Abstract
Background: Mounting evidence has shown the toxic effects of anesthesia to neonatal hippocampus. We used an in vivo mouse model to explore the role of microRNA 34a (miR-34a) in regulating anesthesia-induced hippocampal neurotoxicity. Methods: One-month old C57/BL6 mice received daily intraperitoneal injection of anesthesia (ketamine, 50 mg/kg) for 7 days. One day after, apoptosis was evaluated by TUNEL staining in hippocampal CA1 region, and expression level of miR-34a assessed by real-time quantitative PCR (qPCR). Hippocampal miR-34a was then down-regulated through lentivirus mediated cortical injection prior to anesthesia. The effects of inhibiting hippocampal miR-34a on anesthesia-induced hippocampal apoptosis and memory impairment were further investigated by TUNEL staining and Morris water maze (MWM) test. The predicted molecular target of miR-34a, fibroblast growth factor receptor 1 (FGFR1) was down-regulated in hippocampus through siRNA-mediated cortical injection and its effect on hippocampal apoptosis was also examined. Results: Anesthesia caused severe apoptosis among hippocampal CA1 neurons and upregulated hippocampal miR-34a. On the other hand, lentivirual inhibition of miR-34a protected anesthesia-induced hippocampal apoptosis and memory impairment. Luciferase essay demonstrated FGFR1 was directly regulated by miR-34a in hippocampus. siRNA-induced FGFR1 downregulation further exaggerated anesthesia-induced apoptosis in hippocampus. Conclusions: Overall, we showed that miR-34a negatively modulated anesthesia-induced hippocampal neurotoxicity.
Keywords: Anesthesia, miR-34a, FGFR1, neurotoxicity, hippocampus
Introduction
Clinical and laboratory studies have revealed that commonly used anesthetics would unavoidably induce neurodegeneration in neonatal brains, both in animals and humans [1-3]. Among them, ketamine is an excitatory glutamate N-methyl-D-aspartate (NMDA) receptors antagonist [4,5], and generally used in neonatal anesthesia. Emerging evidence has brought the attention among both physicians and researchers that prolonged or high dosages of ketamine administration would cause severe neurotoxicity in the neonatal hippocampus and induce long-term memory impairment [6-10]. Unfortunately, the exact mechanisms of anesthesia-induced hippocampal neurodegeneration or memory loss are largely unknown.
MicroRNAs (miRNAs) are groups of short-sequenced noncoding ribonucleic acids (RNAs) that abundantly expressed in brain and play essential roles in various aspects of brain development, including neurogenesis and maturation [11,12], regeneration [13], cortical neuropathy and neurodegenerative diseases [14-16]. Among many of the cortically expressed miRNAs, microRNA 34a (miR-34a) belongs to the family of miR-34 (miR-34 a/b/c) and played critical roles in many aspects of cortical development and tumorigenesis [17-19].
In the present study, we set to examine whether miR-34a was involved in the process of anesthesia-induced neurodegeneration in hippocampus. We introduced prolonged administration of ketamine to induce hippocampal neural apoptosis and memory impairment in young animals (one-month old mice). We then examined the corresponding expressional change of miR-34a in mouse hippocampus. Subsequently, we applied lentivirus-mediated miR-34a inhibition and small interfering RNA (siRNA) mediated gene knocking-down to explore the molecular role of miR-34a in ketamine-induced hippocampal apoptosis and memory dysfunction. The purpose of this study is to identify the molecular target to clinically intervene or protect anesthesia-induced neurodegeneration in neonatal cortex.
Experimental procedures
Animals
C57/BL6 mice were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). All animals were maintained in animal facility of the Second Affiliated Hospital of Nantong University. The animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Second Affiliated Hospital of Nantong University in Nantong, Jiangsu Province, China.
In vivo anesthesia
At one-month age, mice received an intraperitoneal injection of 50 mg/kg of body weight of ketamine hydrochloride (Shanghai Sino-west Pharmaceutical Company, Shanghai, China), once a day for seven days. The control group of mice received intraperitoneal injection of normal saline (N.S.).
