Abstract
T cells immunoglobulin mucin 3 (Tim-3) is an important inhibitory stimulatory molecule, which has been reported to play a vital role in the tumor immune escape and be correlated with clinicopathological prognostic stratification in solid tumor. However, the related research is rare of Tim-3 in non-solid tumor, such as acute myeloid leukemia (AML). In this study, we investigated the expression characteristics of Tim-3 on the peripheral blood T cells of newly diagnosed AML patients and its clinical significance. Peripheral blood was obtained from 36 patients with newly diagnosed AML before intervention, with peripheral blood from 20 cases of healthy volunteers collected as normal control. Expression levels of Tim-3 on the peripheral blood T cells were assayed with flow cytometry. We found that Tim-3 expression on the peripheral blood CD4+ T cells and CD8+ T cells in newly diagnosed AML patients were significantly increased compared with that of normal control. CD4+ T cells/CD8+ T cell ratio (CD4/CD8) of peripheral blood in AML patients was significantly correlated with NCCN high risk group. The higher expression level of Tim-3 on CD4+ T cells in the peripheral blood of AML patients had significant correlation with FLT3-ITD mutation, the higher expression level of Tim-3 on CD8+ T cells in AML patients was significantly correlated with NCCN high risk group. To conclude, our results support the concept that Tim-3 is highly expressed on the peripheral blood T cells of AML patients, and Tim-3 expression significantly correlates with clinicopathological prognostic stratification in AMLTim-3, T cell, acute myeloid leukemia, tumor immune escape, clinicopathological prognostic stratification
Keywords: Tim-3, T cell, acute myeloid leukemia, tumor immune escape, clinicopathological prognostic stratification
Introduction
T cells immunoglobulin mucin 3 (Tim-3) is an important inhibitory costimulatory molecules. Tim-3 binding with its ligandgalectin-9, immunemediats the tumor microenvironment inhibition by different mechanisms, and plays an important role in tumorigenesis, development, invasion and metastasis [1]. Several studies have found that Tim-3 is highly expressed on the tumor infiltrating T cells of the solid tumor, and the high expression of Tim-3 is related with the tumorigenesis and tumor development [2,3]. Our team previously showed that TIM-3 expression characterized regulatory T cells in lung cancer tissues and was associated with tumor progression [4]. However, the related research of the expression and role of the Tim-3 in non-solid tumors, such as acute myeloid leukemia (AML) is rare.
In this study, we adopted methods of immunofluorescence and flow cytometry to detect the expression characteristics of Tim-3 on peripheral blood T cells in AML patients and analyzed its clinical significance, in order to investigate the role of Tim-3 in the immune escape mediated by AML tumor cells. The data showed that peripheral blood CD4+ T cells, CD8+ T cells and lymphocyte proportion were decreased significantly in newly diagnosed AML patients than those of normal control. More significantly, the expression level of Tim-3 on the CD4+ T cells and CD8+ T cells of newly diagnosed AML patients were significantly increased compared with normal control. We further analyzed the correlation between Tim-3 expressed level on T cells and the disease diagnosis and prognosis, which suggest that higher expression of Tim-3 on AML patients T cells might be associated with the poor prognosis.
Materials and methods
Patients and specimens
A total of 36 AML cases (except for acute promyelocytic leukemia) were enrolled in the study which were newly diagnosed by bone marrow MICM tests and then treated from 2012 September to 2013 August in the Department of hematology, First Hospital Affiliated to Soochow University. Within the AML cases, there were 21 male cases, 15 female cases, and mean age 37.38±14.12 years. The diagnosis of AML was accorded with the “2008 WHO adult acute myeloid leukemia (non acute promyelocytic leukemia) diagnosis guidelines”, and excluded the patients of autoimmune disease, systemic disease or with other cancers. 20 cases of healthy volunteers were enrolled as normal control with 11 male cases, 9 female cases, and mean age 40.70±15.23 years. There were no statistical significance between the two groups in gender, age difference (P > 0.05).
