Abstract
Diabetic retinopathy (DR) is a well-known serious complication of diabetes mellitus (DM), and can eventually advance to end-stage blindness. In the early stage of DR, endothelial cell barrier disorganized primarily and tight junction (TJ) protein composition transformed subsequently. The small GTPase RhoA and its downstream effector Rho-associated coiled-coil containing protein kinase 1 (ROCK1) regulate a mass of cellular processes, including cell adherence, proliferation, permeability and apoptosis. Although RhoA inhibitors have provided substantial clinical benefit as hypertonicity therapeutics, their use is limited by complex microenvironment as DR. While ample evidence indicates that TJ can be influenced by the RhoA/ROCK1 signaling, the underlying mechanisms remain incompletely understood. Here, we have uncovered a significant signaling network involved in diabetic retinal microvascular endothelial dysfunction (RMVED). Our results indicated that the activation of RhoA/ROCK1 pathway due to high glucose played a key role in microvascular endothelial cell dysfunction (MVED) by way of directly inducing TJ proteins over-expression during DR. We demonstrated that inhibition of RhoA/ROCK1 may attenuate the hypertonicity of endothelial cell caused by high glucose microenvironment meanwhile. Besides, chemical and pharmacological inhibitors of RhoA/ROCK1 pathway may partly block inflammation due to DR. Simultaneously, the apoptosis aroused by high glucose was also prevented considerably by fasudil, a kind of pharmacological inhibitor of RhoA/ROCK1 pathway. These findings indicate that RhoA/ROCK1 signaling directly modulates MVED, suggesting a novel therapeutic target for DR.
Keywords: RhoA, ROCK1, tight junction, apoptosis, fasudil, endothelial dysfunction, diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is a clinically well-defined, sight-threatening, chronic microvascular complication that eventually affects virtually all patients with diabetes [1]. In the early stage of DR, retinal microvascular endothelial cells (RMVECs) barrier are damaged primarily, such as tight junctions (TJs), adherens junctions (AJs), gap junctions (GJs) and complexus adherents (CAs), etc. Among all these biomarkers of endothelial dysfunction, TJs possess a high abundance in RMVECs. Other evidences suggest that the damage of endothelial TJs might be a crucial mechanism underlying the increased permeability of endothelial cells [2]. Simultaneously, the TJ is a multi-protein complex and is the apical most junctional complex in certain epithelial and endothelial cells [3]. The TJ complex is composed of TJ proteins, including the transmembrane proteins claudin-5 and occludin, and the cytoplasmic adaptor proteins such as zonula occludens-1 (ZO-1), the abundance and properties of which determine the magnitude of paracellular solute movement [4,5]. Nonetheless, our understanding of molecular mechanism of TJ modulation remains limited [4] and the understanding of the molecular mechanism responsible for RhoA/ROCK1 remains an enigma hitherto.
Ras, a well-known 21-kDa guanosine triphosphatase (GTPase), consists of a large amount of subfamilies, such as Cdc42, Rac1 and Rho, which have been studied a great deal [6-8]. The Rho proteins, whose prototypical member is RhoA, are molecular switches that respond to cell surface receptors for various cytokines, growth factors, adhesion molecules, and G-protein-coupled receptors by cycling between an inactive guanosine diphosphate (GDP)-bound and an active guanosine triphosphate (GTP)-bound form [9]. Rho-associated coiled-coil containing protein kinases (ROCK1 and ROCK2) [10] are the most extensively studied RhoA effector proteins and regulate actomyosin contractility via a direct phosphorylation of myosin light chain and inactivation of the myosin-binding subunit of myosin phosphatase [9,11]. The RhoA GTPase is crucial in numerous biological and pathological functions [12]. RhoA, the Ras homolog family member A, and its downstream target ROCK1 regulate cellular adherence, migration, proliferation, and apoptosis through the control of the actin cytoskeletal assembly and cell contraction [13,14].
