Abstract
Purpose: Osteopontin (OPN) is known to be a secreted adhesive glycoprotein. Role of OPN in human intrahepatic cholangiocarcinoma (ICC) has not been well understood. This study explored whether genetic variations in the osteopontin gene are associated with ICC risk, progression and metastasis. Material and methods: 260 patients with stages I to IV between 2008 and 2013 were recruited in this study and same number healthy persons were used as control. OPN-66 T/G, -156 G/GG and -443 C/T variants were genotyped using DNA from blood lymphocytes. Chi-square test and a Fisher’s exact test were used to analyze the genotype distribution between healthy subjects and patients, and further its distribution among TNM stages and incidence metastasis in patients. Results: For the variant at nt- 443 (CC), there was a significant difference between the number of patients with stage IV and those with all other stages of ICC (P < 0.01). Patients with -443 (CC) variant had significant higher incidence of lymph and distant metastasis development compared to other genotypes. For the variant at nt- 443 (CT), there was a significant difference between the number of ICC patients with stage III + IV and those with stage I + II (P < 0.01). The survival rates for ICC patients with the C/C genotype were significantly lower than for patients with the other two genotypes (C/T, T/T). Conclusion: OPN -443 C/T polymorphism is a potential predictive marker of metastasis and poor prognosis in ICC patients.
Keywords: Osteopontin, intrahepatic cholangiocarcinoma, genetic variants, metastasis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most frequent form of primary hepatic malignancy in adults after hepatocellular carcinoma [1]. Although significant progress in the diagnostic and surgical approaches has been achieved, the survival rates for patients with ICC remain unfavorable [2-4]. Despite recent advances, the molecular or genetic mechanisms involved in the progression of ICC still remain poorly understood. Thus, to develop effective individualized treatments based on molecular classification are pivotal to improve the prognosis of ICC patients. Although many biomarkers have been evaluated for their prognostic significance in ICC, none of these have been proven to be a predictive power of the prognosis with high specificity and sensitivity for ICC.
Osteopontin (OPN) is a secreted non-collagenous, sialic-acid-rich, chemokine-like extracellular matrix (ECM) protein [5]. OPN binds to αvβ integrins and receptors of the CD44 family to promote cell adhesion, chemotaxis, ECM degradation, angiogenesis, prevention of apoptosis, and indolent tumor growth [6]. Moreover, it plays a crucial role in determining the oncogenic potential of various cancers, contributing to tumor invasion and metastasis [7-10]. Previously lots of studies have demonstrated that OPN is one of the highest overexpressed genes in hepatocellular carcinoma (HCC) and it has been shown the expression of OPN correlates with earlier recurrence, poorer prognosis and metastasis in HCC [11,12]. However, about its role in the ICC, there are few controversy reports so far. Based on our best knowledge, there are three reports about the OPN expression condition in ICC. Terashi et al observed a correlation between low OPN levels and tumor aggressiveness [13], whereas another group found high levels of the glycoprotein in ICC in a rat model system [14]. More interestingly, Holger G Hass identified osteopontin as the most consistently over-expressed gene in intrahepatic cholangiocarcinoma by oligonucleotide microarray and real-time PCR analysis from surgical specimens [15]. Therefore, it deserves further study about whether OPN is a potential prognostic marker and target for anticancer treatment in ICC.
Single nucleotide polymorphisms at oncogene or tumor suppressor gene promoter region sometimes can influence the gene expression significantly, thus causing significant difference on cancer occurrence, metastasis and prognosis, et al. These genetic variants may be good biomarkers for predict prognosis for cancer patients. Previous study has confirmed that OPN promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. However, there are no relative reports about the relationship between OPN polymorphisms with ICC currently. In the present study, we recruited 260 ICC patients and 260 cancer-free control, aim to investigate whether OPN promoter polymorphisms -66 T/G, -156 G/GG, and -443 C/T genotypes affect the occurrence, metastasis and prognosis of ICC patients.