Specimen collection: 5 ml heparin anticoagulant fresh peripheral blood from the AML patients were collected before intervention and 5 ml heparin anticoagulant fresh peripheral blood from the healthy volunteers as normal control were collected in the same term.
This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University, which was in accord with the Helsinki Declaration. The data were analyzed anonymously and informed consent was not required.
Materials and reagents
PeCy5 labeled mouse anti human CD3 monoclonal antibody, FITC labeled mouse anti human CD4 monoclonal antibody and PeCy7 labeled mouse anti human CD8 monoclonal antibody were purhased from eBioscience company, PE labeled mouse anti human Tim-3 monoclonal antibody and PE labeled IgG isotype control were obtained from American company R & D, human lymphocyte separation medium (Ficoll) from Sigma company, flow cytometry was purchased from America Beckman-Coulter company.
Peripheral blood mononuclear cells (PBMC) acquisition from AML patients and healthy volunteers 5 ml fasting venous peripheral blood was acquired and injected to the tube containing heparin anticoagulant. We drew 4 ml human lymphocyte separation medium (Ficoll) with the pipette and place it into a centrifuge tube, inclined the tube, diluted the peripheral blood with equivalent volume of PBS, and then added it slowly along centrifugal pipe wall to the upper side of Ficoll solution, placed at the temperature of 18°C-20°C , and centrifuged at 400 g for 30 min. After centrifugation, there was the opaque tunica layer in the upper and middle junction, which was the mononuclear cell layer (PBMC); gently inserted the capillary straw into the tunica albuginea layers, gently drew the PBMC along the wall of the tube, then moved it into another centrifuge tube, joined the PBS to resuspend the cells, washed 2 times with centrifugal; discard supernatant, resuspend the cells with PBS, and adjusted the cell count as 2×106/ml.
Immunofluorescence detection of the Tim-3 expression on peripheral blood T cells
We separated the PBMC suspension to Ep tubes, each tube with 100 µL, placed them on ice; each pipe added with 5 µL corresponding monoclonal antibody, placed at 4°C, kept away from light for 30 minutes. After washing with PBS for 2 times, added 0.4 ml PBS containing 1% calf serum to resuspend cells and transferred it into the flow tube. Then we analyzed the expression of Tim-3 on peripheral blood T cell surface with Beckman labeled Coulter flow cytometry.
Data analysis and statistical treatment
All the data stream was analyzed with Flowjo software (Tree star company). Statistical analysis was performed with SPSS17.0 software. The measurement data expressed as X̅±s, the comparison of random design using t test. P-values less than 0.05 were considered as being statistically significant.
Results
Clinical data of patients
There were 36 cases of AML patients, male 21 cases, female 15 cases, mean age 37.38±14.12 years old. Among them, 5 cases of acute myeloid leukemia in undifferentiated type (M1 type), 15 cases of acute myeloid leukemia differentiation type (M2), 4 cases of acute myelomonocytic leukemia (M4), 9 cases of acute monocytic leukemia (M5), and 3 cases of acute red leukemia (M6). According to the 2013 American National Comprehensive Cancer Network (NCCN) guidelines based on cytogenetic and molecular characteristics, all the patients were divided into low risk group and high risk group, including 25 cases of patients with low risk group, 11 cases with high risk group. We evaluated the curative effect after 2 cycles of standard induction chemotherapy, 28 cases achieved complete remission (CR1), 8 cases without CR1 (≥ CR2) (data not shown).
Detection of T cell proportion in peripheral blood of AML patients and healthy volunteers
Peripheral blood CD4+ T cells accounted for the proportion of lymphocyte in AML patients was 15.28±10.99%, which was significantly reduced compared with that in healthy volunteers (31.12±2.22%, P < 0.0001). Peripheral blood CD8+ T cells accounted for the proportion of lymphocyte in AML patients was 9.19±7.54%, which was significantly reduced compared with that in healthy volunteers (21.59±4.22%, P < 0.0001). All CD4+ and CD8+ T cells analyzed by flow cytometry were derived from CD3+ T cells, concrete statistical data see Table 1.