The recent study showed the activity of Rho and p-MYPT1 (phospho-myosin phosphatase target protein 1) were markedly increased, and eNOS phosphorylation were downregulated by 57% in the diabetic rat retinas after 2 weeks of diabetes onset [15]. Inhibition of RhoA/ROCK1 signaling significantly reduced endothelial damage and vascular leakage which is stimulated by high glucose/advanced glycation end products (AGEs) in cultured endothelial cells and in the presence of diabetes mellitus [16]. Previous research has indicated a link between the actin cytoskeleton and occludin/ZO-1, in that signaling molecules that regulate contraction of actin filaments are also important for modulation of TJ permeability [17]. Therapeutic benefits of ROCK inhibition may extend to insulin-resistant diabetes [18]. Rikitake et al. found that exposure of endothelial cells to hyperglycemia (12 to 25 mmol/l) increased ROCK1 activity in a concentration-dependent manner and PAI-1 mRNA expression [19]. However, the role of RhoA/ROCK1 pathway targeting TJ proteins in hyperglycemia affecting RMVECs was yet to be adequately resolved.
Our research ranged from the character of TJ proteins at molecule-level resolution to the essence of RhoA/ROCK1 pathway in DR. The study focused on the role of RhoA/ROCK1 signaling pathway playing in injuring endothelial cell barrier caused by high glucose in vitro during the process of DR. We reported that the inhibition of RhoA/ROCK1 pathway may ameliorate the retinal endothelial cell dysfunction induced by hyperglycemia. Meanwhile, a frequently applied clinical drug, fasudil, was found effectively inhibited RhoA/ROCK1 pathway, suggesting a new therapeutic target for the RMVED in DR.
Materials and methods
Reagents and antibodies
Primary antibodies against occludin, claudin-5 and ZO-1 were purchased from cell signaling technology (Danvers, MA, USA), anti-RhoA and anti-ROCK1, anti-p-MYPT1 (Thr853) and anti-MYPT1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Y-27632 (chemical inhibitor of ROCK1) was purchased from Sigma-Aldrich (Sigma, MO, USA). Rho Activation Assay Kit was from Millipore (Bedford, MA, USA). Anti-GAPDH was from cell signaling technology (Danvers, MA, USA).
Cell culture
The rhesus macaque choroid-retinal endothelial cell line, RF/6A cells (purchased from the Cell Bank of the Chinese Academy of Sciences), was cultured in RPMI 1640 Medium (Gibco, Invitrogen, NY, USA), supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Invitrogen, NY, USA), 100 U/ml of penicillin, and 100 μg/ml of streptomycin in 95% humidified air at 37°C with 5% CO2 [20,21]. Since numerous investigators utilized high concentration of glucose (20-35 mmol/l) for in vitro experiments [22,23], we imitated hyperglycemia environment by exposing RF/6A cells to a high level of glucose (HG, 30 mmol/l) for 48 h while to a normal level of glucose (NG, 5 mmol/l) as control.
Western blot analysis
In vitro, Adherent cells grown to subconfluency on tissue culture plates were washed with phosphate-buffered saline (PBS) and lysed in 250 μl of 2x concentrated 12% SDS Lysis Buffer (Beyotime Biotchnology, China). The samples collected were first boiled at 95°C for 5 min and then frozen in ice crush for 5 min in turn. After three times successively manufacturing, cell lysates were electrophoresed in SDS-PAGE, and transferred onto nitrocellulose membrane. The membrane was blocked with 5% nonfat dried milk solution and subsequently incubated overnight in gentle agitation at 4°C with anti-occludin, anti-claudin-5, anti-ZO-1, anti-RhoA and anti-ROCK1, anti-p-MYPT1 (Thr853) and anti-MYPT, respectively. This was followed by incubation with corresponding horseradish peroxidase (HRP) conjugated secondary antibodies. GAPDH was used as internal control. Immunoreactive bands were identified using an enhanced chemiluminescence (ECL) detection system (Pierce Biotechnology, Inc., Rockford, IL, USA).
Real-time PCR
The Real-time PCR primer sequences used were as follows: RhoA forward: 5’-TGGAAAGACATGCTTGCTCAT-3’ and reverse 5’-GCCTCAGGCGATCATAATCTTC-3’, ROCK1 forward 5’-AACATGCTGCTGGATAAATCTGG-3’ and reverse 5’-TGTATCACATCGTACCATGCCT-3’, occludin forward 5’-ACAAGCGGTTTTATCCAGAGTC-3’ and reverse 5’-GTCATCCACAGGCGAAGTTAAT-3’, cla- udin-5 forward 5’-AAATTCTGGGTCTGGTGCTG-3’ and reverse 5’-GCCGGTCAAGGTAACAAAGA-3’, ZO-1 forward 5’-AGGACACCAAAGCATGTGAG-3’ and reverse 5’-GGCATTCCTGCTGGTTACA-3’, ICAM-1 forward 5’-GACTAAGCCAAGAGGAAG-3’ and reverse 5’-CTCAGCATACCCAATAGG-3’.