Patients and methods
Patients
This study comprises a total of 260 primary ICC patients who had not undergone preoperative treatment (with a mean age of 59 ± 11.2 years) seen at the Clinic of General Surgery and Transplantation of the 301 Hospital, between August 2008 and June 2013. The diagnosis of ICC was based on histology obtained by preoperative or intraoperative biopsy or by examination of resected liver specimens. Patients with gallbladder carcinoma or mixed hepatocellular carcinoma/ICC and cirrhosis were excluded from this study. Available data included age, sex, tumor location, tumor size, histological differentiation, tumor stroma, vascular invasion (portal vein, hepatic vein, hepatic artery, and bile duct invasion), lymphatic permeation, perineural invasion, intrahepatic metastasis, and lymph node metastasis (Table 1).
Table 1.
Clinicopathologic characteristics of patients with ICC and healthy controls
| No. of patients or controls | |||
|---|---|---|---|
|
|
|||
| Characteristics | Case (n) | controls (n) | P |
| No. | 260 | 260 | |
| Age, y | > 0.05 | ||
| Median | 57.2 | 56.3 | |
| Range | 24-81 | 23-87 | |
| Gender | > 0.05 | ||
| Male | 149 | 147 | |
| Female | 111 | 113 | |
| Alcohol abusing | < 0.01 | ||
| Never | 170 | 218 | |
| Former | 26 | 13 | |
| Current | 64 | 29 | |
| Location | - | - | |
| Hilar | 89 | - | |
| Peripheral | 171 | - | |
| Histology | - | ||
| Differentiated | 85 | - | |
| Undifferentiated | 175 | - | |
| UICC stage grouping (sixth edition) | - | ||
| I | 49 | - | |
| II | 67 | ||
| III (A-C) | 115 | - | |
| IV | 29 | -- | |
| Lymph node | -- | ||
| Positive | 160 | - | |
| Negative | 100 | - | |
| Tumor size (cm) | - | ||
| ≤ 4 | 138 | - | |
| > 4 | 122 | - | |
| Distant metastasis | |||
| Yes | 29 | ||
| No | 231 | ||
Healthy control group consisted of a random sample of 260 age-matched and sex-matched ethnic Han Chinese from Beijing.
All the participants agreed to participate in this study and had adequate blood DNA for genotyping and all had complete follow-up and clinical information. There was no significant difference in the distribution of demographic information between patients enrolled and patients who did not. Written informed consent was obtained from each participant for the use of their DNA and clinical information. The study was approved by the Institutional Review Board of 301 Hospital, Beijing, China.
SNP genotyping
Genomic DNA was extracted from 5-mL blood sample that was collected from each patient and healthy subject upon recruitment. The OPN-66 T/G, -156 G/GG (rs17524488), and -443 C/T (rs11730582) variants were genotyped by direct sequencing of the sense and anti-sense strands following polymerase chain reaction (PCR) amplification of the promoter regulatory region -473 to -3 (forward primer 50-CAA GCT ACT GCA TAC TCG AAA TCA CA-30; reverse primer 50-ACA ACC AAG CCC TCC CAG AAT TTA-30), as previously described [16]. PCR was performed using 50 ng DNA as a template under the following conditions: 95°C for 10 min, then 36 cycles of 94°C for 30 s, an annealing temperature for 60 s, and 72°C for 60 s, with a final extension at 72°C for 15 min. After affinity membrane purification using the QIAquick Gel Extraction kit (Qiagen, Carlsbad, CA, USA), the PCR products were subjected to cycle sequencing with the respective forward and reverse primer using an automated ABI 3100 DNA sequencer by GeneCore Bio Technologies (Shanghai China). A 15% blind, random sample of study subjects was genotyped twice by different persons and the reproducibility was 100%.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software. Quantitative variables departing from the normal distribution, including age, gender and alcohol abusing status were summarized. Comparison of age between cases and controls was assessed using an independent Student’s t-test. Comparison of gender, alcohol abusing status and genotype frequencies between cases and controls was assessed using a chi-square test and a Fisher’s exact test. Survival was calculated by the Kaplan-Meier method. All probability (P) values were two-tailed and statistical significance was indicated as P < 0.05.