Table 1.
Peripheral blood CD4+, CD8+ T cells accounted for the proportion of lymphocyte of AML patients and healthy volunteers
| Groups | Case number | CD4+ (%) | P value | CD8+ (%) | P value |
|---|---|---|---|---|---|
| AML patients | 36 | 15.28±10.99 | 9.19±7.54 | ||
| healthy volunteers | 20 | 31.12±2.22 | < 0.0001 | 21.59±4.22 | < 0.0001 |
The comparison CD4+ T cell proportion between the two groups; t=7.475, P < 0.0001, the difference was statistically significant; comparison the percentage of CD8+ T cells between the two groups, t=5.822; P < 0.0001, the difference was statistically significant. The P-values were calculated using the two sample t test.
Expression of Tim-3 on peripheral blood T cells in patients with AML and healthy volunteers
The expression level of Tim-3 on CD4+ T cells was 4.77±3.56% in newly diagnosed AML patients, which was significantly higher than that of healthy volunteers (0.73±0.62%, P < 0.0001); all CD4+ T cells analyzed by flow analysis were derived from CD3+ T cells, concrete statistical data, see Table 2 and Figure 1.
Table 2.
Expression level of Tim-3 on the peripheral blood CD4+ T cells of AML patients and healthy volunteers
| Groups | Case number | Tim-3+/CD4+ (%) | P value |
|---|---|---|---|
| AML patients | 36 | 4.77±3.56 | |
| healthy volunteers | 20 | 0.73±0.62 | < 0.0001 |
The difference of the expression level of Tim-3 on the peripheral blood CD4+ T cells of AML patients and healthy volunteers was statistically significant (t=4.033, P < 0.0001). The P-values were calculated using the two sample t test.
Figure 1.
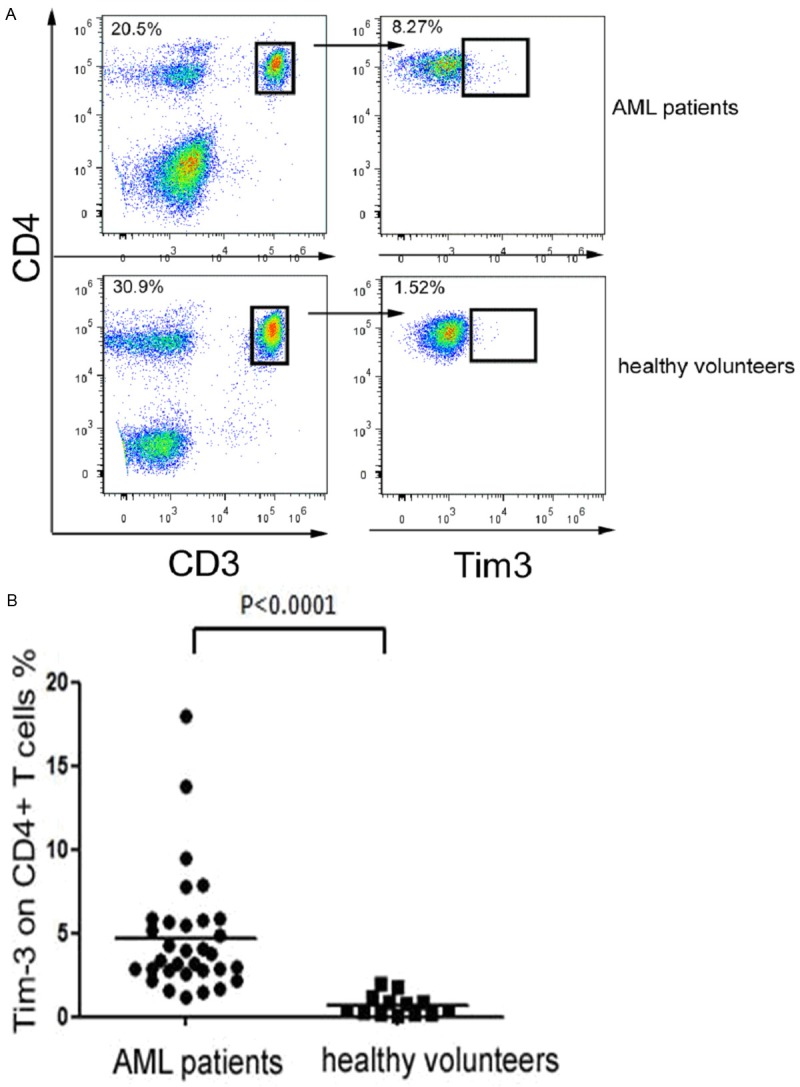