Hippocampal terminal transferase dUTP nick end labeling (TUNEL) staining
Twenty-four hours after the completion of ketamine administration, mice were sacrificed and hippocampal slices (300 μm) were prepared by a vibratone (Leica, Germany). Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining was then performed by an In-situ cell death kit (Roche, USA) to examine the apoptosis in hippocampal CA1 region. An antibody against neuronal marker microtubule-associated protein MAP2 (1:100, Santa Cruz, USA) was also applied to identify neurons in hippocampal CA1 region. The percentages (mean ± SEM) of neurons with apoptotic nuclei (both MAP2+ and TUNEL+) were then quantified.
RNA isolation and real-time quantitative PCR (qPCR)
After mice were sacrificed, hippocampus was quickly extracted, frozen on dry ice and homogenized with a Trizol Reagent (Invitrogen, USA). 200 μL chloroform was added. Tissue samples were centrifuged for 20 min and the aqueous tissue was isolated with RNase-free tubes (Solarbio, China). Total RNA was then washed by 75% ethanol and collected in 50 μL pure water. The RNA concentration was assessed by a nanodrop spectrophotometer (260/280 nm, Ocean Optics, USA) followed by 2% agarose gel electrophoresis. Hippocampal messenger RNAs, miR-34a and internal control U6, FGFR1 and internal control GAPDH, were analyzed with a miRNAs qPCR Quantitation Kit (GenePharma, Shanghai, China) with a DNA Engine Opticon 2 Two-color real-time PCR Detection System (Bio-Rad, USA) according to manufacturer’s manuals.
Lentivirus production
The inhibition of hippocampal miR-34a was performed with lentivirual conduction. The coding oligonucleotides of mouse antisense miR-34a inhibitor, miR-34a mimics and non-specific control were purchased from RiboBio (Shanghai, China). The sequences were then cloned into feline immunodeficiency virus (FIV) based lentivirus expression vector, Pcdh-CMV-MCS-EF1-copGFP (SBI, USA) and co-expressed with pPACK packaging system in 293 TN cells to produce viral particles of miR-34a antisense inhibitor (miR34a-I), miR-34a mimics (miR34a-mimics) and non-specific control vector (miR-NC).
SiRNA production
Short interfering RNA (siRNA), including FGFR1 siRNA (FGFR1_siRNA) and control siRNA (Ctrl_siRNA) were purchased from Stanta Cruz (Santa Cruz Biotechnology, USA). The concentration of in vivo application was 50 uM.
In vivo hippocampal injection
Twenty-four hours prior to anesthesia, lentiviruses (miR34a-I and miR-NC) or siRNAs (FGFR1_siRNA and Ctrl_siRNA) were delivered to mouse hippocampus through an injection on on the right side of the brain. Under a surgical microscope, a tiny hole (0.05 mm in diameter) was drilled on the right cortex just above hippocampus. A Hamilton syringe was applied to inject total volume of 2 μL lentiviruses or siRNAs into dorsal hippocampus (from bregma: dorso-ventral, -2.0 mm, medio-lateral, +2.4 mm; anterio-posterior, -1.8 mm).
Luciferase reporter assays
Hippocampal cDNA was produced by regular PCR. The wild-type 3’-UTR and mutant 3’-UTR of FGFR1 were then amplified and cloned into a pMIR-REPORT luciferase reporter vector (Ambion, USA) to produce Luc- FGFR1 and Luc- FGFR1-mu constructs. The pMIR-REPORT control vector, and Luc- FGFR1 and Luc- FGFR1-mu were then co-transfected with β-galactosidase and lentivirus miR34a-mimics into HEK293 cells with Lipofectamine 2000 reagent according to manufacturer’s manual (Invitrogen, USA). The luciferase reporter assay (Promega, USA) was then conducted 24 hours after transfection. The fluorescent intensities were measured in triplicates and normalized to the intensity of β-galactosidase of control vector.