Short interfering RNA (siRNA)
For knockdown of ROCK1, we performed targeted gene silencing using siRNA technique. The sequence of siRNA targeting ROCK1 is as follows: CAGCAAAUCCUAAUGAUAA. siRNA targeting ROCK1 and control siRNA were purchased from QIAGEN (Vienna, Austria). RF/6A cells were transfected with 100 nmol/L of siRNA using HiPerFect Transfection Reagent (QIAGEN, Vienna, Austria) according to the manufacturer’s instructions. Efficiency of ROCK1 knock-down was confirmed by real-time PCR analysis of mRNA (Data are not shown).
Endothelial cell permeability
For the detection of endothelial permeability, albumin diffusion across endothelial monolayers was measured as described previously [24-26]. The permeability of the RF/6A cells monolayer was quantified as a percentage clearance of bovine serum albumin (BSA) from insert to lower well, as described by Quan and Godfrey [27] and Bonner and O’Sullivan [28]. Finally, the clearance rates of Trypan blue-labelled albumin through the cell monolayers were measured using a spectrophotometer.
Transendothelial electrical resistance
Transendothelial electrical resistance (TEER) was determined as previously described [29]. We partly modified that method like Pavan B et al. depicted before [30]. RF/6A cells were seeded to form confluent monolayers to develop low electrical resistance on Transwell-permeable supports (area of 0.33 cm2) in 24-well plates [31]. TEER was measured using an epithelial voltmeter (WPI, Sarasota, FL).
Analysis of apoptosis
To detect early stages of apoptosis, an Annexin-VFLUOS staining kit (Roche) was used according to the manufacturer’s instructions. Briefly, cells were washed with PBS and incubated with Annexin-V/propidium iodide (PI)/Hoechst 33342 for 15 min at room temperature. Cells were visualized with a fluorescence microscope and Annexin-V positive and PI negative cells were assessed as apoptotic. The percentage of apoptotic cells was calculated by dividing Annexin-V positive cells by the total number of cells visualized by Hoechst staining. Three randomly chosen optical fields per preparation were digitized and each experimental condition was done in triplicate. The mean percentage of apoptotic cells was averaged over a total of three independent experiments.
Statistical analysis
The results are expressed as the means ± standard deviation (SD). Group means were compared by one-way ANOVA using the GraphPad Prism 4.0 software system (GraphPad, San Diego, CA) and the statistical software program SPSS version 17.0 for Windows (SPSS, Chicago, IL). In all comparisons, a value of P < 0.05 was considered to indicate a statistically significant difference.
Results
RhoA and ROCK1 are activated by high glucose in RF/6A cells
Previously, we demonstrated that high glucose increased RhoA activity. Compared with NG (normal glucose, 5 mmol/L) treated group, HG (high glucose, 30 mmol/L) group showed an increase in the RhoA activity by measurement of RhoA-GTP/total RhoA ratio, (P < 0.05) (Figure 1A). Mnt (mannitol, 30 mmol/L) group was without effect and fasudil inhibited the response effectively (P < 0.05) in contrast (Figure 1A). Subsequently, real-time PCR analysis of RhoA proved the same effect (Figure 1B).
Figure 1.