Tim-3 was significantly higher expressed on CD4+ T cells in newly diagnosed AML patients. CD4+ T cells were harvested from peripheral blood of the newly diagnosed AML patients and healthy volunteers. Cells were then stained for CD3, CD4, and Tim-3. A. Representative dot-plots show Tim-3 expression on CD4+ T cells in newly diagnosed AML patients and healthy volunteers. Data shown are representative of ten independent experiments; B. Summarized results of the percentage (%) of Tim-3 expression on CD4+ T cells from AML patients and healthy volunteers are shown. Horizontal bars depict the mean percentage of Tim-3 expression on CD4+ T cells. The P-values were calculated using the two sample t test.
The expression level of Tim-3 on CD8+ T cells was 5.90±4.91% in newly diagnosed AML patients, which was significantly higher than that of healthy volunteers (0.96±0.54%, P=0.02); all CD8+ T cells analyzed by flow analysis were derived from CD3+ T cells, concrete statistical data, see Table 3 and Figure 2.
Table 3.
Expression level of Tim-3 on the peripheral blood CD8+ T cells of AML patients and healthy volunteers
| Groups | Case number | Tim-3+/CD8+ (%) | P value |
|---|---|---|---|
| AML patients | 36 | 5.90±4.91 | |
| healthy volunteers | 20 | 0.96±0.54 | 0.02 |
The difference between the expression level of Tim-3 on the peripheral blood CD8+ T cells of AML patients and healthy volunteers was statistically significant (t=3.301, P=0.02). The P-values were calculated using the two sample t test.
Figure 2.
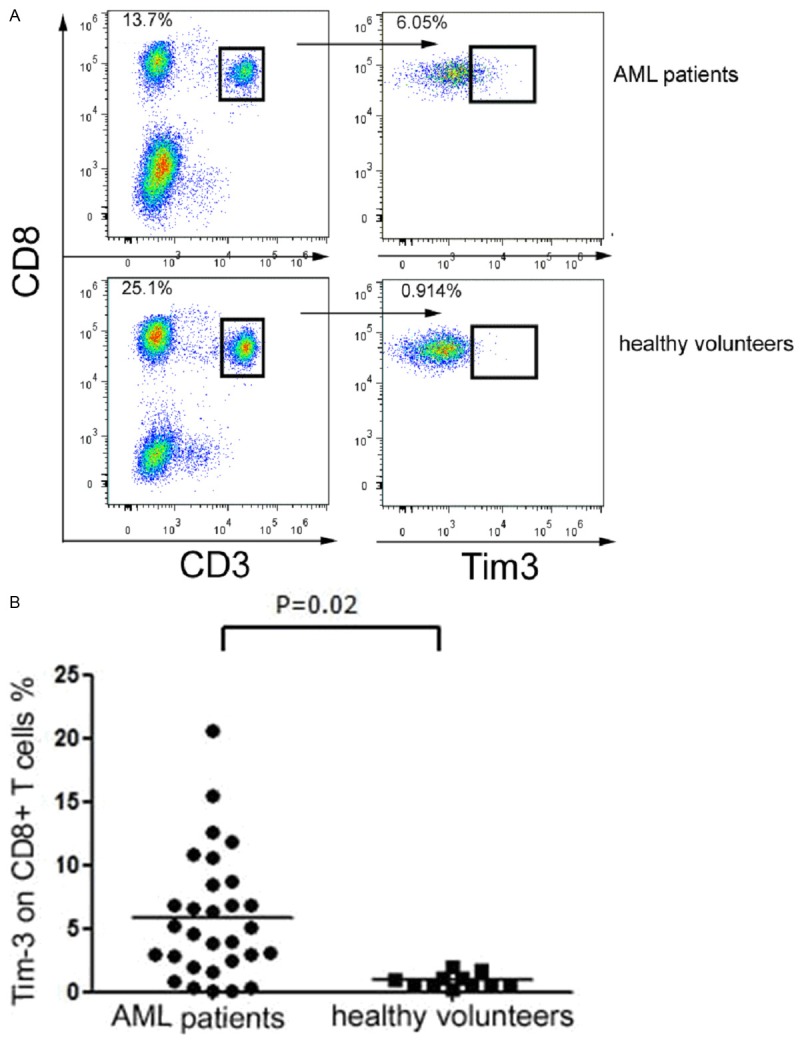