Morris water maze (MWM) test
The MWM test was conducted according to previous study with minor modification [20]. The MWM was built as a circular pool (200 cm diameter), filled with warm water at 21 C. Mice, at 2-month age, were trained to swim in water to a submerged platform (1.5 cm×1.5 cm. 1.5 cm beneath water surface) guided by distal cues attached to the walls. A computerized tracking/analyzing video system was used to record all animal movements. The acquisition phase included four training sessions per day for four consecutive days. Mice were and allowed to swim freely until they reached the platform in 2 minutes and rested on it for 30 seconds. If mice did not locate the platform then, they were manually placed on the platform for 30 seconds. On 5th day, animals was conducted an examining session, in which the path length and path time to reach the platform were recorded.
Statistical analysis
All data were presented as mean ± standarddeviations (SEM). All experiments were repeated at least three times. Statistic analysis was conducted with a MS-Windows-based SPSS software (version 13.0). The statistic differences were evaluated by student’s t-test, and the significance was defined while P < 0.05.
Results
Anesthesia caused apoptosis in hippocampal CA1 neurons and upregulated miR-34a
It was shown previously in an in vivo rat experiment that consecutive treatment of high concentrations of ketamine caused neuronal damage in hippocampus in young brain [6]. In the present study, we used similar approach and successfully induced hippocampal apoptosis in the developing mouse brain. Yong C57/BL6 mice (one-month old) were conducted with intraperitoneal (I.P.) injection of ketamine (50 mg/kg per day) for 7 days. One day after the last administration of ketamine, mice were sacrificed and the hippocampal slices were prepared to examine the status of apoptosis among neurons. In control group, mice were injected with normal saline (N.S.) and prepared as the same way as ketamine-treated mice. The TUNEL staining, along with immuno-staining of neuronal marker MAP2 were conducted in CA1 regions of the prepared hippocampal slices (Figure 1A). Under N.S. condition, there seemed to be little or no apoptotic CA1 neurons, based on double-positive staining of TUNEL and MAP2. However, in hippocampi treated with ketamine, considerable amount of apoptotic CA1 neurons were identified with double-staining of TUNEL and MAP2 (arrows), suggesting significant apoptosis induced by anesthesia. The qPCR was also conducted one day after the last administration of ketamine to compare the expression levels of miR-34a between ketamine-treated and control (N.S.) hippocampi. It demonstrated that miR-34a was markedly upregulated due to ketamine administration (Figure 1B, *: P < 0.05, as compared to N.S.).
Figure 1.
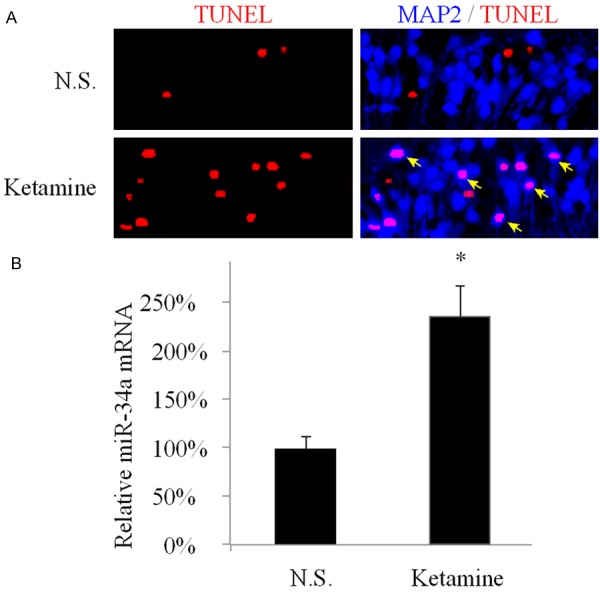
In vivo anesthesia lead to hippocampal apoptosis and upregulation of miR-34a C57/BL6 mice were conducted with I.P. injection of 50 mg/kg ketamine once a day for seven consecutive days. Control mice were injected with normal saline (N.S.). A. One day after treatment, hippocampal slices under both ‘ketamine’ and ‘N.S.’ conditions were prepared. In CA1 area, a neuronal marker of MAP2 (Blue, 1:100, Sigma-Aldrich, USA) was used to visualize CA1 neurons and TUNEL staining (Red) to visualize apoptotic nuclei. Cells positive to both TUNEL and MAP2 signals are identified as apoptotic CA1 neurons (arrows); B. One day after treatment, hippocampal tissues under both ‘ketamine’ and ‘N.S.’ conditions were examined by qPCR to compare the expression levels of miR-34a. (*: P < 0.05, as compared to N.S.).
