration: role in neointimal formation after vascular injury. Am J Physiol Cell Physiol. 2013;305:C591–C600. doi: 10.1152/ajpcell.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin N, Shi J, Wang D, Tong T, Wang M, Fan F, Zhan Q. IQGAP1 interacts with Aurora-A and enhances its stability and its role in cancer. Biochem Biophys Res Commun. 2012;421:64–69. doi: 10.1016/j.bbrc.2012.03.112. [DOI] [PubMed] [Google Scholar]
- 17.Dong PX, Jia N, Xu ZJ, Liu YT, Li DJ, Feng YJ. Silencing of IQGAP1 by shRNA inhibits the invasion of ovarian carcinoma HO-8910PM cells in vitro. J Exp Clin Cancer Res. 2008;27:77. doi: 10.1186/1756-9966-27-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 19.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 20.Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1027. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urao N, Razvi M, Oshikawa J, McKinney RD, Chavda R, Bahou WF, Fukai T, Ushio-Fukai M. IQGAP1 is involved in post-ischemic neovascularization by regulating angiogenesis and macrophage infiltration. PLoS One. 2010;5:e13440. doi: 10.1371/journal.pone.0013440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel JB, Li Z, Lacolley P. Smooth muscle cells and vascular diseases. Cardiovasc Res. 2012;95:135–137. doi: 10.1093/cvr/cvs172. [DOI] [PubMed] [Google Scholar]
- 23.Naoum JJ, Hunter GC, Woodside KJ, Chen C. Current advances in the pathogenesis of varicose veins. J Surg Res. 2007;141:311–316. doi: 10.1016/j.jss.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Sung HJ, Eskin SG, Sakurai Y, Yee A, Kataoka N, McIntire LV. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann Biomed Eng. 2005;33:1546–1554. doi: 10.1007/s10439-005-7545-2. [DOI] [PubMed] [Google Scholar]
- 25.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang RH, Zheng XL, Callis TE, Stansfield WE, He J, Baldwin AS, Wang DZ, Selzman CH. Myocardin inhibits cellular proliferation by inhibiting NF-κB (p65)-dependent cell cycle progression. Proc Natl Acad Sci U S A. 2008;105:3362–3367. doi: 10.1073/pnas.0705842105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, Shi Z, Kilsdonk EP, Gui Y, Wang DZ. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long X, Bell RD, Gerthoffer WT, Zlokovic BV, Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1505–1510. doi: 10.1161/ATVBAHA.108.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rij AM, Jones GT, Hill GB, Jiang P. Neovascularization and recurrent varicose veins: more histologic and ultrasound evidence. J Vasc Surg. 2004;40:296–302. doi: 10.1016/j.jvs.2004.04.031. [DOI] [PubMed] [Google Scholar]