Tim-3 was significantly higher expressed on CD8+ T cells in newly diagnosed AML patients. CD8+ T cells were harvested from peripheral blood of the newly diagnosed AML patients and healthy volunteers. Cells were then stained for CD3, CD8, and Tim-3. A. Representative dot-plots show Tim-3 expression on CD8+ T cells in newly diagnosed AML patients and healthy volunteers. Data shown are representative of ten independent experiments; B. Summarized results of the percentage (%) of Tim-3 expression on CD8+ T cells from AML patients and healthy volunteers are shown. Horizontal bars depict the mean percentage of Tim-3 expression on CD8+ T cells. The P-values were calculated using the two sample t test.
The relationship between T cell ratio in peripheral blood at diagnosis and the prognosis of AML patients
Statistical analysis showed that, the proportion of CD4+ T cells and CD8+ T cells in peripheral blood of AML patients had no significant correlation with FLT3-ITD mutation, alleviate degree and NCCN risk group (P > 0.05); the CD4+ T cells and CD8+ T cell ratio (CD4/CD8) in peripheral blood of AML patients had no significant correlation with FLT3-ITD mutation and degree of relief (P > 0.05), but had significantly correlated with NCCN risk group (P < 0.05) (Table 4).
Table 4.
Relationship between T cell ratio in peripheral blood and diagnosis and prognosis of AML patients
| Case number=36 | CD4+ (%) | P value | CD8+ (%) | P value | CD4/CD8 | P value | |
|---|---|---|---|---|---|---|---|
| FLT3-ITD | |||||||
| positive | 10 | 16.8 | 11.12 | 1.62 | |||
| negative | 26 | 15.53 | 0.5067 | 11.3 | 0.4357 | 1.91 | 0.5357 |
| CR | |||||||
| CR1 | 28 | 17.86 | 12.32 | 1.88 | |||
| ≥ CR2 | 8 | 9.42 | 0.1501 | 6.53 | 0.444 | 1.48 | 0.2573 |
| NCCN groups | |||||||
| Low and medium risk | 25 | 15.23 | 9.52 | 1.9 | |||
| High risk | 11 | 14.79 | 0.7922 | 12.53 | 0.1877 | 1.31 | 0.0469* |
P < 0.05.