Inhibition of hippocampal miR-34a reduced neuronal apoptosis and memory impairment
As we discovered that miR-34a upregulation was associated with anesthesia-induced hippocampal apoptosis, we wondered whether miR-34a had a functional role in it. For that purpose, we constructed lentivirual vector containing antisense oligonucleotides to specifically knock know miR-34a (miR34-I) and injected it into mouse hippocampus. The control mice received hippocampal injection of non-specific lentivirual vector (miR-NC). One day following hippocampal injection, two groups of mice were conducted with ketamine administration for 7 days, followed by immuno-staining of TUNEL essay in 24 hours. As expected, with non-specific miR-NC treatment, ketamine induced severe apoptosis in hippocampal CA1 regions (Figure 2A, upper panel, arrows). However, while mice were pretreated with miR34a-I to down-regulate miR-34a in hippocampus, significantly less CA1 neurons were dying after ketamine administration (Figure 2A, lower panel, arrows). This result suggested that inhibition of miR-34a had a protective effect on neuronal death in anesthesiainduced hippocampal apoptosis.
Figure 2.
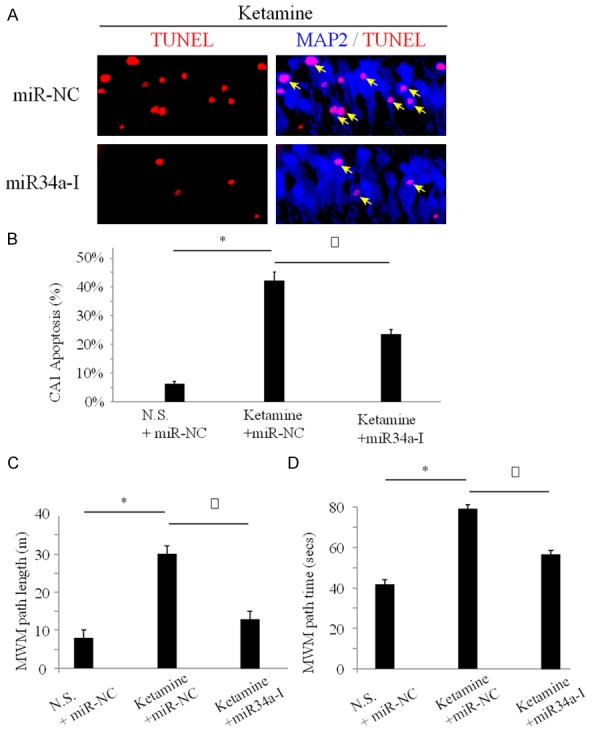
Knocking down miR-34a reduced hippocampal apoptosis and memory impairment One day before ketamine administration, C57/BL6 mice received cortical injection of lentivirus vector of miR-34a inhibitor (miR34a-I) to specifically knock down miR-34a in hippocampus. Control mice were injected with non-specific lentivirus (miR-NC). After ketamine administration, hippocampal slices were examined and apoptotic CA1 neurons were identified as both positive to TUNEL and MAP2 immuno-stainings (arrows) (A). Three groups of mice received different combination of lentivirual and ketamine administration, including those received miR-NC hippocampal injection and normal saline treatment (N.S.+miR-NC), those received miR-NC hippocampal injection and ketamine administration (Ketamine+miR-NC) and those received miR34a-I hippocampal injection and ketamine administration (Ketamine+miR34a-I). One day after ketamine administration, quantification of apoptotic CA1 neurons was performed (B) (*: P < 0.05; Δ: P < 0.05). One month after ketamine administration, cognitive memory test of Morris Water Maze (MWM) test was performed to measure the path length (C) and path time (D) for mice to swim to the hidden platform (*: P < 0.05; Δ: P < 0.05).