Results
Patient characteristics and clinical outcomes
This study recruited 260 patients with ICC and 260 healthy controls. The baseline clinical characteristics of patients are summarized in Table 1. There were no significant differences in terms of distribution of age and gender, but significant on alcohol abusing status, suggest alcohol abusing is one of risk factors. Clinicopathologic characteristics of the patients and controls are shown in Table 1.
SNPs in the promoter region of human OPN gene
Direct sequencing of DNA fragments between nt-473 and nt-3 in patients and age- and gender-matched controls revealed 3 SNPs in the OPN promoter, located at nt -156 [GG/GG homozygotes, GG/G-(deletion) heterozygotes, G-/G- homozygotes], nt- 443 [CC homozygotes, CT heterozygotes, TT homozygotes], and nt -66 (Figure 1), as shown in Table 2. There was no significant difference in the distribution of these SNPs (nt -66, -156, -443) between patients and controls. The distribution of genotypes for TNM stages in ICC patients is shown in Table 3. However, regarding tumor-node-metastasis TNM stages, we found that for the SNP at nt- 443, there was significant difference on distribution of three genotypes among four stages (P < 0.001, Table 3). Among patients with the CT genotype, there was a significant difference between patients with stages I + II and stages III + IV (P < 0.01), data was shown in Table 4. Similarly, among patients with the CC genotype at nt- 443, there was a significant difference between patients with stages III + IV and stages I + II (P < 0.01) and between stages IV and combination of stage I to stage III (P < 0.01; Table 4). There were no significant differences among the TNM stages and the other two SNPs (nt -66 and nt -156) of the OPN promoter.
Figure 1.
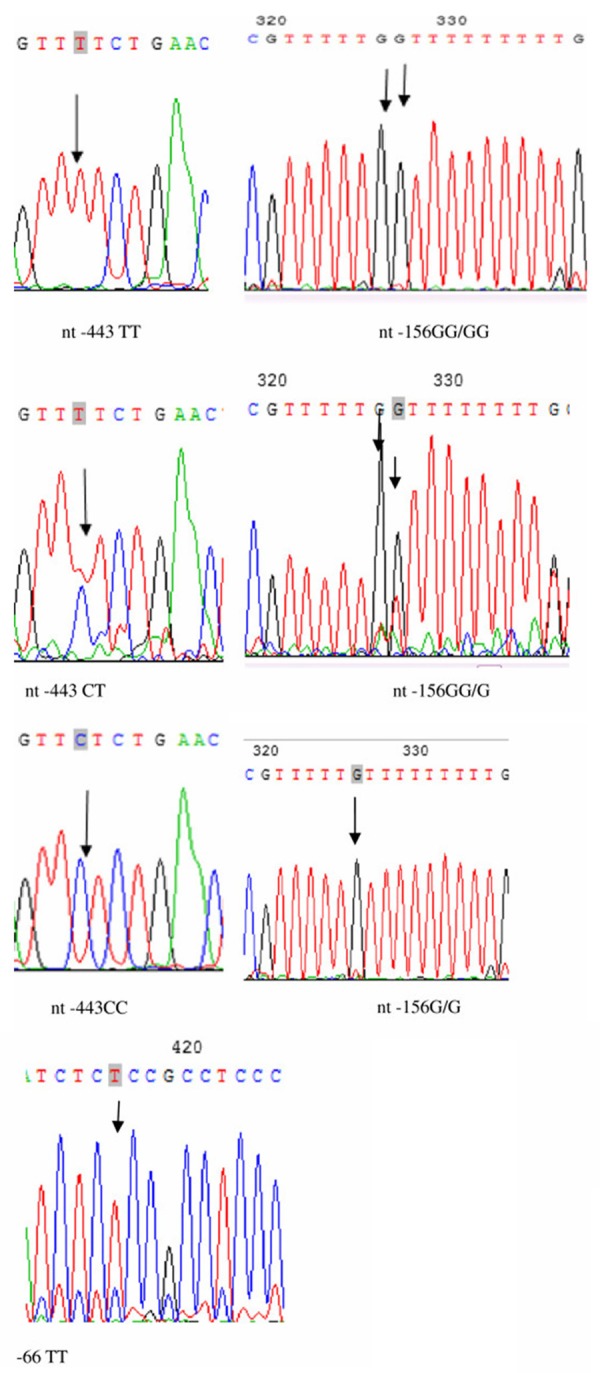
Schematic diagram and sequencing data of the OPN promoter. Representative figure for the sequencing analysis on the promoter. The SNP nt- 443 has the following alleles: CC, CT, and TT. There is a small insertion at nt-156, which has three alleles: G/G, G/GG, GG/GG. The SNP nt-66 has only one allele: TT.