Relationship between the expression level of Tim-3 on blood T cells at diagnosis and the prognosis of AML patients
The expression level of Tim-3 on blood CD4+ T cells in AML patients had no obvious correlation with disease remission (CR1 or ≥ CR2) and NCCN risk group (P > 0.05), but was significantly correlated with FLT3-ITD mutation (P < 0.05); The expression level of Tim-3 on blood CD8+ T cells in AML patients had no obvious correlation with disease remission (CR1 or ≥ CR2) and FLT3-ITD mutation (P > 0.05), but was significantly correlated with NCCN risk group (P < 0.05) (Table 5).
Table 5.
Relationship between the expression level of Tim-3 on peripheral blood T cells and diagnosis and prognosis of AML patients
| Case number=36 | Tim-3+/CD4+(%) | P value | Tim-3+/CD8+ (%) | P value | |
|---|---|---|---|---|---|
| FLT3-ITD | |||||
| positive | 10 | 11.51 | 18.52 | ||
| negative | 26 | 2.34 | 0.0021* | 8.47 | 0.3363 |
| CR | |||||
| CR1 | 28 | 5.7 | 14.75 | ||
| ≥ CR2 | 8 | 0.03 | 0.242 | 5.22 | 0.191 |
| NCCN groups | |||||
| Low and medium risk | 25 | 1.63 | 3.93 | ||
| High risk | 11 | 4.8 | 0.1321 | 11.65 | 0.0203* |
P < 0.05.
Discussion
Inhibitory costimulatory molecule Tim-3 is a member of the T cell immunoglobulin mucin (Tim) family [5], which was earliest found expression on the differentiated and maturated Th1 cells, through combining with its ligand galectin-9 produce inhibitory signals, mediated Th1 cell death, suggesting that Tim-3 is a negaive regulatory molecules involved in the cellular immune response of Th1 cell [6]. Subsequent researches found that Tim-3 expressed on dendritic cells, monocytes, CD8+ T cells and other lymphocyte subsets, and mediate immune suppression through different mechanisms. Tim-3 as an immune regulatory molecule, is further studied in the field of immunology, including immune regulation in solid tumor [2-4], but in the hematological malignancy it had been seldom explored, especially the role of Tim-3 in AML immune regulation was rare researched.
T cell mediated immune response is the basis of anti-tumor immune responses. T cell activation and proliferation can effectively kill or eliminate tumor cells. However, T cell activation and function is regulated and influenced by many factors of the microenvironment in vivo [2-4]. Recent years, as the research on the mechanism of tumor microenvironment affecting T cell activation and function goes deeper, anti immune checkpoints molecules have aroused widespread concern. Anti immune checkpoints molecules affect T cell immune responses through down-regulate or up-regulate T cell function [7,8]. In the tumor occurrence and development process, they mainly manifest the inhibitory effect on T cell function, resulting in T cell energy/depletion/dysfunction, thus weakening the tumor immune response. Cancer immunotherapy aims to specific monoclonal antibody blocking immune checkpionts molecule or its ligands to reverse the anergic T cells/depletion/dysfunction status and to replay the function of immune surveillance and destruction or removal of tumor cells. In the tumor microenvironment, tumor infiltrating lymphocytes (TILs) can be the reaction of the body antitumor immune responses, which has an important significance on the prognosis of patients with tumors. Studies have found that, Tim-3 is high expressed on the TILs [9]. Animal experiments have also confirmed, TILs of tumor bearing mices (colon cancer, breast cancer, melanoma, AML) express Tim-3 with high level [9,10], and the high expression of Tim-3 is related to tumor development and stage [10].