We then gave mice combined treatment of lentivirus and ketamine, and conducted both in vitro quantitative apoptosis essay and in vivo memory function essay, to further verify this hypothesis. In one group, mice received miRNC hippocampal injection followed with normal saline administration (N.S.+miR-NC). In second group, mice received miR-NC hippocampal injection followed by ketamine administration (Ketamine+miR-NC). In third and final group, mice received miR34a-I hippocampal injection followed by ketamine administration (Ketamine+miR34a-I).
One day after ketamine administration, hippocampus of three groups of mice were prepared in slices and examined with TUNEL staining. Quantitative measurement demonstrated that between two groups of mice received pre- hippocampal injection of control siRNA (miR-NC), ketamine induced larger number of apoptotic CA1 neurons (Figure 2B, *: P < 0.05). It also showed that, between two groups of mice receiving ketamine administration, lentivirual inhibitor of miR-34a (miR34a-I) significantly reduced the number of apoptotic CA1 neurons (Figure 2B, Δ: P < 0.05). One month after ketamine administration, three groups of mice were examined with Morris Water Maze (MWM) test. The results demonstrated that between two groups of mice received pre-hippocampal injection of control siRNA (miR-NC), ketamine caused significant memory impairment with increased path length and path time for mice to reach the center platform (Figure 2C, 2D, *: P < 0.05). It also showed that, between two groups of mice receiving ketamine administration, lentivirual inhibitor of miR-34a (miR34a-I) significantly rescued the memory impairment with improved path length and path time for mice to reach the center platform (Figure 2C, 2D, Δ: P < 0.05).
FGFR1 was likely the target of miR-34a in protecting hippocampus from anesthesia-induced damage
We then investigated the possible molecular pathway involved in the inhibition of miR-34a on protecting anesthesia-induced hippocampal damage. Based on some of the internet-based miRNA target predicting services, such as TargetScan (http://www.targetscan.org/) and miRANDA (http://www.microrna.org/microrna/home.do), we identified that a member of the fibroblast growth factor receptor (FGFR) family, FGFR1, was a potential target of miR-34a (Figure 3A). We thus used a luciferase assay, including the construct containing mouse hippocampal FGFR1 (luc-FGFR1) and its 3’-UTR mutated construct (Luc-FGFR1-mu) to verify that it was indeed the case for FGFR1 to be targeted with miR-34a in mouse hippocampus (Figure 3B).
Figure 3.
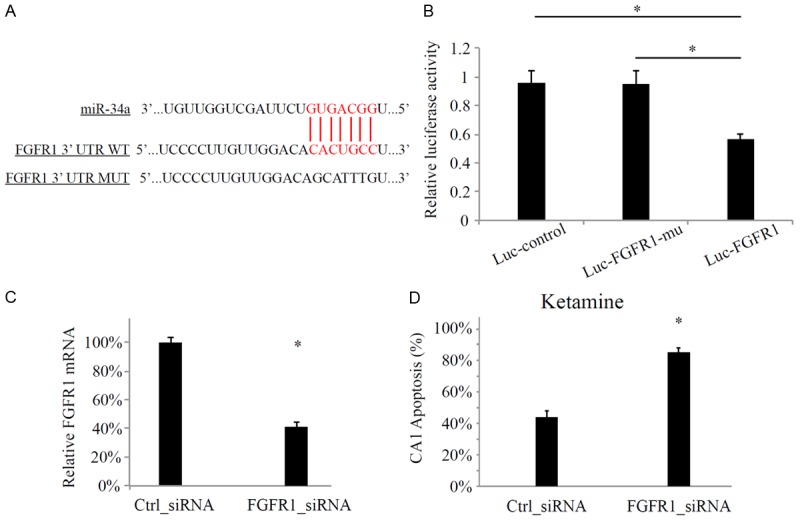
miR-34a targeted FGFR1. A. The predicted binding site between miR-34a and FGFR1 3’-UTR. Also listed is the mutated sequence of 3’-UTR of FGFR1 (FGFR1-mu); B. Luciferase essay transfected HEK293 cells with β-galactosidase, miR34a-mimics and one of the reporter, the control construct (Luc-control), mutant 3’-UTR FGFR1 (Luc-FGFR1-mu) or wild-type 3’-UTR FGFR1 (Luc-FGFR1). The measurments were normalized to β-galactosidase activity of Luc-control. (*: P < 0.05). Mouse hippocampus was injected with FGFR1-specific siRNA (FGFR1_siRNA, 50 uM) or negative control siRNA (Ctrl_siRNA, 50 uM); C. The hippocampal expressions of FGFR1were measured by qPCR 24 hours after cortical injection of Ctrl_siRNA or FGFR1_siRNA (*: P < 0.05); D. Mice received cortical siRNA injections and then 7-day ketamine administration. One day after ketamine administration, TUNEL staining was performed on hippocampal slices and apoptosis of CA1 neurons were compared between mice received Ctrl_siRNA and FGFR_siRNA (*: P < 0.05).