Table 2.
Comparison of OPN promoter between ICC patients and healthy controls
| controls | Patients | ICC | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| n | n | P | LN (+) | LN (-) | P | DM (-) | DM (+) | P | |
| -66 T/G | |||||||||
| TT | 251 | 256 | 1.00 | 158 | 99 | 1.00 | 228 | 28 | 1 |
| TG | 9 | 4 | 0.261 | 2 | 1 | 0.637 | 3 | 1 | 0.537 |
| -156 | |||||||||
| G/G | 107 | 111 | 1.00 | 53 | 35 | 1.00 | 101 | 13 | 1 |
| G/GG | 110 | 101 | 0.563 | 74 | 45 | 0.775 | 92 | 10 | 0.826 |
| GG/GG | 43 | 48 | 0.804 | 33 | 20 | 0.860 | 38 | 6 | 0.786 |
| -443 | |||||||||
| TT | 114 | 120 | 1 | 49 | 47 | 1.00 | 111 | 8 | 1.000 |
| CT | 115 | 111 | 0.709 | 63 | 38 | 0.116 | 102 | 9 | 0.803 |
| CC | 31 | 29 | 0.773 | 48 | 15 | 0.002 | 17 | 12 | < 0.001 |
Note: LN: Lymph node metastasis; P value was calculated by chi-square test and a Fisher’s exact test. DM: distant metastasis.
Table 3.
The distribution of genotypes for TNM stages among ICC patients
| The TNMs of ICC | |||||
|---|---|---|---|---|---|
|
|
|||||
| Genotypes | I | II | III | IV | P |
| -66 | 0.603 | ||||
| TT | 48 | 67 | 113 | 28 | |
| TG | 1 | 0 | 2 | 1 | |
| -156 | 0.730 | ||||
| G/G | 21 | 34 | 46 | 10 | |
| G/GG | 18 | 24 | 47 | 12 | |
| GG/GG | 10 | 9 | 22 | 7 | |
| -443 | < 0.001 | ||||
| TT | 36 | 35 | 41 | 8 | |
| CT | 11 | 28 | 63 | 9 | |
| CC | 2 | 4 | 11 | 12 | |
Note: P value refers to significance value among all the groups on SNP, and was calculated by person chi-square test.
Table 4.
The genotype distribution of nt- 443 in the OPN promoter by ICC TNM stage
| The TNM stages of ICC | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Genotypes | I + II | III + IV | P | I + II + III | IV | P |
| -443 | ||||||
| TT | 71 | 49 | 1.000 | 111 | 8 | 1.000 |
| CT | 39 | 72 | < 0.001 | 102 | 9 | 0.803 |
| CC | 6 | 23 | < 0.001 | 17 | 12 | < 0.001 |
Effect of SNPs on lymph and distant metastasis
As shown in Tables 2 and 3, there were total 29 patients who had CC genotype at nt- 443, among them, 12 cases were at stage IV and had distant metastasis. By compared with TT genotype, it demonstrated that CC genotype at nt- 443 might significantly increase the risk of development of distant metastasis (P < 0.01). We also found that significant association between the -443 genotypes in the OPN promoter and lymph node metastasis, type CC had more risks to develop lymph node metastasis (Table 2).