We previously found that Tim-3 highly expressed in lung cancer tissues and was associated with tumor progression [4]. However, research is rare that focus on the Tim-3 in non-solid tumors, such as acute myeloid leukemia (AML). This paper analyzed the proportion of CD4+ and CD8+ T cells in AML peripheral blood and the expression characteristics of Tim3 on blood T cells. Results showed that, the peripheral blood CD4+ T cells, CD8+ T cells and lymphocyte proportion were decreased significantly in newly diagnosed AML patients than those of normal control (P < 0.05), and peripheral blood CD4+ T cells, CD8+ T cell ratio (CD4/CD8) in AML is significantly correlated with NCCN risk groups (P < 0.05). More significantly, the expression level of Tim-3 on the CD4+ T cells and CD8+ T cells of newly diagnosed AML patients were (4.77±3.56)% and (5.90±4.91)%, were significantly increased compared with normal people [(0.73±0.62)%, (0.96±0.54)%)] (P < 0.05). We further analyzed the correlation between Tim-3 expressed level on T cells and the disease diagnosis and prognosis. Results suggest that, 1) Tim-3 expression level on CD4+ T cell patients with the FLT3-ITD mutation positive was increased significantly than that of FLT3-ITD mutation negative patients, whereas the FLT3-ITD mutation as a driven role play in the pathogenesis of AML, is a poor prognosis sign [9]; 2) Tim-3 expression level on CD8+ T cells in patients was increased significantly in NCCN high-risk group than that of the low risk group. These results suggest that high expression of Tim-3 on AML patients T cells might be associated with the prognosis of patients.
Recent years, more and more studies have confirmed that the relationship between Tim-3 and exhaustion of effector T cells. Exhausted T cell is a kind of damage of T cell, which was first discovered in virus infected mice [11]. Subsequent studies found that exhausted T cell can appear in human chronic viral infection and tumor [12]. The function of exhausted T cells lose progressively, and there cell phenotype different from the effector T cells and memory T cells, lack of proliferation ability and the response to antigenic stimulation, the ability to decline or loss of the secretion of cytokines (such as IL-2, IFN- γ, TNF- α) [13]. Immune checkpoint molecule PD-1 was first identified as a marker of exhausted T cells in the tumor [14], which persistent expressed on tumor infiltrating lymphocytes in patients with tumors and mediated T cell dysfunction. Subsequent researches found that, Tim-3 is another important marker of exhausted T cells [15]. Both in cancer patients and animal experiments, Tim-3 and PD-1 often showed co-expression, both considered as common symbols of T cell exhaustion molecules [3,16]. Our study suggested that, in the tumor response of AML, due to the continued stimulation of leukemia cell antigen and the induced inhibition of the tumor microenvironment, T cells also exhibited the status of exhaustion/dysfunction, as one of the important immune suppression in the tumor microenvironment, weaken the immune response to AML cells, which played an important role in AML development.
In our study, CD4+ and CD8+ T cell proportion were significantly decreased in AML patients, and the ratio of CD4+ and CD8+ T cell (CD4/CD8) was associated with the AML NCCN risk stratification. So we speculated that high expression of Tim-3 on T cells might also lead to T cells more susceptible to death, which might be one of the factors leading to the development and prognosis of AML.
In summary, high expression of Tim-3 on blood T cells in AML patients was associated with clinicopathological prognostic stratification. The negative immune regulation of Tim-3 involved in the AML may be mediated through the T cell exhaustion, but its exact function and mechanism remains to be further studied.
Acknowledgements
This work was funded by Jiangsu Provincial Special Program of Medical Science (BL2012005), Jiangsu Province’s Key Medical Center (ZX201102), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the National Natural Science Foundation of China (31170866) and Jiangsu Natural Science Fund (BK 2011289).
Disclosure of conflict of interest
None.
References
- 1.Huang X, Bai X, Cao Y, Wu J, Huang M, Tang D, Tao S, Zhu T, Liu Y, Yang Y, Zhou X, Zhao Y, Wu M, Wei J, Wang D, Xu G, Wang S, Ma D, Zhou J. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregμlation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regμlates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 7.Pentcheva-Hoang T, Corse E, Allison JP. Negative regμlators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimμlatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 9.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 13.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue FY, Gyenes G, Wong D, Klein MB, Saeed S, Benko E, Kovacs C, Kaul R, Ostrowski MA. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol. 2010;40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 14.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8 T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2013;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]