Based on that result, we then conducted in vivo hippocampal injection of FGFR1 specific siRNA (FGFR1_siRNA, 50 uM) in one-month old mice to further examine whether FGFR1 would directly play a role in anesthesia associated hippocampal apoptosis. The negative control siRNA (Ctrl_siRNA, 50 uM) was also injected to examine the efficiency of siRNA modulation. It demonstrated by qPCR that the expression level of FGFR1 was significantly down-regulated by FGFR1_siRNA, but not by Ctrl_siRNA (Figure 3C, *: P < 0.05).
Finally, one day after hippocampal injection of siRNA to genetically knock down FGFR1 in hippocampus, mice were administrated with 7 days of ketamine followed by TUNEL staining and quantitative measurement of hippocampal apoptosis. The result revealed that the more apoptotic CA1 neurons were found in mice received FGFR1_siRNA, as compared to mice just received control siRNA (Figure 3D). Thus, our results strongly supported the hypothesis that FGFR1 was directly targeted by miR-34a to regulate anesthesia-induced hippocampal apoptosis.
Discussion
General anesthetics are extensively used in modern days and generally considered to be effectively safe. However, more and more clinical or experimental studies have brought caution among the anesthetic society that, prolonged or high dosage application of anesthetics would induce neurotoxicity or neurodegeneration in developing brains. In the present study, we used an in vivo animal model to introduce prolonged and continuous administration of ketamine (50 mg/kg per day, seven days) in young mice. The induced toxic effects, including severe apoptosis among hippocampal CA1 neurons and long-term memory impairment are in line with previous studies showing similar neurodegenerative effects of ketamine on neonatal brains [6,7], further solidify the notation that caution shall be taken while putting infants under general anesthetics.
We found that, under the condition of anesthesia-induced hippocampal neurodegeneration, miR-34a was significantly upregulated. We then used lentivirual tools to genetically down-regulate the expression of miR-34a in hippocampus, and discovered that inhibition of miR-34a was protective against ketamine-induced hippocampal apoptosis and memory impairment. Previous reports showed that miR-34a was acting as tumor-suppressor that commonly downreuglated and induced apoptosis in tumor cells [17,19]. In hippocampus, miR-34 family (miR-34a/b/c) is the targets of p53 and they are essential for cortical development as deficit of miR-34a lead to under-development of neuronal differentiation and synaptogenesis [21]. Furthermore, hippocampal miR-34a was upregulated under pathological conditions, such as epilepsy or neurodegenerative disease [22,23]. Thus, the results of our experiment showing mir-34a inhibition protected hippocampal apoptosis are in line with previous ones demonstrating miR-34a downregulating reduced caspase-3 and protected apoptosis in Alzheimer’s disease [23], suggesting a protective role of miR-34a in cortical development against apoptosis or neurodegeneration.
Also in the present study, we showed that FGFR1 was very likely to be the direct modulatory target of miR-34a in hippocampus and downregulation of FGFR1 exaggerated the hippocampal apoptosis induced by anesthesia. The fibroblast growth factor family, including fibroblast growth factor receptor 1 (FGFR1), is essential for the development of hippocampus. Previous reports have demonstrated that under the condition of social defeat, FGFs were significantly downregulated in hippocampus [24], and long-term activation of FGFR1 reduced apoptosis in retinal pigmented epithelial cells [25]. Although we did not present direct evidence of FGFR1 activation or upregulation reduced/protected anesthesia-induced apoptosis, the results of our study showing knocking down FGFR1 facilitated apoptosis in hippocampal CA1 neurons would support this hypothesis and future experiment exploring the correlation between miR-34a and FGFR1, as well as the direct effect of FGFR1 against hippocampal neurodegeneration would certainly further our understanding on their regulation on neurodegeneration in neonatal cortex.