Associations between genotypes in the OPN promoter region and survival
Kaplan-Meier estimates of different genotypes at nt- 443 in the OPN promoter are shown in Figure 2. The survival rates for patients with the C/C genotype were significantly lower than the survival rates for patients with the other two genotypes (C/T, T/T). There were no significant associations between survival and genotypes at the other sites (nt -156 and nt -66).
Figure 2.
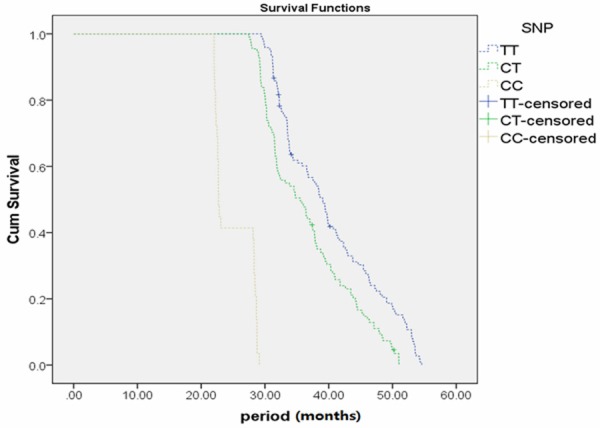
Kaplan-Meier survival is significantly lower in ICC patients with the C/C genotype as compared to the other two genotypes at nt -443 in OPN promoter.
Discussion
Based on my knowledge, it is first time to report the relationship between OPN polymorphisms and risk of ICC patients. Lots of evidence suggests that OPN plays a role in the regulation of tumor metastasis and that OPN expression is particularly high in metastatic tumors [17-19]. OPN is overexpressed in cancers that have a high propensity for forming distant metastases. Moreover, high OPN expression in the primary tumor is associated with early metastasis and poor clinical outcome in hepatocellular carcinoma and other cancers [16,17,20-23].
There is arguing about the role of OPN in ICC patients. Dr Tomohiro Iguchi selected OPN expression as a risk factor in the split of the survival tree model in ICC and demonstrated that lower expression of OPN was the best predictor of the patients’ prognosis [1]. In the present study, we focused on the association of these SNPs with TNM stages of ICC, especially for distant metastasis. Although the distribution of genotypes (CC) at nt- 443 in the OPN promoter was not significantly different between ICC patients and healthy controls, there were significant difference in the distribution of genotypes between patients with stage IV and other stage ICC (Table 4).
Recent study proved that the haplotype -443C/-156 G/-66 T of OPN gene is associated with significantly enhanced promoter activity compared to five other allelic variants tested [24]. A recent study on melanoma metastases found that those homozygous for the -443C allele expressed significantly higher levels of OPN mRNA compared to those that were either heterozygous (CT) or homozygous for the -443 T allele [25]. Transcription factor c-Myb binds to the region of the OPN promoter in an allele-specific manner and induces enhanced activity of the -443C compared to the -443 T OPN promoter [26]. Taken together, these data suggest that the variation at nt- 443 in the OPN promoter plays a role in GC progression and metastasis, especially for the CC genotype at nt- 443 in the OPN promoter. Whether the polymorphisms of OPN are related to expression of OPN in cancer patients remain unknown. Over-expression of OPN was found in ICC samples in a previous study [15], and the alteration of the -443 T → C promoter region could significantly increase the promoter activity by Dual Luciferase Reporter Assay System [16].
In the present study, we found that the CT genotype at nt- 443 in the OPN promoter showed significant differences between stages III + IV and stage I + II ICC, but no significant difference between stage IV and sum of other stages of ICC (Table 4); and for the CC genotype, there was significant difference between stage IV and other single stages or combination of any other stages. The main reason for this may be due to the limited number of patients in CC type subgroups. It is also possible that the CC genotype has more enhanced transcription activity of the region of the OPN promoter compared to CT genotypes [25]. Among total 29 CC genotype patients, 12 patients were diagnosed as distant metastasis, it is extremely high, but there is no significant difference on the ratio of CC type between ICC patients and healthy controls. The main reason for this, we hypothesize that OPN is a not key factor for initiating ICC, but once the carcinogenesis occurred, OPN will enhance this process effectively, especially for distant metastasis and lymph metastasis, which is consistent with previous study [27]. However, the further study is needed to investigate this hypothesis. Meanwhile, the current study also provides another evidence to suggest over-expression of OPN may correlate with the poor prognosis of ICC, which is different from Terashi’s report.