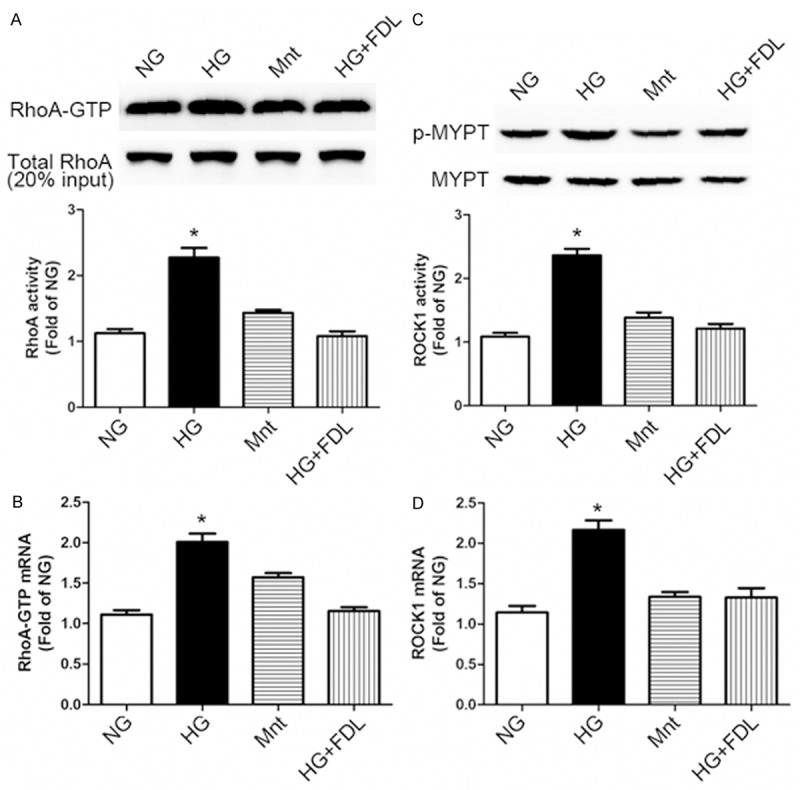
High glucose induced RhoA/ROCK1 activation and fasudil inhibited this response in RF/6A cells. A: Western blotting (up) and quantification (down) of RhoA activity (RhoA-GTP/Total RhoA ratio) profiles in cell treatment groups: NG (normal glucose, 5 mmol/L), HG (high glucose, 30 mmol/L), Mnt (mannitol, 30 mmol/L) and HG + FDL (high glucose with fasudil) for 48 h. 20% of total protein lysate from each sample served as loading controls. B: Real-time PCR analysis of RhoA activity profiles in each groups. C: ROCK1 activity was measured by western blotting (up) and quantification (down) using the p-MYPT1 (Thr853)/MYPT1 ratio. D: Real-time PCR analysis of ROCK1 mRNA profiles. (A-D: *P < 0.05 HG vs. others, n = 3). Data are presented as means ± SDs.
Additionally, an increase of ROCK1 activity which quantified by MYPT1 (Thr853) phosphorylation was observed after RhoA activation. Regarded as a specific Rho-kinase target [32,33], MYPT1 phosphorylation was markedly increased (P < 0.05) in the HG treated cells group while compared with the NG control. In comparison, treatment with fasudil (HG + FDL group) significantly reduced the increase of p-MYPT1 (P < 0.05) (Figure 1C). Also, we examined the ROCK1 mRNA expression level and acquired the same verification as showed in Figure 1D.
Tight junction damage in response to high glucose requires RhoA/ROCK1 signaling
As previous study described, classic TJ proteins consisted of claudin-5, occludin and ZO-1 etc [34]. In our research, we checked the expression level of these proteins by ways of western blotting and real-time PCR respectively. Meanwhile, our experiments focused on whether RhoA/ROCK1 signaling pathway is involved in the high glucose treatment.
Descendant occludin expression level was discovered after stimulating by HG for 48 hours compared with NG control (Figure 2A). To test the hypothesis that the involvement of RhoA/ROCK1 signaling pathway resulting in decreased occludin protein level, a specific ROCK1-siRNA and a chemical inhibitor of ROCK1, Y-27632, were used as negative controls. As to the pharmacological inhibitor, an extensively applied clinical drug fasudil was selectively chosen afterwards.
Figure 2.
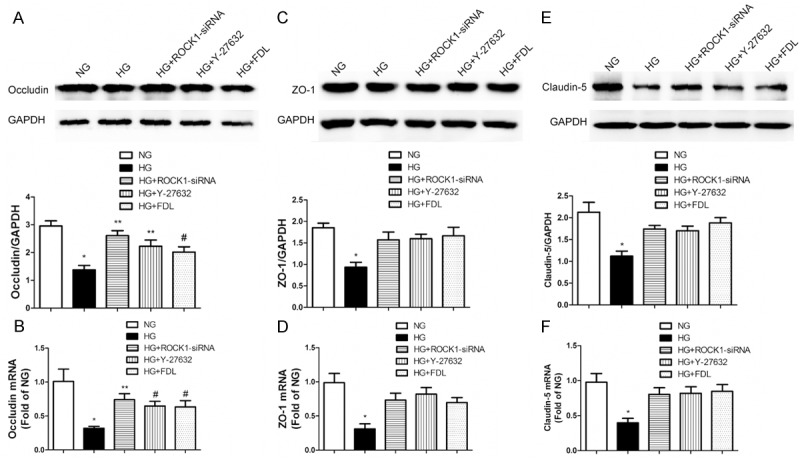
TJ destruction owing to high glucose in RF/6A cells required RhoA/ROCK1 signaling pathway. A: Western blotting (up) and quantification (down) of occludin profiles in five cell treatment groups: NG, HG, high glucose with ROCK1-siRNA (HG + ROCK1-siRNA), high glucose with Y-27632 (HG + Y-27632) and high glucose with fasudil (HG + FDL). B: Real-time PCR analysis of occludin mRNA profiles (A, B: *P < 0.01 vs. NG, **P < 0.01 vs. HG, #P < 0.05 vs. HG, n = 3). C: Western blotting (up) and quantification (down) of ZO-1 profiles. D: Real-time PCR analysis of ZO-1 mRNA profiles. E: Western blotting (up) and quantification (down) of claudin-5 profiles. F: Real-time PCR analysis of ZO-1 mRNA profiles (C-F: *P < 0.05 vs. others, n = 3). Data are presented as means ± SDs.