Overall, our study demonstrated a new mechanism of miR-34a negatively regulating anesthesia-induced hippocampal apoptosis and its regulation was very likely through FGFR1 in hippocampus.
Disclosure of conflict of interest
None.
References
- 1.Flick RP, Nemergut ME, Christensen K, Hansen TG. Anesthetic-related neurotoxicity in the young and outcome measures: the devil is in the details. Anesthesiology. 2014;120:1303–1305. doi: 10.1097/ALN.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen EA, Brambrink AM. Anesthetic neurotoxicity in the newborn and infant. Curr Opin Anaesthesiol. 2013 doi: 10.1097/01.aco.0000433061.59939.b7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 4.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 5.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Liu Y, Jin W, Ji X, Dong Z. Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res. 2012;1476:164–171. doi: 10.1016/j.brainres.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Liu Y, Zhang P, Kang R, Li X, Bo L, Dong Z. In vitro dose-dependent inhibition of the intracellular spontaneous calcium oscillations in developing hippocampal neurons by ketamine. PLoS One. 2013;8:e59804. doi: 10.1371/journal.pone.0059804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng S, Zhang Y, Zhang J, Wang H, Ren B. Effect of ketamine on ERK expression in hippocampal neural cell and the ability of learning behavior in minor rats. Mol Biol Rep. 2010;37:3137–3142. doi: 10.1007/s11033-009-9892-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr. Preclinical assessment of ketamine. CNS Neurosci Ther. 2013;19:448–453. doi: 10.1111/cns.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W Jr, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L. MicroRNAs tune cerebral cortical neurogenesis. Cell Death Differ. 2012;19:1573–1581. doi: 10.1038/cdd.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda RC. MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis. Front Genet. 2012;3:77. doi: 10.3389/fgene.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, Murashov AK. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front Mol Neurosci. 2013;6:35. doi: 10.3389/fnmol.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh P, Soon PS, Feige JJ, Chabre O, Zhao JT, Cherradi N, Lalli E, Sidhu SB. Dysregulation of microRNAs in adrenocortical tumors. Mol Cell Endocrinol. 2012;351:118–128. doi: 10.1016/j.mce.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Bithell A, Johnson R, Buckley NJ. Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington’s disease. Biochem Soc Trans. 2009;37:1270–1275. doi: 10.1042/BST0371270. [DOI] [PubMed] [Google Scholar]
- 16.Goedeke L, Fernandez-Hernando C. microRNAs: A connection between cholesterol metabolism and neurodegeneration. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2014.05.034. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathod SS, Rani SB, Khan M, Muzumdar D, Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–495. doi: 10.1016/j.fob.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silber J, Jacobsen A, Ozawa T, Harinath G, Pedraza A, Sander C, Holland EC, Huse JT. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS One. 2012;7:e33844. doi: 10.1371/journal.pone.0033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 20.Patil SS, Sunyer B, Hoger H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009;198:58–68. doi: 10.1016/j.bbr.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW, Ventura A, Concepcion CP, Han YC, Candi E, Knight RA, Mak TW, Melino G. microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A. 2011;108:21099–21104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L, Li Y, Xiao B. MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci. 2012;13:115. doi: 10.1186/1471-2202-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y, Zhang L, Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res Bull. 2009;80:268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008;430:147–150. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryckaert M, Guillonneau X, Hecquet C, Courtois Y, Mascarelli F. Both FGF1 and bcl-x synthesis are necessary for the reduction of apoptosis in retinal pigmented epithelial cells by FGF2: role of the extracellular signal-regulated kinase 2. Oncogene. 1999;18:7584–7593. doi: 10.1038/sj.onc.1203200. [DOI] [PubMed] [Google Scholar]