There are also some drawbacks in the present study, one of them is because all the subjects are Chinese individuals, the results should be interpreted with caution and need to be confirmed in larger and ethnically divergent population samples. On the other hand, the number of stage IV patients in the current study is not high enough, so the large-population research is needed to make stronger conclusion about the association between distant metastasis formation and -433 polymorphisms.
In summary, -443 C/T of OPN is a potential biomarker for predicting prognosis of ICC especially for lymph and distant metastasis.
Acknowledgements
This study was supported by a grant from the Foundation of the ‘Twelfth Five-year Plan’ for the Medical Science Development of People’s Liberation Army (grant no. CWS11J109).
Disclosure of conflict of interest
None.
References
- 1.Iguchi T, Yamashita N, Aishima S, Kuroda Y, Terashi T, Sugimachi K, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. A comprehensive analysis of immunohistochemical studies in intrahepatic cholangiocarcinoma using the survival tree model. Oncology. 2009;76:293–300. doi: 10.1159/000207506. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 4.Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:231–246. doi: 10.1016/j.soc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou HJ, Dai C, Sun HJ, Qin Y, Zhang WD, Ren N, Ye QH, Qin LX. Osteopontin promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013;57:1024–1034. doi: 10.1002/hep.26103. [DOI] [PubMed] [Google Scholar]
- 6.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathology international. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 8.Pan HW, Ou YH, Peng SY, Liu SH, Lai PL, Lee PH, Sheu JC, Chen CL, Hsu HC. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–127. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 9.Jin Y, Tong DY, Tang LY, Chen JN, Zhou J, Feng ZY, Shao CK. Expressions of Osteopontin (OPN), alphanubeta3 and Pim-1 Associated with Poor Prognosis in Non-small Cell Lung Cancer (NSCLC) Chin J Cancer Res. 2012;24:103–108. doi: 10.1007/s11670-012-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai WC, Lee HS, Jin JS, Gao HW, Chao TK, Chen A, Nieh S, Chan DC, Chang FN, Lin CK. Association between Osteopontin and EGFR Expression with Clinicopathological Parameters in Hepatocellular Carcinoma. Chin J Physiol. 2012;55:412–420. doi: 10.4077/CJP.2012.BAA082. [DOI] [PubMed] [Google Scholar]
- 12.Chen RX, Xia YH, Cui JF, Xue TC, Ye SL. Osteopontin, a single marker for predicting the prognosis of patients with tumor-node-metastasis stage I hepatocellular carcinoma after surgical resection. J Gastroenterol Hepatol. 2010;25:1435–1442. doi: 10.1111/j.1440-1746.2010.06277.x. [DOI] [PubMed] [Google Scholar]
- 13.Terashi T, Aishima S, Taguchi K, Asayama Y, Sugimachi K, Matsuura S, Shimada M, Maehara S, Maehara Y, Tsuneyoshi M. Decreased expression of osteopontin is related to tumor aggressiveness and clinical outcome of intrahepatic cholangiocarcinoma. Liver Int. 2004;24:38–45. doi: 10.1111/j.1478-3231.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 14.Takemura F, Inaba N, Miyoshi E, Furuya T, Terasaki H, Ando S, Kinoshita N, Ogawa Y, Taniguchi N, Ito S. Optimization of liver biopsy RNA sampling and use of reference RNA for cDNA microarray analysis. Anal Biochem. 2005;337:224–234. doi: 10.1016/j.ab.