High glucose down-regulating occludin through RhoA/ROCK1 pathway was confirmed by HG + ROCK1-siRNA group, which revealed a clear increase in western blotting and real-time PCR (Figure 2A, 2B). Whereafter HG + Y-27632 and HG + FDL groups both demonstrated that discrepancy again (P < 0.05).
The other TJ proteins, ZO-1 and claudin-5, which have been verified soon afterwards (Figure 2C-F) also demonstrated the similar tendency like occludin. The decreased level of these two proteins due to high glucose was expectedly restored by ROCK1-siRNA and Y-27632 (P < 0.05). Likewise, cells treated with fasudil displayed a meaningful reverse of these two protein levels (P < 0.05), suggesting a potential clinical therapy target for DR.
Fasudil inhibits high glucose induced microvascular endothelial cell hypertonicity
Figure 3A, 3B shows that high glucose could significantly increased endothelial permeability, compared with NG control as 100% (Figure 3A; P < 0.01; n = 3). Treatment by HG + FDL evidently decreased the BSA clearance rate compared with NG control basal values (P < 0.01; n = 3). As we predicted before, negative controls such as HG + ROCK1-siRNA and HG + Y-27632 groups both showed distinct discrepancy compared with HG groups (P < 0.05; n = 3).
Figure 3.
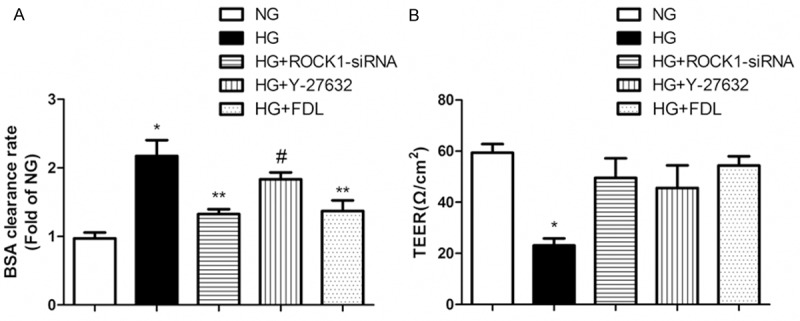
The permeability of RF/6A cells was increased by high glucose and reversed by fasudil. A: The BSA clearance rate of RF/6A cells in five cell treatment groups: NG, HG, HG + ROCK1-siRNA, HG + Y-27632 and HG + FDL (*P < 0.01 vs. NG, **P < 0.01 vs. HG, #P < 0.05 vs. HG, n = 3). B: Utilization of TEER to measure RF/6A cells permeability (*P < 0.01 vs. others, n = 3). Data are presented as means ± SDs.
For purpose of certifying the hypertonicity in consequence of high glucose could be reversed by fasudil once again, we utilized TEER measurement of RF/6A cells to test the hypothesis on the other hand. Corresponded to BSA clearance rate, TEER was also restored approach to baseline in HG + FDL group compared to HG group (Figure 3B; P < 0.01; n = 3). Certain differences were observed in HG + ROCK1-siRNA and HG + Y-27632 groups as well as BSA clearance rate (P < 0.01; n = 3).