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Hass HG, Nehls O, Jobst J, Frilling A, Vogel U, Kaiser S. Identification of osteopontin as the most consistently over-expressed gene in intrahepatic cholangiocarcinoma: detection by oligonucleotide microarray and real-time PCR analysis. World J Gastroenterol. 2008;14:2501–2510. doi: 10.3748/wjg.14.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujihara CK, Arcos-Fajardo M, Brandao De Almeida Prado E, Jose Brandao De Almeida Prado M, Sesso A, Zatz R. Enhanced glomerular permeability to macromolecules in the Nagase analbuminemic rat. Am J Physiol Renal Physiol. 2002;282:F45–50. doi: 10.1152/ajprenal.2002.282.1.F45. [DOI] [PubMed] [Google Scholar]
- 18.Deuther-Conrad W, Franke S, Sommer M, Henle T, Stein G. Differences in the modulating potential of advanced glycation end product (AGE) peptides versus AGE proteins. Kidney Int Suppl. 2001;78:S63–66. doi: 10.1046/j.1523-1755.2001.59780063.x. [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen BL, Kamps WA, Hartel RM, Veth RP, Sluiter WJ, Hoekstra HJ. Effect of single chemotherapeutic agents on lhe growing skeleton of the rat. Ann Oncol. 2000;11:1121–1126. doi: 10.1023/a:1008352620870. [DOI] [PubMed] [Google Scholar]
- 20.Belovari T, Bulic-Jakus F, Juric-Lekic G, Maric S, Jezek D, Vlahovic M. Differentiation of rat neural tissue in a serum-free embryo culture model followed by in vivo transplantation. Croat Med J. 2001;42:611–617. [PubMed] [Google Scholar]
- 21.Rennen HJ, Makarewicz J, Oyen WJ, Laverman P, Corstens FH, Boerman OC. The effect of molecular weight on nonspecific accumulation of (99m)T-labeled proteins in inflammatory foci. Nucl Med Biol. 2001;28:401–408. doi: 10.1016/s0969-8051(01)00208-6. [DOI] [PubMed] [Google Scholar]
- 22.Mirshafiey A, Mehrabian F, Razavi A, Shidfar MR, Namaki S. Novel therapeutic approach by culture filtrate of Cryptococcus neoformans var. gattii (CneF) in experimental immune complex glomerulonephritis. Gen Pharmacol. 2000;34:311–319. doi: 10.1016/s0306-3623(00)00075-6. [DOI] [PubMed] [Google Scholar]
- 23.Largo R, Gomez-Garre D, Santos S, Penaranda C, Blanco J, Esbrit P, Egido J. Renal expression of parathyroid hormone-related protein (PTHrP) and PTH/PTHrP receptor in a rat model of tubulointerstitial damage. Kidney Int. 1999;55:82–90. doi: 10.1046/j.1523-1755.1999.00241.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunimasa JI, Itoga Y, Yasuhara M, Hori R, Inui KI. Pharmacokinetics and pharmacological effect of recombinant human granulocyte colony-stimulating factor conjugated to poly(styrene-co-maleic acid) in rats. J Pharm Pharmacol. 1999;51:777–782. doi: 10.1211/0022357991773131. [DOI] [PubMed] [Google Scholar]
- 25.Huang JS, Guh JY, Hung WC, Yang ML, Lai YH, Chen HC, Chuang LY. Role of the Janus kinase (JAK)/signal transducters and activators of transcription (STAT) cascade in advanced glycation end-product-induced cellular mitogenesis in NRK-49F cells. Biochem J. 1999;342:231–238. [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas ME, Brunskill NJ, Harris KP, Bailey E, Pringle JH, Furness PN, Walls J. Proteinuria induces tubular cell turnover: A potential mechanism for tubular atrophy. Kidney Int. 1999;55:890–898. doi: 10.1046/j.1523-1755.1999.055003890.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Liu H, Wu W, Li Y, Li J. Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J Exp Clin Cancer Res. 2013;32:45. doi: 10.1186/1756-9966-32-45. [DOI] [PMC free article] [PubMed] [Google Scholar]