Inhibition of RhoA/ROCK1 reverses the endothelial dysfunction induced by high glucose
As mentioned before [35], high glucose triggered two indicators of endothelial dysfunction: intracellular adhesion molecule-1 (ICAM-1) up-regulation and increased cell death by apoptosis. As shown in Figure 4A, 4B, western blotting and real-time PCR both revealed a crucial increase of ICAM-1 protein level in cell group incubated with high glucose (P < 0.01). Meanwhile, treatment of RF/6A cells with RhoA/ROCK1 inhibition in three distinct aspects such as ROCK1-siRNA, Y-27632 and fasudil all chiefly inhibited the ICAM-1 up-regulation owing to hyperglycemia.
Figure 4.

High glucose induced endothelial dysfunction and fasudil restored the response. A: Western blotting (up) and quantification (down) of ICAM-1 profiles in five cell treatment groups: NG, HG, HG + ROCK1-siRNA, HG + Y-27632 and HG + FDL (*P < 0.01 vs. NG, #P < 0.05 vs. HG, n = 3). B: Real-time PCR analysis of ICAM-1 profiles (*P < 0.01 vs. NG, #P < 0.05 vs. HG, **P < 0.01 vs. HG, n = 3). C, D: Fluorescence microscope and quantification analysis of RF/6A cells apoptosis levels (*P < 0.01 vs. others). Data are presented as means ± SDs.
On the other side, RF/6A cells maintained in high glucose for 48 hours showed a significant increase in cell death numbers, as compared with those maintained in normal glucose (Figure 4D). This increase trend was attenuated in negative controls and pharmacological groups respectively.
Discussion
Diabetic retinopathy, a prevalent complication of diabetes, is a leading cause of vision loss [36,37]. Early nonproliferative stages of diabetic retinopathy are characterized by retinal microvascular damage that leads to vascular hyper-permeability [37] and, a certain extent of inflammation. The correlation between diabetes and MVED has been demonstrated in various studies before [38,39]. MVED in DM results in reduced activation of endothelial nitric oxide synthase (eNOS), reduced generation and bioavailability of nitric oxide (NO) and increased production of reactive oxygen species (ROS) [40]. Nonetheless, the mechanism between TJs and hyperglycemia induced RMVED in DR hasn’t yet been researched sufficiently, especially the potentially involved signaling pathways.
The endothelial TJ is representative of a structural barrier with selective paracellular permeability to solutes and larger molecules, and its destruction enhances microvascular permeability [41,42]. Simultaneously, TJ reflects a pathophysiological consequence of disease is also regulated by intracellular signaling pathways such as RhoA etc. Occludin, claudin-5 and ZO-1 are the major transmembrane proteins localizing at the TJ [43]. Increasing evidence suggests that the TJ is a dynamically regulated structure. At the other hand, ICAM-1 stands for the function of the TJ and initial inflammation during DR as well.
RhoA, one of the best characterized Rho GTPase, is known to regulate the formation of F-actin stress fibers and focal adhesion complexes [44]. It is well-known that RhoA small GTPase is an upstream activator of ROCK1 [45]. RhoA and its downstream effectors, ROCK1 and ROCK2, regulate a number of cellular processes, including cell motility, proliferation, survival, and permeability [9]. First we found that the increased level of RhoA and ROCK1 correlated with high glucose, and fasudil can effectively reversed these responses. Furthermore, we showed here that high glucose led to the up-regulation of biomarkers of hypertonicity, BSA clearance rate and TEER, which subsequently been restored mostly by ROCK1 inhibition.
As far as clinical significance to be concerned, we selectively sorted out three classic TJ proteins to explore the correlation between the ascended permeability owing to hyperglycemia and the RhoA/ROCK1 pathway. For the purpose of testing the previous hypothesis, we took advantage of three negative control groups of ROCK1 such as small interference RNA (ROCK1-siRNA), chemical inhibitor (Y-27632) and pharmacological inhibitor (Fasudil) to detect TJ proteins expression level. Above all, RhoA/ROCK1 activity activated by high glucose was clearly verified. Soon afterwards, the potential role of RhoA/ROCK1 signaling in early stage of endothelial dysfunction was examined. Similar but not completely equivalent of forepassed experiments, these negative groups all demonstrated meaningful distinction compared with positive group (P < 0.05). Of importance, we also found that RhoA/ROCK1 inhibition resulted in elevated level of TJ proteins.
Inspired considerably by these observations, we went on to assess the forward dysfunction of endothelium described above, such as permeability, ICAM-1 and apoptosis. The results shown in figures were completely and clearly in favor of our incipient hypothesis. Most of all, both the increase and recovery of permeability and apoptosis are mediated by fluctuations of RhoA/ROCK1 activity, opening a new perspective for the prevention, treatment and control of diabetic retinopathy.
In conclusion, our results provide molecular insight into the regulation of the endothelial dysfunction induced by high glucose in RF/6A cells. Using in vitro models, we demonstrate the crucial role of RhoA/ROCK1 in the control of inflammation and apoptosis through high glucose dependent pathways. We also provide evidence that fasudil inhibited the RhoA/ROCK1 resulting from the high glucose. Our data suggest that RhoA/ROCK1 can be a potential therapeutic target for treatments aimed at reducing hypertonicity and high apoptosis of high glucose. A deep insight into the molecular details of endothelial function regulation could be obtained by recent studies using live cells and advanced experimental techniques.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (81271032).
Disclosure of conflict of interest
None.
References
- 1.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasiotis H, Kolosov D, Bui P, Kelly SP. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: a review. Respir Physiol Neurobiol. 2012;184:269–281. doi: 10.1016/j.resp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Takayama N, Kai H, Kudo H, Yasuoka S, Mori T, Anegawa T, Koga M, Kajimoto H, Hirooka Y, Imaizumi T. Simvastatin prevents large blood pressure variability induced aggravation of cardiac hypertrophy in hypertensive rats by inhibiting RhoA/Ras-ERK pathways. Hypertens Res. 2011;34:341–347. doi: 10.1038/hr.2010.229. [DOI] [PubMed] [Google Scholar]
- 7.Chew TW, Liu XJ, Liu L, Spitsbergen JM, Gong Z, Low BC. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33:2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Casey PJ, Kumar AP, Pervaiz S. Deciphering the signaling networks underlying simvastatin-induced apoptosis in human cancer cells: evidence for non-canonical activation of RhoA and Rac1 GTPases. Cell Death Dis. 2013;4:e568. doi: 10.1038/cddis.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D’Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riou P, Kjaer S, Garg R, Purkiss A, George R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, O’Reilly N, McDonald NQ, Parker PJ, Ridley AJ. 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell. 2013;153:640–653. doi: 10.1016/j.cell.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, Hung MC, Pandolfi PP, Lin HK. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 14.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng H, Luo P, Li Y, Wang C, Liu X, Ye Z, Li C, Lou T. Simvastatin alleviates hyperpermeability of glomerular endothelial cells in early-stage diabetic nephropathy by inhibition of RhoA/ROCK1. PLoS One. 2013;8:e80009. doi: 10.1371/journal.pone.0080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Lim SB, Ng MY, Ali SM, Kausalya JP, Limviphuvadh V, Maurer-Stroh S, Hunziker W. ZO-1 regulates Erk, Smad1/5/8, Smad2, and RhoA activities to modulate self-renewal and differentiation of mouse embryonic stem cells. Stem Cells. 2012;30:1885–1900. doi: 10.1002/stem.1172. [DOI] [PubMed] [Google Scholar]
- 18.Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57:1683–1692. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]
- 19.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin J, Yuan F, Shen MQ, Feng YF, He QL. Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem. 2013;381:267–272. doi: 10.1007/s11010-013-1710-y. [DOI] [PubMed] [Google Scholar]
- 21.adlapatla RK, Vadlapudi AD, Pal D, Mukherji M, Mitra AK. Ritonavir inhibits HIF-1alpha-mediated VEGF expression in retinal pigment epithelial cells in vitro. Eye (Lond) 2014;28:93–101. doi: 10.1038/eye.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59:3159–3166. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo I, Viretto M, Barale C, Mattiello L, Doronzo G, Pagliarino A, Cavalot F, Trovati M, Anfossi G. High glucose inhibits the aspirin-induced activation of the nitric oxide/cGMP/cGMP-dependent protein kinase pathway and does not affect the aspirin-induced inhibition of thromboxane synthesis in human platelets. Diabetes. 2012;61:2913–2921. doi: 10.2337/db12-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Apostolova MD, Cherian MG, Chakrabarti S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab Invest. 2000;80:1311–1321. doi: 10.1038/labinvest.3780139. [DOI] [PubMed] [Google Scholar]
- 25.Dang L, Seale JP, Qu X. Reduction of high glucose and phorbol-myristate-acetate-induced endothelial cell permeability by protein kinase C inhibitors LY379196 and hypocrellin A. Biochem Pharmacol. 2004;67:855–864. doi: 10.1016/j.bcp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Dang L, Seale JP, Qu X. High glucose-induced human umbilical vein endothelial cell hyperpermeability is dependent on protein kinase C activation and independent of the Ca2+-nitric oxide signalling pathway. Clin Exp Pharmacol Physiol. 2005;32:771–776. doi: 10.1111/j.1440-1681.2005.04266.x. [DOI] [PubMed] [Google Scholar]
- 27.Quan X, Godfrey HP. Falcon Technical Bulletin 413. Becton Dickinson, Franklin Lakes, NJ: 1998. In Vitro Study of Cytokine-Mediated Activation of Endothelial Cell Permeability Using Falcon Cell Culture Inserts. [Google Scholar]
- 28.Bonner SM, O’Sullivan MA. Endothelial cell monolayers as a model system to investigate dengue shock syndrome. J Virol Methods. 1998;71:159–167. doi: 10.1016/s0166-0934(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 29.Dalpiaz A, Paganetto G, Pavan B, Fogagnolo M, Medici A, Beggiato S, Perrone D. Zidovudine and ursodeoxycholic acid conjugation: design of a new prodrug potentially able to bypass the active efflux transport systems of the central nervous system. Mol Pharm. 2012;9:957–968. doi: 10.1021/mp200565g. [DOI] [PubMed] [Google Scholar]
- 30.Pavan B, Capuzzo A, Forlani G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp Eye Res. 2014;120:50–54. doi: 10.1016/j.exer.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Peiris D, Pacheco I, Spencer C, MacLeod RJ. The extracellular calcium-sensing receptor reciprocally regulates the secretion of BMP-2 and the BMP antagonist Noggin in colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2007;292:G753–766. doi: 10.1152/ajpgi.00225.2006. [DOI] [PubMed] [Google Scholar]
- 32.Filina JV, Gabdoulkhakova AG, Safronova VG. RhoA/ROCK downregulates FPR2-mediated NADPH oxidase activation in mouse bone marrow granulocytes. Cell Signal. 2014;26:2138–2146. doi: 10.1016/j.cellsig.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Bouchareb R, Boulanger MC, Fournier D, Pibarot P, Messaddeq Y, Mathieu P. Mechanical strain induces the production of spheroid mineralized microparticles in the aortic valve through a RhoA/ROCK-dependent mechanism. J Mol Cell Cardiol. 2014;67:49–59. doi: 10.1016/j.yjmcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez SH, Fan S, Dykstra H, Rom S, Mercer A, Reichenbach NL, Gofman L, Persidsky Y. Inhibition of glycogen synthase kinase 3beta promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. PLoS One. 2013;8:e55972. doi: 10.1371/journal.pone.0055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617–623. doi: 10.1016/j.exer.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21:127–144. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 37.Zheng B, Li T, Chen H, Xu X, Zheng Z. Correlation between ficolin-3 and vascular endothelial growth factor-to-pigment epithelium-derived factor ratio in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 2011;152:1039–1043. doi: 10.1016/j.ajo.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Saker S, Stewart EA, Browning AC, Allen CL, Amoaku WM. The effect of hyperglycaemia on permeability and the expression of junctional complex molecules in human retinal and choroidal endothelial cells. Exp Eye Res. 2014;121:161–167. doi: 10.1016/j.exer.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Leal EC, Aveleira CA, Castilho AF, Serra AM, Baptista FI, Hosoya K, Forrester JV, Ambrosio AF. High glucose and oxidative/nitrosative stress conditions induce apoptosis in retinal endothelial cells by a caspase-independent pathway. Exp Eye Res. 2009;88:983–991. doi: 10.1016/j.exer.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Balakumar P, Chakkarwar VA, Krishan P, Singh M. Vascular endothelial dysfunction: a tug of war in diabetic nephropathy? Biomed Pharmacother. 2009;63:171–179. doi: 10.1016/j.biopha.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim Biophys Acta. 2008;1778:646–659. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Wang T, Gui P, Yao C, Sun W, Wang L, Wang H, Xie W, Yao S, Lin Y, Wu Q. Resolvin D1 reverts lipopolysaccharide-induced TJ proteins disruption and the increase of cellular permeability by regulating IkappaBalpha signaling in human vascular endothelial cells. Oxid Med Cell Longev. 2013;2013:185715. doi: 10.1155/2013/185715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 44.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 45.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]


